
Computed tomography characteristics of acute pancreatitis based on different etiologies at different onset times: a retrospective cross-sectional study
Introduction
Acute pancreatitis (AP) is a clinical condition resulting from inflammation of the pancreas and its systemic repercussions (1). The main causes of AP are cholelithiasis (gallstones), alcoholism, high blood triglycerides, high blood calcium, and idiopathic cases (2). Previous studies have shown that the severity, mortality, and prognosis are different across the various etiologies of AP (3-6).
As the first-choice imaging examination to diagnose AP, computed tomography (CT) is suitable to observe the dynamic changes of different etiologies at different onset times (7-9). Previous studies showed that the proportion of necrotizing pancreatitis (NP), imaging scores (such as CT severity index score), and local complications (pancreatic fluid collections) were different across the various etiologies (10-18). The time from symptom onset to imaging of these studies also varied. The imaging characteristics of different etiologies and trends of changes at different onset times of different etiologies remain unclear. Therefore, we conducted this study to investigate the CT characteristics of AP based on different etiologies, including: (I) the CT findings of AP with different etiologies; (II) the CT characteristics for different etiologies at different onset times; and (III) the comparison of CT with clinical characteristics based on different etiologies in different phases of AP. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1231/rc).
Methods
Study design and setting
This is a retrospective cross-sectional study evaluating the CT characteristics of the first attack of AP based on different etiologies at different onset times. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Affiliated Hospital of North Sichuan Medical College (No. 2021ER[A]017), and individual consent for this retrospective analysis was waived.
Identification of patients
Between 1 January 2015 and 31 December 2019, patients with a first episode of AP admitted to 3 tertiary referral centers based in 3 different prefecture-level cities in the province of Sichuan were included. These centers were the Affiliated Hospital of North Sichuan Medical College, Chinese People’s Liberation Army Western Theater General Hospital, and Suining Central Hospital.
For the diagnosis of AP, the presence of at least 2 of the following criteria was required: (I) consistent abdominal pain, (II) serum amylase and/or lipase above 3 times the upper limit of normal, and (III) typical imaging findings of AP.
The inclusion criteria of our study were as follows: (I) inpatient, (II) patients diagnosed with primary AP for the first time, (III) patients who underwent both plain and contrast-enhanced CT (CECT), and (IV) patients who had corresponding clinical and laboratory data within 3 days before or after the CT examination to diagnose the clinical severity.
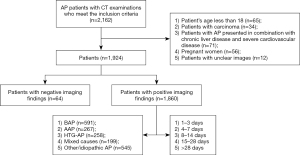
The exclusion criteria were as follows: (I) the age of the patient was less than 18 years; (II) patients with pancreatic carcinoma or any tumor that affects metabolism; (III) patients with AP who presented in combination with chronic liver disease and severe cardiovascular disease; (IV) pregnant patients; and (V) patients with unclear images.
Different disease etiologies were diagnosed according to the following criteria. Biliary AP represents the main etiological background of AP globally and it is diagnosed by imaging techniques (1). Alcoholic AP is caused by excessive alcohol consumption prior to onset or with a clinical history of >5 years and alcohol consumption >50–100 g/day (1). Hypertriglyceridemic AP is related to triglycerides (TGs) over 11.3 mmol/L or above 5.65 mmol/L with emulsion serum (normal TGs ≤1.70 mmol/L) (5,19). Moreover, we grouped patients who had 2 or 3 of the above 3 etiologies as mixed-cause cases. Other causes of AP include endoscopic retrograde cholangiopancreatography, pancreas divisum, genetics, polymorphisms, and drug intake, among others. We grouped the other etiologies and idiopathic etiologies into a subgroup. Therefore, we ultimately included 5 etiology subgroups.
All patients were managed according to the Evidence-based guidelines for the management of acute pancreatitis by the International Association of Pancreatology and the American Pancreatic Association working group guidelines of 2013 (20).
Clinical observations
The clinical charts of all cases were reviewed to check age, gender, etiology, length of hospital stay, intensive care unit (ICU) admission, clinical severity using scores according to the 2012 revised Atlanta classification (RAC) (Tables S1,S2) and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score (Table S3) for each patient.
CT technique
The CT images were acquired with the patient in the supine position, head advanced, and with a scanning range from the xiphoid process to the iliac crest plane or lower. Patients underwent an abdominopelvic CT study with one of the 5 following multidetector-row CT systems (Table S4): SOMATOM Definition AS + 128 (Siemens Healthineers, Erlangen, Germany), LightSpeed VCT 128 (GE Health care, Boston, MA, USA), Brilliance 64 (Philips Health Care, Amsterdam, The Netherlands), Toshiba Aquilion ONE 320 (Toshiba Medical Systems, Tokyo, Japan), and SOMATOM Definition Flash (Siemens Healthineers, Germany). The acquisition parameters were set at 80–140 kVp; 155–250 mAs; pitch, 0.5–1.0; collimation, 0.625–5 mm; and slice thickness, 1.5–5 mm.
Definition and imaging evaluation
The CT images of all cases were independently reviewed by 2 radiologists (with at least 3 years abdominal CT experience) who were blinded to the patients’ clinical and pathological information (including the onset time). The major CT findings were described by using the 2012 RAC, using terms including interstitial edematous pancreatitis (IEP), NP, subtypes of NP [parenchymal necrosis alone, peripancreatic necrosis alone, and a combined type (peripancreatic and parenchymal necrosis)], and local complications, including acute peripancreatic fluid collections, acute necrotic collections, pancreatic pseudocysts, and walled-off necrosis (21). The severity of AP on CT was graded using the modified CT severity index (MCTSI) score (Table S5) for every AP patient. An MCTSI score was used because it is better than the CT severity index score at avoiding the missed diagnosis of moderate to severe AP (22).
