
Temporal changes in plaque characteristics after treatment and their relationship with stroke recurrence: a quantitative study using magnetic resonance imaging
Introduction
Acute ischemic stroke (AIS) is a leading cause of death and long-term disability worldwide (1). Intracranial atherosclerotic disease (ICAD) is the major cause of ischemic stroke, which accounts for 30–50% of strokes in Asian and Black populations and 8–10% in White populations (2,3). Theoretically, atherosclerotic lesions should regress and symptoms should improve when patients receive medical treatment after the first stroke attack. However, around 15% of patients still experience recurrent ischemic events despite aggressive medical treatment (optimal antiplatelet therapy, intensive management of vascular risk factors, and lifestyle modification) (4). Exploring the clinical and neuroimaging characteristics of these patients could shed light on the mechanisms leading to stroke recurrence.
Conventional imaging modalities, such as magnetic resonance angiography (MRA), computed tomographic angiography, and digital subtraction angiography, are commonly used in routine clinical practice to identify and assess ICAD. However, these imaging modalities focus on lumen information and reveal minimal information about atherosclerotic plaque characteristics; as a result, they are of limited value in making accurate predictions for stroke recurrence. Studies have shown that vessel wall magnetic resonance imaging (VWMRI) is an effective tool for evaluating the wall features of ICAD (5-7). Research has reported that plaque characteristics such as contrast enhancement, intraplaque hemorrhage, wall remodeling, and plaque morphological features are strongly associated with stroke occurrence (8-11); however, these studies were largely cross-sectional. As atherosclerosis is a dynamic process, longitudinal studies are needed to further elucidate the evolution of plaque features and their association with recurrent events.
Recently, longitudinal follow-up studies by Kim et al. (12) and Song et al. (13) reported that baseline plaque enhancement can predict stroke recurrence. However, these studies did not explore the dynamic change in the degree of plaque enhancement at the time of stroke recurrence. Another study did not find that plaque enhancement (neither at baseline nor the progression of enhancement) predicted recurrent stroke (14). Therefore, the evolution of plaque features after medical treatment and their prognostic value are still largely unknown.
In this study, we aimed to investigate the temporal changes in atherosclerotic plaques after medical treatment and to explore their relationship with the recurrence of ischemic stroke using VWMRI. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-210/rc).
Methods
Study population
This retrospective cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2021-SRFA-111), and individual consent for this retrospective analysis was waived. From November 2016 to May 2021, patients presenting with a transient ischemic attack (TIA) or AIS caused by middle cerebral artery (MCA) atherosclerosis who received treatment and underwent both baseline and follow-up VWMRI examinations at the First Affiliated Hospital of Nanjing Medical University were recruited.
The detailed inclusion criteria were as follows: (I) definite culprit MCA plaque confirmed on baseline VWMRI; (II) patients received routine antiplatelet aggregation (clopidogrel and/or aspirin) and/or lipid-lowering therapy (statins) treatments after the first onset of symptoms; (III) complete information of clinical and laboratory examinations was available; (IV) patients had ≥1 atherosclerotic risk factors, including hypertension, diabetes mellitus, hyperlipidemia, or cigarette smoking; and (V) high quality images were available for analysis. The exclusion criteria were as follows: (I) the stroke/TIA was caused by nonatherosclerotic diseases such as arterial dissection, aneurysm, moyamoya disease, or cardioembolism; (II) the patient had experienced a new TIA/stroke at the follow-up that was different from the original attack; (III) coexisting carotid stenosis >30%, or definite vulnerable plaque of carotid artery identified on VWMRI at the ipsilateral of stroke/TIA; (IV) the patient received a bypass or stent therapy during follow-up; (V) incomplete clinical information; (VI) a follow-up interval of less than 3 months; and (VII) poor VWMRI image quality.
Clinical information and outcomes
Relevant demographic and clinical data were collected at baseline for each patient, including gender, age, clinical symptoms, risk factors of atherosclerosis (hypertension, diabetes mellitus, hyperlipidemia, or current cigarette smoker), laboratory indicators [low-density lipoprotein (LDL), high density lipoprotein (HDL), cholesterol (CHOL), and triglyceride (TG)], and pharmaceutical treatment (use of clopidogrel, aspirin, or statins).