The different phases of AP onset
Onset time was defined as the time from symptoms until CT examination and not the time of admission to the hospital (21). The first symptoms included common abdominal pain, abdominal discomfort, or accompanying symptoms such as nausea and vomiting. Existing research shows that there are several key time points from the onset of AP. Day 3 is when the diagnostic value of CT is still being explored. Assessment within 3 days of symptom onset can provide crucial information about the expected course (23). However, another study pointed the ideal time for assessing severe AP or complications related to AP with CT is after 3 days (7). Day 7 is when the 2012 RAC distinguished the early phase and the late phase of AP onset (21). Day 14 is the time within which half of all deaths occur. These deaths are mainly due to failure of multiple organ systems (1). Day 28 is when local complications of AP begin to differ among patients according to the 2012 RAC (21). Therefore, patients with NP, local complications, and severe disease (graded by MCTSI score, RAC, APACHE II score) were divided into the following 5 phases according to the time points from AP onset to CT examination: Phase I (patients who received CT within 1–3 days after onset), Phase II (patients who received CT within 4–7 days after onset), Phase III (patients who received CT within 8–14 days after onset), Phase IV (patients who received CT within 15–28 days after onset), and Phase V (patients who received CT more than 28 days after onset) (Figure 1). For patients who underwent 2 or more CT examinations, the 2 interobserver values were based on all CT scans until the end-point CT for each patient, and the end result for comparison between patients of different etiology was the value when the clinical features or imaging appearance were most severe.
Data analysis
Statistical analyses were carried out using SPSS 25.0 (IBM Corp., Armonk, NY, USA). Categorical data were reported as n (%), and continuous data were expressed as the median [interquartile range (IQR)] or mean ± standard deviation (SD). Intragroup correlation coefficients (ICCs) were calculated for the MCTSI score, and kappa statistics were calculated for 2012RAC and necrosis types to evaluate the variability between observers. For the comparison of qualitative variables (necrosis, local complications), grade variable (RAC), and nonnormally distributed data (APACHE II, MCTSI score), the Kruskal–Wallis H test or Bonferroni method were used for etiology subgroups in different phase comparisons. The correlations between clinical and imaging scores were evaluated by Spearman rank correlation tests, and r was used to represent the correlation coefficient. There was a significant difference when P<0.05.
Results
Patient characteristics
This retrospective study included 1,924 AP patients, the average age was 49.97±14.68 years; 58.4% (1,124/1,924) of the patients were men, and 41.6% (800/1,924) were women. The median hospital stay was 12 days (IQR, 8 to 18 days). Only 1.1% (21/1,924) died during the study period, and these deaths all occurred during the first year.
In the 1,924 patients with AP, the etiologies included biliary AP in 32.1% of patients (618/1,924), alcoholic AP in 14.3% of patients (276/1,924), hypertriglyceridemic AP in 13.7% of patients (264/1,924), mixed cause AP in 10.4% of patients (201/1,924), and “other/idiopathic” AP in 29.4% of patients (565/1,924).
The clinical characteristics of patients with AP of different etiologies are shown in Table 1. The average age in the biliary AP group was 56.72±15.39 years, which was older than that in other etiology subgroups with an average age of 40–50 years (P<0.05). The median age in the hypertriglyceridemic AP group was 41.94±10.34 years, which was similar to that in the alcoholic AP group and lower than that in the other 3 etiology subgroups (P<0.05).
Table 1
| Characteristics | All patients (n=1,924) | BAP (n=618) | AAP (n=276) | HTG-AP (n=264) | Mixed causes (n=201) | Other/idiopathic AP (n=565) | P value |
|---|---|---|---|---|---|---|---|
| Age, average (SD), year | 49.97 (14.68) | 56.72 (15.39) | 45.49 (11.97) | 41.94 (10.34) | 46.91 (11.74) | 49.61 (14.62) | 0.00 |
| Gender (male), n (%) | 1,124 (58.4) | 235 (38.0) | 267 (97.5) | 173 (65.5) | 182 (90.5) | 267 (47.3) | 0.00 |
| Hospital stay [IQR], d | 12 [8, 18] | 14 [10, 19] | 11 [7, 16] | 12 [8, 18] | 13 [9, 18.5] | 11 [8, 17] | 0.00 |
| Patients in intensive care unit, n (%) | 162 (8.4) | 61 (9.9) | 16 (5.8) | 23 (8.7) | 24 (11.9) | 38 (6.7) | 0.05 |
SD, standard deviation; IQR, interquartile range; AP, acute pancreatitis; BAP, biliary acute pancreatitis; AAP, alcoholic acute pancreatitis; HTG-AP, hypertriglyceridemic acute pancreatitis.
There was a significant difference in etiology distribution between the male group and the female group (P<0.05). The proportion of male patients was the highest in the alcoholic AP subgroup (97.5%, 267/276), while the proportion of female patients was highest in the biliary AP subgroup (62.0%, 383/618). Regarding the length of hospital stay during the index admission, among the 5 etiology subgroups, the median length of hospital stay for patients with biliary AP was 14 days (IQR, 10 to 19 days), which was longer than that for patients with alcoholic AP (11 days), hypertriglyceridemic AP (12 days), and “other/idiopathic” AP (11 days) (P<0.05). There were 8.4% (162/1,924) of AP patients admitted to the ICU. Patients with mixed cause AP (11.9%, 24/201) were more likely to be admitted to the ICU than the other 4 etiology subgroups (P<0.05). Patients with alcoholic AP (5.8%, 16/276) were less likely to be admitted to the ICU than those with biliary AP (9.9%, 61/618) (P<0.05).
The positive rate of CT for AP diagnosis and interobserver agreement
Of the 1,924 patients who met the criteria of AP, 96.7% (1,860/1,924) had positive CT findings as well as at least 1 of the other 2 criteria, and 3.3% (64/1,924) of patients met the first 2 criteria for AP diagnosis only, meaning that these patients had negative imaging findings. Among the 1,860 AP patients with positive CT findings, the etiologies included biliary AP in 31.8% (591/1,860), alcoholic AP in 14.4% (267/1,860), hypertriglyceridemic AP in 13.9% (258/1,860), mixed cause AP in 10.7% (199/1,860), and “other/idiopathic” AP in 29.3% (545/1,860).
As the 2012RAC includes the evaluation standard for local complications on imaging, based on the same clinical information, we used the 2012RAC to compare the endpoint of the total value of mild, moderately severe, and severe AP. Interobserver agreement was excellent for the 2012 RAC [Kappa, 0.939; 95% confidence interval (CI): 0.925 to 0.953], MCTSI score (ICC, 0.909; 95% CI: 0.901 to 0.916), NP (Kappa, 0.915; 95% CI: 0.897 to 0.933), and necrotizing subtypes (Kappa, 0.899; 95% CI: 0.763 to 0.811) (P<0.001).
The CT findings
The CT findings of AP based on different etiologies
Among the 1,860 AP patients with positive CT findings, 66.8% (1,243/1,860) had IEP, and 33.2% (617/1,860) had NP. The prevalence of IEP for hypertriglyceridemic AP patients (72.5%, 187/258) was higher than that for those with alcoholic AP (63.7%, 170/267) and “other/idiopathic” AP (62.9%, 343/545) (P<0.05). Among the 617 NP patients, the proportion of the combined type (peripancreatic and parenchymal necrosis) (63.7%, 393/617) was higher than that of peripancreatic necrosis alone (28.4%, 175/617) and parenchymal necrosis alone (7.9%, 49/617).
Among the 1,860 AP patients, the prevalence of NP in “other/idiopathic” AP patients was 37.1% (202/545), which was higher than that of those with biliary AP (31.0%, 183/591) and hypertriglyceridemic AP (27.5%, 71/258) (P<0.05). However, the prevalence of NP was not significantly different among patients with alcoholic AP (36.3%, 97/267), biliary AP (31.0%, 183/591), hypertriglyceridemic AP (27.5%, 71/258), and mixed causes (32.3%, 64/199) (P>0.05). The proportion of those 3 necrotic subtypes had no significant differences in etiology distribution (P>0.05; Figure 2).
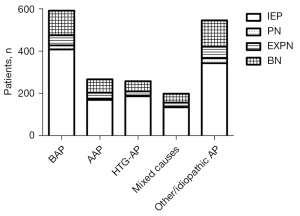
There were 617 NP patients among the 1,860 AP patients with positive CT examinations. In the 617 NP patients, “other/idiopathic” AP and biliary AP were more common, accounting for 32.7% (202/617) and 29.7% (183/617), respectively. Alcoholic AP, hypertriglyceridemic AP, and mixed causes accounted for 15.7% (97/617), 11.5% (71/617), and 10.4% (64/617), respectively.
Among the 617 NP patients, the percentage of biliary AP increased from Phase I (17.1%, 19/111) to Phase IV (42.9%, 42/98) (P<0.05). However, the percentage of hypertriglyceridemic AP decreased from Phase I (24.3%, 27/111) to Phase V (1.3%, 1/76), and the percentage of mixed causes decreased from Phase I (18.0%, 200/111) to Phase III (6.3%, 13/206) (P<0.05). There was no significant difference in the percentage of alcoholic AP and “other/idiopathic” AP among Phases I–V NP patients (P>0.05; Table 2).
Table 2
| Groups | All patients | 1–3 days | 4–7 days | 8–14 days | 15–28 days | >28 days | P value† |
|---|---|---|---|---|---|---|---|
| All patients (n) | 617 | 111 | 126 | 206 | 98 | 76 | |
| BAP | 29.7% (183/617) | 17.1% (19/111) | 23.0% (29/126) | 31.1% (64/206) | 42.9% (42/98) | 38.2% (29/76) | <0.05* |
| AAP | 15.7% (97/617) | 12.6% (14/111) | 14.3% (18/126) | 17.5% (36/206) | 15.3% (15/98) | 18.4% (14/76) | >0.05 |
| HTG-AP | 11.5% (71/617) | 24.3% (27/111) | 17.5% (22/126) | 7.8% (16/206) | 5.1% (5/98) | 1.3% (1/76) | <0.05** |
| Mixed causes | 10.4% (64/617) | 18.0% (20/111) | 15.1% (19/126) | 6.3% (13/206) | 8.2% (8/98) | 5.3% (4/76) | <0.05*** |
| Other/idiopathic AP | 32.7% (202/617) | 27.9% (31/111) | 30.2% (38/126) | 37.4% (77/206) | 28.6% (28/98) | 36.8% (28/76) | >0.05 |
†, the Bonferroni-adjusted P value. *, the Bonferroni method showed significant difference between 1–3 and 15–28 days (P<0.05, 95% CI: 0.15–0.52), 1–3 and >28 days (P<0.05, 95% CI: 0.17–0.66), 4–7 and 15–28 days (P<0.05, 95% CI: 0.22–0.71). **, the Bonferroni method showed significant difference between 1–3 and 8–14 days (P<0.05, 95% CI: 1.95–7.46), 1–3 and 15–28 days (P<0.05, 95% CI: 2.20–16.23), 1–3 and >28 days (P<0.05, 95% CI: 3.20–181.74), 4–7 and 15–28 days (P<0.05, 95% CI: 1.43–10.81), 4–7 and >28 days (P<0.05, 95% CI: 2.10–120.31). ***, the Bonferroni method showed significant difference between 1–3 and 8–14 days (P<0.05, 95% CI: 1.56–6.85), 4–7 and 8–14 days (P<0.05, 95% CI: 1.25–5.55). NP, necrotizing pancreatitis; CT, computed tomography; BAP, biliary AP; AAP, alcoholic AP; HTG-AP, hypertriglyceridemic AP; AP, acute pancreatitis.
The local complications
Among the 1,860 AP patients with positive CT findings, 64.6% (1,202/1,860) had local complications. Among the 1,202 patients with local complications, the acute peripancreatic fluid collections (52.7%, 634/1,202) was more common than acute necrotic collections (39.7%, 477/1,202), walled-off necrosis (15.2%, 183/1,202), and pancreatic pseudocysts (0.2%, 3/1,202).
Among the 1,860 patients with positive CT findings, according to the etiology of AP, the prevalence of local complications in mixed causes (71.9%, 143/199), hypertriglyceridemic AP (69.0%, 178/258), and alcoholic AP (68.2%, 182/267) patients was higher than that in biliary AP patients (59.7%, 353/591; P<0.05).