Cases of stroke or TIA were classified by a neurologist if symptoms could be localized to an arterial territory and showed corresponding sensory dysfunction, dyskinesia, language barriers, or visual disturbances. The patients were divided into the following 3 groups according to the time interval from the symptoms to the baseline VWMRI: (I) AIS, less than 4 weeks; (II) subacute ischemic stroke, 4–8 weeks; and (III) chronic ischemic stroke, more than 8 weeks.
Recurrence was considered if cases had TIA/stroke symptoms or new hyperintensity infarcts on diffusion-weighted imaging (DWI) on the ipsilateral side of the original attack during the follow-up (15). Cases who reported nonspecific discomfort such as insomnia, headache, and dizziness were not considered to have experienced recurrent cerebrovascular events. The second MRI was defined as the last VWMRI exam in patients without stroke recurrence, or the VWMRI exam closest to the recurrence event in patients with stroke recurrence. The follow-up interval was calculated as the number of days from the baseline scan to the second VWMRI exam.
VWMRI acquisition
The VWMRI was performed with a 3.0 Tesla MRI scanner (Siemens Skyra; Siemens Healthineers, Erlangen, Germany) equipped with a 20-channel head/neck coil. Our protocols included the following. (I) 3-dimensional (3D) time-of-flight MRA (TOF-MRA): repetition time/echo time (TR/TE), 22/3.6 ms; flip angle, 18°; field of view (FOV), 210×190 mm2; and acquired resolution, 0.55×0.55×0.55 mm3. (II) 3D T1-weighted sampling perfection with application optimized contrast using different angle evolutions (SPACE) sequence before and after contrast administration: TR/TE, 900/4.2 ms; FOV, 240×216 mm2 (covering from carotid artery bifurcation to all intracranial arteries in order to assess intracranial and carotid plaques simultaneously); turbo-spin factor, 43 echoes; echo spacing, 4.2 ms; and acquired resolution, 0.75×0.75×0.75 mm3 and 0.6×0.6×0.6 mm3 (data after November 2019). Contrast-enhanced 3D SPACE was started with an approximately 5-minute delay time after administration of 0.1 mmol/kg contrast agent (gadodiamide, GE Healthcare, Dublin, Ireland) with an injection rate of 4.5 mL/s. (III) Axial DWI: b-value, 0 and 1,000 mm2/s; FOV, 230 mm × 230 mm; section thickness, 5 mm; and matrix, 192×192.
Culprit plaque identification and analysis
All the baseline VWMRI images were first analyzed by 2 experienced neuroradiologists (with 22 and 9 years of experience, respectively). According to the clinical information, a culprit plaque was identified when it was (I) the only lesion within the vascular territory of the stroke/TIA or (II) the most stenotic lesion when multiple plaques were present within the same vascular territory of the stroke/TIA (16). Any disagreement was solved by consensus reading. After that, 2 neuroradiologists (with 5 and 3 years of experience, respectively) who were blinded to the clinical information but aware of the culprit plaque independently analyzed the plaque features of all the initial and follow-up VWMRI images. During this procedure, the optimal plane, which was perpendicular to the vessel axis at the most stenotic site of the culprit plaque, was reconstructed for plaque analyses.
The detailed definitions and measurements of plaque and wall features included the following. (I) Stenosis ratio based on lumen diameter: the lumen diameter at the most narrowed lumen (MNL) and at the reference site (the nearest plaque-free segment proximal to the stenotic vessel) on TOF-MRA. If a proximal reference site was not available, then the neighboring distal site was used instead. Stenosis (diameter) (%) = (1 − lumen diameter at the MNL site/reference lumen diameter) × 100%. (II) Stenosis ratio based on lumen area (LA): stenosis (area) (%) = (1 − LA at the MNL site/reference LA) × 100%. (III) Plaque burden (PB): the LA and the outer wall area (WA), which were manually delineated on T1-weighted SPACE at the most stenotic site. PB was calculated as (1 − LA/WA) × 100%. (IV) Grade of plaque enhancement, which was visually assessed using the following grading scheme (16): grade 0, enhancement similar to or less than that of normal-appearing intracranial arterial wall in the same individual; grade 1, enhancement greater than that of grade 0 but less than that of the pituitary infundibulum; and grade 2, enhancement similar to or greater than that of the infundibulum. (V) Enhancement ratio (ER): a circular region of interest drawn at the brightest area of the plaque. The mean signal intensity (SI) of the plaques were obtained. ER = (SIpost − SIpre)/SIpre × 100%. All measurements were performed using commercially available software (Carestream Vue PACS v12.1, Carestream Health, Rochester, NY, USA).