There were 1,202 patients with local complications in the 1,860 patients with positive CT examinations. Among the 1,202 patients, the percentage of biliary AP increased from Phase I (25.2%, 102/404) to Phase IV (44.0%, 55/125) (P<0.05). However, the percentage of hypertriglyceridemic AP decreased from Phase I (22.5%, 91/404) to Phase V (1.2%, 1/84) (P<0.05). There was no significant difference in the percentage of alcoholic AP, “other/idiopathic” AP, or mixed causes among Phases I–V patients with local complications (P>0.05; Table 3).
Table 3
| Groups | All CTs | 1–3 days | 4–7 days | 8–14 days | 15–28 days | >28 days | P value† |
|---|---|---|---|---|---|---|---|
| All CTs (n) | 1,202 | 404 | 299 | 290 | 125 | 84 | |
| BAP | 29.4% (353/1,202) | 25.2% (102/404) | 26.1% (78/299) | 29.3% (85/290) | 44.0% (55/125) | 39.3% (33/84) | <0.05* |
| AAP | 15.1% (182/1,202) | 13.4% (54/404) | 13.4% (40/299) | 18.3% (53/290) | 16.8% (21/125) | 16.7% (14/84) | >0.05 |
| HTG-AP | 14.8% (178/1,202) | 22.5% (91/404) | 17.1% (51/299) | 9.7% (28/290) | 5.6% (7/125) | 1.2% (1/84) | <0.05** |
| Mixed causes | 11.9% (143/1,202) | 26.5% (50/404) | 29.1% (43/299) | 32.4% (30/290) | 22.4% (14/125) | 35.7% (6/84) | >0.05 |
| Other/idiopathic AP | 28.8% (346/1,202) | 12.4% (107/404) | 14.4% (87/299) | 10.3% (94/290) | 11.2% (28/125) | 7.1% (30/84) | >0.05 |
†, the Bonferroni-adjusted P value. *, the Bonferroni method showed significant difference between 1–3 and 15–28 days (P<0.05, 95% CI: 0.28–0.63), 4–7 and 15–28 days (P<0.05, 95% CI: 0.17–0.66), 8–14 and 15–28 days (P<0.05, 95% CI: 0.34–0.82). **, the Bonferroni method showed significant difference between 1–3 and 8–14 days (P<0.05, 95% CI: 1.73–4.29), 1–3 and 15–28 days (P<0.05, 95% CI: 2.21–10.88), 1–3 and >28 days (P<0.05, 95% CI: 3.31–175.74), 4–7 and 15–28 days (P<0.05, 95% CI: 0.77–4.24), 4–7 and >28 days (P<0.05, 95% CI: 1.19–66.19). CT, computed tomography; BAP, biliary AP; AAP, alcoholic AP; HTG-AP, hypertriglyceridemic AP; AP, acute pancreatitis.
The MCTSI score
Among the 1,860 AP patients, the average MCTSI score was 5.41±2.38 points. According to the etiology of AP, the MCTSI score was 5.45±2.47 points in alcoholic AP, 5.45±2.42 points in biliary AP, 5.45±2.29 points in the mixed causes subgroup, 5.39±2.42 points in “other/idiopathic” AP, and 5.27±2.17 points in hypertriglyceridemic AP. However, there was no significant difference in etiology distribution in the MCTSI score (P>0.05).
Among the 1,860 patients with positive CT examinations, 391 patients had severe AP graded by the MCTSI score (8–10 points). The percentages of biliary AP in Phases I-V were 20.0%, 25.3%, 34.9%, 39.7%, and 40.7%, respectively, which showed an increasing trend from Phase I to Phase V (P<0.05; Figure 3). However, the percentage of hypertriglyceridemic AP decreased from Phase I to Phase V, and the percentages were 22.7%, 17.3%, 7.8%, 6.9%, and 1.9%, respectively (P<0.05; Figure 4). There were no significant differences in the percentage of alcoholic AP, “other/idiopathic” AP, or mixed causes among Phases I-V of severe AP graded by the MCTSI score (P>0.05).
Clinical characteristics
Time from onset of symptoms to discharge
Among the 1,860 AP patients, for the time from onset of symptoms to clinical discharge, among the 5 etiology subgroups, the median days for biliary AP was 18 days (IQR, 13 to 28 days), which was longer than that of the other 4 subgroups (P<0.05). The median days for hypertriglyceridemic AP was 13 days (IQR, 10 to 19.25 days), which was shorter than that for the biliary AP (18 days), “other/idiopathic” AP (16 days), and mixed causes subgroups (16 days) (P<0.05).
The severity of AP graded by the 2012 RAC
Among the 1,860 AP patients, moderately severe AP (MSAP) (63.8%, 1,187/1,860) was more common than mild AP (MAP) (27.2%, 506/1,860) and severe AP (SAP) (9.0%, 167/1,860) as graded by the 2012 RAC. According to the etiology of AP, the percentage of SAP was 8.3% (49/591) in the biliary AP subgroup, 9.0% (24/267) in the alcoholic AP subgroup, 9.3% (24/258) in the hypertriglyceridemic AP subgroup, 12.6% (25/199) in the mixed causes subgroup, and 8.3% (45/545) in the “other/idiopathic” AP subgroup. There were no significant differences in etiology distribution in the severity of AP graded by the 2012 RAC (P>0.05; Figure 5).
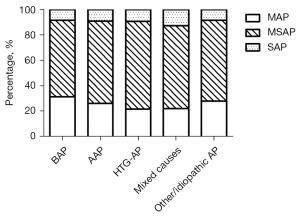
There were 167 patients with SAP among the 1,860 patients with positive CT examinations. Among these 167 patients, biliary AP and “other/idiopathic” AP were more common, with percentages of 29.3% (49/167) and 26.9% (45/167), while alcoholic AP, hypertriglyceridemic AP, and mixed causes accounted for 14.4% (24/167), 14.4% (24/167), and 15.0% (25/167), respectively.
Among the 167 patients with SAP, the clinical trend of the percentage of hypertriglyceridemic AP decreased from Phase I (34.4%, 11/32) to Phase V (0.0%, 0/15) (P<0.05). There was no significant difference in the percentage of alcoholic AP, biliary AP, “other/idiopathic” AP, and mixed causes among Phases I-V of severe AP graded by the 2012RAC (P>0.05; Table 4).
Table 4
| Groups | All CTs | 1–3 days | 4–7 days | 8–14 days | 15–28 days | >28 days | P value† |
|---|---|---|---|---|---|---|---|
| All patients (n) | 167 | 32 | 53 | 41 | 26 | 15 | |
| BAP | 29.3% (49/167) | 21.9% (7/32) | 22.6% (12/53) | 34.1% (14/41) | 30.8% (8/26) | 53.3% (8/15) | >0.05 |
| AAP | 14.4% (24/167) | 6.3% (2/32) | 11.3% (6/53) | 14.6% (6/41) | 26.9% (7/26) | 20.0% (3/15) | >0.05 |
| HTG-AP | 14.4% (24/167) | 34.4% (11/32) | 15.1% (8/53) | 9.8% (4/41) | 3.8% (1/26) | 0.0% (0/15) | <0.05* |
| Mixed causes | 15.0% (25/167) | 25.0% (8/32) | 20.8% (11/53) | 4.9% (2/41) | 11.5% (3/26) | 6.7% (1/15) | >0.05 |
| Other/idiopathic AP | 26.9% (45/167) | 12.5% (4/32) | 30.2% (16/53) | 36.6% (15/41) | 26.9% (7/26) | 20.0% (3/15) | >0.05 |
†, the Bonferroni-adjusted P value. *, the Bonferroni method showed significant difference between 1–3 and 4–7 days (P<0.05, 95% CI: 1.03–8.40), 1–3 and 8–14 days (P<0.05, 95% CI: 1.37–17.14), 1–3 and 15–28 days (P<0.05, 95% CI: 1.56–109.95). SAP, severe AP; RAC, revised Atlanta classification; BAP, biliary AP; AAP, alcoholic AP; HTG-AP, hypertriglyceridemic AP; AP, acute pancreatitis.
The APACHE II scores
Among the 1,860 AP patients, the average APACHE II score was 4.95±3.50 points. According to the etiology of AP, the average APACHE II score was higher in the biliary AP (5.98±3.42 points) than in those with “other/idiopathic” AP (4.88±3.28 points), mixed causes (4.65±3.16 points), alcoholic AP (4.11±4.76 points), and hypertriglyceridemic AP (3.84±2.91 points; P<0.05).
There were 387 patients with severe AP graded by APACHE II score (at least 8 points) among the 1,860 patients with positive CT examinations. Among the 387 patients, the percentage of hypertriglyceridemic AP in Phases I–V was 17.6%, 5.4%, 1.1%, 5.3%, and 0.0%, respectively, and the trend of the percentage of hypertriglyceridemic AP decreased from Phase I to Phase V (P<0.05). There was no significant difference in the percentage of alcoholic AP, biliary AP, “other/idiopathic” AP, or mixed causes among Phases I-V of severe AP graded by APACHE II score (P>0.05).
Correlation between the clinical and imaging characteristics
Among the 1,860 AP patients with positive CT findings, according to Spearman’s rank correlation coefficients, the MCTSI score showed positive correlations with the 2012 RAC and APACHE II score. The correlation coefficients between the MCTSI and RAC were higher than those between the MCTSI and APACHE II score (Table 5).
Table 5
| Groups | Overall | 1–3 days | 4–7 days | 8–14 days | 15–28 days | >28 days |
|---|---|---|---|---|---|---|
| All patients (n) | 1,860 | 654 | 500 | 414 | 169 | 123 |
| MCTSI/RAC | ||||||
| All patients | 0.620 | 0.665 | 0.607 | 0.598 | 0.463 | 0.604 |
| BAP | 0.615 | 0.622 | 0.543 | 0.624 | 0.623 | 0.663 |
| AAP | 0.653 | 0.740 | 0.784 | 0.469 | –* | –* |
| HTG-AP | 0.584 | 0.565 | 0.585 | 0.592 | –* | –* |
| Mixed causes | 0.603 | 0.625 | 0.539 | 0.730 | –* | 0.714 |
| Other/idiopathic AP | 0.634 | 0.741 | 0.600 | 0.603 | 0.426 | 0.444 |
| MCTSI/APACHE II | ||||||
| All patients | 0.167 | 0.199 | 0.157 | 0.210 | –* | –* |
| BAP | 0.150 | –* | 0.187 | 0.300 | –* | –* |
| AAP | 0.128 | –* | –* | –* | –* | –* |
| HTG-AP | 0.239 | 0.243 | 0.243 | –* | –* | –* |
| Mixed causes | 0.286 | 0.274 | 0.274 | –* | –* | –* |
| Other/idiopathic AP | 0.204 | 0.276 | 0.276 | 0.261 | –* | –* |
*, spearman rank correlation tests showed no correlation (P>0.05). RAC, revised Atlanta classification; APACHE II, acute Physiology and Chronic Health Evaluation II; MCTSI, modified CT severity index; BAP, biliary AP; AAP, alcoholic AP; HTG-AP, hypertriglyceridemic AP; AP, acute pancreatitis.
The correlation coefficient between the MCTSI and RAC was the highest in Phase I (r=0.665) and the lowest in Phase IV (r=0.463). The correlation coefficient between the MCTSI and RAC was the highest in alcoholic AP (r=0.653) and the lowest in hypertriglyceridemic AP (r=0.584) among the 5 etiological subgroups. For each etiology-subgroup, the correlation coefficient between MCTSI and RAC was the highest in Phase I for “other/idiopathic” AP (r=0.741), Phase II for alcoholic AP (r=0.784), Phase III for hypertriglyceridemic AP (r=0.592) and mixed causes AP (r=0.730), and Phase V for biliary AP (r=0.663). This shows that the correlation between MCTSI and RAC in different etiological subgroups reached its highest at different phases following the different onset times of the disease.