The temporal change in each imaging characteristic over time was calculated using the following formula: percentage change = (initial − follow-up / initial) × 100%. The measurement results of continuous variables were averaged for subsequent analysis. Any difference of categorical variables between the 2 readers was solved by consensus reading with the help of a senior neuroradiologist (with 22 years of experience).
Statistical analysis
Quantitative data conforming to the normal distribution are represented as the means ± standard deviations, otherwise as the medians [interquartile range (IQR) presented as the 25th and 75th percentile]. Categorical data were described as numbers and corresponding percentages. The inter-reader reproducibility for the plaque characteristics assessment was evaluated using kappa analysis or intraclass correlation coefficient (ICC). Reliabilities <0.4 were characterized as poor, those 0.4–0.75 were considered fair to good, and those >0.75 were considered excellent.
The clinical and plaque characteristics before and after medical treatment were compared by paired sample t-test or Wilcoxon signed rank test. The baseline and percentage changes of clinical and plaque characteristics were calculated and compared between the patients with and without recurrence using an independent sample t-test, Mann-Whitney U test or chi-squared test as appropriate. Variables reaching significance on univariable analysis (P<0.05) were entered into the multivariable logistic regression analysis. To avoid collinearity in statistics, collinearity diagnostics were first performed. A variance inflation factor (VIF) was calculated. Then, a stepwise backward method in multivariable logistic regression was used to analyze clinical or plaque features associated with stroke recurrence after adjustment for age, gender, and laboratory indicators. Odds ratios (ORs) with 95% confidence interval (CI) were calculated. All statistical analyses were performed using SPSS software version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Patient demographics
A total of 95 patients were assessed using the screening criteria. Twenty-eight patients were excluded, as detailed in the flowchart (Figure 1). As a result, 67 patients (mean age: 54.42±15.08, 41 males) were finally included for analysis, including 36, 17, and 14 patients with AIS, TIA, and chronic ischemic stroke at baseline, respectively.
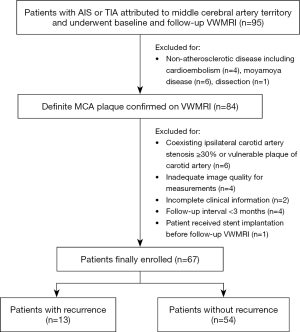
At the follow-up, 13 cases (19.4%) had a recurrence on the ipsilateral side of the original attack, including 4 cases with AIS and 9 patients with TIA. The median follow-up intervals between the initial and follow-up VWMRIs were 349 days (180–558 days) and 330 days (185–440 days) for the patients with and without stroke recurrence, respectively. No significant difference was found between the recurrence group and nonrecurrence group for demographics, risk factors, or clinical characteristics. The detailed demographics, clinical features, and comparisons between the 2 groups are listed in Table 1.