Discussion
In this study, 96.7% of AP patients with CECT examinations had positive CT findings. We found that biliary AP was the most common etiology of AP, which is consistent with the results of previous studies (24,25). The proportion of “other/idiopathic” AP in the present study was 29.4%, which is higher than that in present studies in China that range from 17% to 24% (3,26-28). A possible reason for this is that our results were based on CT and did not include all clinical AP patients. Clinically, some patients with mild AP were not diagnosed by CT, and the proportion of the etiology of these patients was uncertain.
We observed NP in 33.2% of AP patients with positive CECT findings and it was mostly seen in “other/idiopathic” AP patients, while IEP was most common in hypertriglyceridemic AP patients. Biliary AP showed an increasing percentage, while hypertriglyceridemic AP showed a decreasing percentage of NP on CT from Phase I to Phase IV. There were 64.6% of AP patients with local complications as shown by CT. Compared with parenchymal necrosis, local peri-pancreatic complications occurred more frequently in non-biliary disease. Biliary AP showed an increasing percentage of local complications, while hypertriglyceridemic AP showed a decreasing percentage from Phase I to Phase IV. Biliary AP and hypertriglyceridemic AP also showed changes of severe AP graded by MCTSI scores. For severe AP graded by RAC and APACHE II scores, only hypertriglyceridemic AP showed a decreasing percentage from Phase I to Phase V. The correlation coefficients between MCTSI and RAC were higher than those between the MCTSI and APACHE II score. Our results may serve to renew some basic data about CT characteristics according to the 2012 RAC and provide evidence for different rules for CT features based on the common etiologies of AP at different onset times. This may be helpful for the accurate recognition and management of AP.
In this study, 96.7% of patients with AP had positive CT findings based on CECT, and this percentage was higher than that of a previous study in 2006 (29). This may be because the current imaging methods available are better than those used previously, and pancreatologists and radiologists have reached a new consensus and understanding about AP after publication of the 2012 RAC (21). Hence, we can better observe the abnormality on images and use clearer definitions.
A study in 2019 suggested that up to 10–20% of patients with AP also had NP (30). However, in our study, that proportion was 33.2%. The possible reason for this is that the identification of necrosis has become more accurate with advances in imaging technology (31). For example, previously, patients with peripancreatic necrosis alone were likely to be categorized as having pancreatic fluid collections (32). Furthermore, the 33.2% proportion was based on patients with positive CT findings, but some patients with mild AP or IEP did not undergo any CT scan, which may also have contributed to the high proportion of NP results.
Consistent with Huang et al. (33), IEP was the most common in hypertriglyceridemic AP patients. A metaanalysis pointed out that NP was more common in alcoholic AP than biliary AP (10), which contradicted the results of Hughey et al. (12). In our results, the prevalence of NP in alcoholic AP was higher than that in biliary AP but with no significant difference, which is consistent with a previous study (34). This may be because some studies that conducted metaanalysis included not only primary AP but also recurrent AP (10). A study indicated that the recurrence rate was approximately 38% for alcoholic AP and 17% for biliary AP (35).
Patients with NP usually have a protracted and variable disease course because pancreatic and peripancreatic necrosis may liquefy or remain solid, become infected or remain sterile, or persist or disappear over time (21,36). In our results, biliary AP showed an increasing percentage of NP on CT from Phase I to Phase IV, while hypertriglyceridemic AP showed a decreasing percentage from Phase I to IV. Our results indicate that compared with other etiological subgroups, biliary AP has a longer time from onset to discharge, while hypertriglyceridemic AP has a shorter time.
In this study, CT showed that 64.6% of AP patients had local complications, which was relatively higher than that of previous studies (15,37). This may be because of the recent development of imaging modalities that enabled us to closely observe fluid collections in this study. Previous studies have reported that alcoholic AP is closely related to the development of local complications, including walled-off necrosis and pancreatic pseudocysts (15,37,38). Our study also found that the prevalence of local complications in alcoholic AP was higher than that in biliary AP.
Biliary AP is an inflammation of the pancreas that occurs due to obstruction of the common channel that drains both the biliary and pancreatic ducts (1). The symptoms of older biliary AP patients may be far less obvious in their respective manifestations (39), the admission time will be postponed, and persistent inflammatory stimulation results in slow improvement or stops the local morphological changes around the pancreas. This may be why CT showed that the local complications of biliary AP recovery were slower.
In this study, we found that hypertriglyceridemic AP patients recovered faster than others regardless of CT or clinical examination. The reason for this may be because hypertriglyceridemia dose aggravates the severity and related local complications of AP, and TG levels are high in the early stage but decrease significantly in the late stage, as many hypertriglyceridemic AP patients receive aggressive intravenous fluid administration and have compromised caloric intake (40-42). However, biliary AP patients are often older and have many systemic complications, and those factors would not change with onset time, which may explain why biliary AP patients have a high APACHE II score and the percentage of biliary AP was not significantly different at different phases of SAP graded by the RAC and APACHE II score.
Some studies have shown that there is no correlation between imaging scores (CTSI, MRSI score) and the APACHE II score (7,43). However, Zhou et al. (32) demonstrated that extrapancreatic inflammation on CT scores have weak positive correlations with the APACHE II score. In addition, Peng et al. (44) reported that the pleural effusion volume was correlated with the APACHE II score, and Jiang et al. (45) found a significant correlation between the incidence of vascular involvement and AP severity on the basis of APACHE II score. In our study, the MCTSI score had a positive correlation with the RAC and APACHE II score. The possible reason may be that the previous studies only scored imaging in the early phase of the disease (usually within 72 h) when necrotic tissues were not yet clearly visible.
Our study had some limitations. First, this study used retrospective data collection, and partial information could not be collected for the clinical and imaging characteristics at the same time, which might have led to the loss of data and affected the results. Second, the patients were divided into 5 phases, and the patients in each subgroup were observed together, which was an ideal state. However, some patients had individual differences leading to errors, so it is worthwhile to conduct a prospective study to investigate the dynamic course of disease in the same patient in the future.
Conclusions
The AP patients in this study had a very high rate of positive CECT findings, and the proportion of NP shown on CT was as high as 33.2% of positive CECT findings. Biliary AP recovery was slower, while hypertriglyceridemic AP recovery was faster on CT than in other etiological subgroups. There may be differences in the imaging and clinical manifestations of different etiologies of AP, and these may be related to the onset time of AP symptoms.
Acknowledgments
Funding: This work was supported by the Project of Nanchong City School Cooperative Scientific Research (No. 19SXHZ 0287) and the Scientific Research Project of Affiliated Hospital of North Sichuan Medical College (No. 2020ZD013 to XL).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1231/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1231/coif). XL reports that this work was supported by the Project of Nanchong City Schoo1 Cooperative Scientific Research (No. 19SXHZ 0287) and the Scientific Research Project of Affiliated Hospital of North Sichuan Medical College (No. 2020ZD013 to XL). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Affiliated Hospital of North Sichuan Medical College (No. 2021ER[A]017), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Forsmark ChE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med 2017;376:598-9. [PubMed]
- Jin M, Bai X, Chen X, Zhang H, Lu B, Li Y, Lai Y, Qian J, Yang H. A 16-year trend of etiology in acute pancreatitis: The increasing proportion of hypertriglyceridemia-associated acute pancreatitis and its adverse effect on prognosis. J Clin Lipidol 2019;13:947-953.e1. [Crossref] [PubMed]
- Zheng Y, Zhou Z, Li H, Li J, Li A, Ma B, Zhang T, Liao Q, Ye Y, Zhang Z, Yang Y, Wang Z, Zhang Z, Yang J, Li F. A multicenter study on etiology of acute pancreatitis in Beijing during 5 years. Pancreas 2015;44:409-14. [Crossref] [PubMed]
- He F, Zhu HM, Li BY, Li XC, Yang S, Wang Z, Zhang M. Factors predicting the severity of acute pancreatitis in elderly patients. Aging Clin Exp Res 2021;33:183-92. [Crossref] [PubMed]
- Wang YH, Xu ZH, Zhou YH, Sun SL, Xu ZW, Qi X, Zhou WJ, Sheng HQ, Zhao B, Mao EQ. The clinical characteristic of biliary-hyperlipidemic etiologically complex type of acute pancreatitis: a retrospective study from a tertiary center in China. Eur Rev Med Pharmacol Sci 2021;25:1462-71. [PubMed]
- Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas 2006;33:323-30. [Crossref] [PubMed]
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012;262:751-64. [Crossref] [PubMed]
- Fung C, Svystun O, Fouladi DF, Kawamoto S. CT imaging, classification, and complications of acute pancreatitis. Abdom Radiol (NY) 2020;45:1243-52. [Crossref] [PubMed]
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002;223:603-13. [Crossref] [PubMed]
- Bálint ER, Fűr G, Kiss L, Németh DI, Soós A, Hegyi P, Szakács Z, Tinusz B, Varjú P, Vincze Á, Erőss B, Czimmer J, Szepes Z, Varga G, Rakonczay Z Jr. Assessment of the course of acute pancreatitis in the light of aetiology: a systematic review and meta-analysis. Sci Rep 2020;10:17936. [Crossref] [PubMed]
- Lankisch PG, Assmus C, Pflichthofer D, Struckmann K, Lehnick D. Which etiology causes the most severe acute pancreatitis? Int J Pancreatol 1999;26:55-7. [Crossref] [PubMed]
- Hughey M, Taffel M, Zeman RK, Patel S, Hill MC. The diagnostic challenge of the sequelae of acute pancreatitis on CT imaging: a pictorial essay. Abdom Radiol (NY) 2017;42:1199-209. [Crossref] [PubMed]
- Uhl W, Isenmann R, Curti G, Vogel R, Beger HG, Büchler MW. Influence of etiology on the course and outcome of acute pancreatitis. Pancreas 1996;13:335-43. [Crossref] [PubMed]
- Barauskas G, Ignatavičius P, Vitkauskienė A, Pundzius J, Dambrauskas Ž. Impact of etiology on course and outcomes of severe acute pancreatitis. Medicina (Kaunas) 2015;51:167-72. [Crossref] [PubMed]
- Cui ML, Kim KH, Kim HG, Han J, Kim H, Cho KB, Jung MK, Cho CM, Kim TN. Incidence, risk factors and clinical course of pancreatic fluid collections in acute pancreatitis. Dig Dis Sci 2014;59:1055-62. [Crossref] [PubMed]
- Kim YS, Kim Y, Kim SK, Rhim H. Computed tomographic differentiation between alcoholic and gallstone pancreatitis: Significance of distribution of infiltration or fluid collection. World J Gastroenterol 2006;12:4524-8. [Crossref] [PubMed]
- Yasuda H, Horibe M, Sanui M, Sasaki M, Suzuki N, Sawano H, et al. Etiology and mortality in severe acute pancreatitis: A multicenter study in Japan. Pancreatology 2020;20:307-17. [Crossref] [PubMed]
- Chen CH, Dai CY, Hou NJ, Chen SC, Chuang WL, Yu ML. Etiology, severity and recurrence of acute pancreatitis in southern taiwan. J Formos Med Assoc 2006;105:550-5. [Crossref] [PubMed]
- Mosztbacher D, Hanák L, Farkas N, Szentesi A, Mikó A, Bajor J, et al. Hypertriglyceridemia-induced acute pancreatitis: A prospective, multicenter, international cohort analysis of 716 acute pancreatitis cases. Pancreatology 2020;20:608-16. [Crossref] [PubMed]
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13:e1-15. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SSAcute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, Mortele KJ. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. AJR Am J Roentgenol 2011;197:386-92. [Crossref] [PubMed]
- Porter KK, Zaheer A, Kamel IR, Horowitz JM, Arif-Tiwari H, Bartel TB, Bashir MR, Camacho MA, Cash BD, Chernyak V, Goldstein A, Grajo JR, Gupta S, Hindman NM, Kamaya A, McNamara MM, Carucci LR. ACR Appropriateness Criteria® Acute Pancreatitis. J Am Coll Radiol 2019;16:S316-30. [Crossref] [PubMed]
- Sun C, Li Z, Shi Z, Li G. Current diagnosis and treatment of acute pancreatitis in China: a real-world, multicenter study. BMC Gastroenterol 2021;21:210. [Crossref] [PubMed]
- Zilio MB, Eyff TF, Azeredo-Da-Silva ALF, et al. A systematic review and meta-analysis of the aetiology of acute pancreatitis. HPB (Oxford) 2019;21:259-67. [Crossref] [PubMed]
- Zhu Y, Pan X, Zeng H, He W, Xia L, Liu P, Zhu Y, Chen Y, Lv N. A Study on the Etiology, Severity, and Mortality of 3260 Patients With Acute Pancreatitis According to the Revised Atlanta Classification in Jiangxi, China Over an 8-Year Period. Pancreas 2017;46:504-9. [Crossref] [PubMed]
- Han T, Cheng T, Liao Y, He Y, Liu B, Lai Q, Pan P, Liu J, Cao Y, Yu H. The ratio of red blood cell distribution width to serum calcium predicts severity of patients with acute pancreatitis. Am J Emerg Med 2022;53:190-5. [Crossref] [PubMed]
- Pu W, Luo G, Chen T, Jing L, Hu Q, Li X, Xia H, Deng M, Lü M, Chen X A. 5-Year Retrospective Cohort Study: Epidemiology, Etiology, Severity, and Outcomes of Acute Pancreatitis. Pancreas 2020;49:1161-7. [Crossref] [PubMed]
- Zhang XM, Feng ZS, Zhao QH, Xiao CM, Mitchell DG, Shu J, Zeng NL, Xu XX, Lei JY, Tian XB. Acute interstitial edematous pancreatitis: Findings on non-enhanced MR imaging. World J Gastroenterol 2006;12:5859-65. [Crossref] [PubMed]
- Garg PK, Zyromski NJ, Freeman ML. Infected Necrotizing Pancreatitis: Evolving Interventional Strategies From Minimally Invasive Surgery to Endoscopic Therapy-Evidence Mounts, But One Size Does Not Fit All. Gastroenterology 2019;156:867-71. [Crossref] [PubMed]
- Xiao B, Xu HB, Jiang ZQ, Zhang J, Zhang XM. Current concepts for the diagnosis of acute pancreatitis by multiparametric magnetic resonance imaging. Quant Imaging Med Surg 2019;9:1973-85. [Crossref] [PubMed]
- Zhou T, Chen Y, Wu JL, Deng Y, Zhang J, Sun H, Lan C, Zhang XM. Extrapancreatic Inflammation on Magnetic Resonance Imaging for the Early Prediction of Acute Pancreatitis Severity. Pancreas 2020;49:46-52. [Crossref] [PubMed]
- Huang SW, Mao EQ, Wang HS, Zhao B, Chen Y, Qu HP, Chen EZ. Clinical characteristics of 5375 cases of acute pancreatitis from a single Chinese center, 1996-2015. Chin Med J (Engl) 2019;132:1233-6. [Crossref] [PubMed]
- Verdonk RC, Sternby H, Dimova A, Ignatavicius P, Koiva P, Penttila AK, Ilzarbe L, Regner S, Rosendahl J, Bollen TL. Short article: Presence, extent and location of pancreatic necrosis are independent of aetiology in acute pancreatitis. Eur J Gastroenterol Hepatol 2018;30:342-5. [Crossref] [PubMed]
- Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015;149:1490-1500.e1. [Crossref] [PubMed]
- Maatman TK, Mahajan S, Roch AM, Lewellen KA, Heimberger MA, Colgate CL, Ceppa EP, House MG, Nakeeb A, Schmidt CM, Zyromski NJ. High Rates of Readmission in Necrotizing Pancreatitis: Natural History or Opportunity for Improvement? J Gastrointest Surg 2019;23:1834-9. [Crossref] [PubMed]
- Lankisch PG, Weber-Dany B, Maisonneuve P, Lowenfels AB. Pancreatic pseudocysts: prognostic factors for their development and their spontaneous resolution in the setting of acute pancreatitis. Pancreatology 2012;12:85-90. [Crossref] [PubMed]
- Sarathi Patra P, Das K, Bhattacharyya A, Ray S, Hembram J, Sanyal S, Dhali GK. Natural resolution or intervention for fluid collections in acute severe pancreatitis. Br J Surg 2014;101:1721-8. [Crossref] [PubMed]
- Carvalho JR, Fernandes SR, Santos P, Moura CM, Antunes T, Velosa J. Acute pancreatitis in the elderly: a cause for increased concern? Eur J Gastroenterol Hepatol 2018;30:337-41. [Crossref] [PubMed]
- Yang AL, McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology 2020;20:795-800. [Crossref] [PubMed]
- Olesen SS, Harakow A, Krogh K, Drewes AM, Handberg A, Christensen PA. Hypertriglyceridemia is often under recognized as an aetiologic risk factor for acute pancreatitis: A population-based cohort study. Pancreatology 2021;21:334-41. [Crossref] [PubMed]
- Berberich AJ, Ziada A, Zou GY, Hegele RA. Conservative management in hypertriglyceridemia-associated pancreatitis. J Intern Med 2019;286:644-50. [Crossref] [PubMed]
- Cho JH, Kim TN, Chung HH, Kim KH. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol 2015;21:2387-94. [Crossref] [PubMed]
- Peng R, Zhang L, Zhang ZM, Wang ZQ, Liu GY, Zhang XM. Chest computed tomography semi-quantitative pleural effusion and pulmonary consolidation are early predictors of acute pancreatitis severity. Quant Imaging Med Surg 2020;10:451-63. [Crossref] [PubMed]
- Jiang ZQ, Xiao B, Zhang XM, Xu HB. Early-phase vascular involvement is associated with acute pancreatitis severity: a magnetic resonance imaging study. Quant Imaging Med Surg 2021;11:1909-20. [Crossref] [PubMed]