Table 1
| Characteristics | Patients with recurrence (n=13) | Patients without recurrence (n=54) | P value |
|---|---|---|---|
| Male, n (%) | 8 (61.5) | 33 (61.1) | 0.977 |
| Age, years (mean ± SD) | 59.00±15.72 | 53.32±14.86 | 0.225 |
| Baseline symptom, n (%) | 0.139 | ||
| AIS | 6 (46.1) | 30 (55.5) | |
| TIA | 6 (46.1) | 11 (20.3) | |
| CIS | 1 (7.6) | 13 (24.0) | |
| Risk factors, n (%) | |||
| Hypertension | 12 (92.3) | 38 (70.3) | 0.202 |
| Diabetes | 6 (46.1) | 13 (24.0) | 0.214 |
| Hyperlipidemia | 4 (30.7) | 13 (24.0) | 0.886 |
| Smoking | 0.553 | ||
| Current smoker | 2 (15.3) | 13 (24.0) | |
| Previous smoker | 2 (15.3) | 4 (7.4) | |
| Non-smoker | 9 (69.2) | 37 (68.5) | |
| Laboratory test, mmol/L (mean ± SD) | |||
| LDL | 2.35±0.73 | 2.47±0.91 | 0.642 |
| HDL | 1.01±0.16 | 1.09±0.28 | 0.157 |
| CHOL | 3.76±0.86 | 4.02±1.14 | 0.438 |
| TG | 1.61±0.92 | 1.51±0.69 | 0.687 |
| Medications, n (%) | |||
| Aspirin | 13 (100.0) | 47 (87.0) | 0.386 |
| Clopidogrel | 12 (92.3) | 45 (83.3) | 0.703 |
| Statins | 12 (92.3) | 53 (98.1) | 0.353 |
| Follow-up interval, days† | 349 (180, 558) | 330 (185, 440) | 0.949 |
†, data are expressed as median (IQR), IQR presented as the 25th and 75th percentile. AIS, acute ischemic stroke; TIA, transient ischemic attacks; CIS, chronic ischemic stroke; LDL, low density lipoprotein; HDL, high density lipoprotein; CHOL, cholesterol; TG, triglyceride; IQR, interquartile range.
Temporal changes in clinical and plaque characteristics after treatment
The inter-reader agreement for evaluating the initial and follow-up plaque features were good to excellent, with ICC values of 0.762 and 0.736 (stenosis-diameter), 0.780 and 0.794 (stenosis-area), 0.856 and 0.831 (plaque thickness), 0.712 and 0.735 (PB), and 0.874 and 0.887 (ER), and kappa values of 0.834 and 0.861 (enhancement grade).
After medical treatment, the laboratory indicators (LDL, CHOL, and TG), stenosis ratio (area), PB, and ER of patients without recurrence (n=54) all showed significant decreases (all P<0.05). In addition, the number of patients with grade 2 enhancement dropped from 42.5% to 16.6%. In contrast, no significant change in plaque features before and after treatment was found for patients with recurrence, despite a trend of markedly declining LDL values (2.35±0.73 vs. 1.90±0.47, P=0.070). Detailed changes in clinical and plaque characteristics before and after treatment are shown in Table 2.
Table 2
| Characteristics | Patients with recurrence (n=13) | Patients without recurrence (n=54) | |||||
|---|---|---|---|---|---|---|---|
| Initial | Follow-up | P value | Initial | Follow-up | P value | ||
| Clinical characteristics | |||||||
| LDL, mmol/L | 2.35±0.73 | 1.90±0.47 | 0.070 | 2.47±0.91 | 2.10±0.68 | 0.001 | |
| HDL, mmol/L | 1.01±0.16 | 0.99±0.21 | 0.686 | 1.09±0.28 | 1.14±0.29 | 0.087 | |
| CHOL, mmol/L | 3.76±0.86 | 3.15±0.69 | 0.038 | 4.02±1.14 | 3.70±0.83 | 0.014 | |
| TG, mmol/L | 1.61±0.92 | 1.44±0.42 | 0.415 | 1.51±0.69 | 1.31±0.60 | 0.001 | |
| Plaque characteristics | |||||||
| Stenosis (diameter) (%) | 70.54±27.26 | 76.46±28.89 | 0.380 | 59.20±26.19 | 54.35±29.83 | 0.092 | |
| Stenosis (area) (%) | 68.89±24.99 | 75.54±23.82 | 0.153 | 69.22±23.84 | 57.89±27.43 | <0.001 | |
| Plaque thickness, mm | 1.28±0.34 | 1.43±0.33 | 0.057 | 1.37±0.37 | 1.31±0.38 | 0.071 | |
| PB (%) | 87.21±9.96 | 89.48±8.57 | 0.216 | 85.70±10.68 | 82.46±11.80 | <0.001 | |
| ER (%) | 99.20±56.45 | 90.62±45.28 | 0.553 | 87.03±65.88 | 54.96±50.72 | <0.001 | |
| Enhancement grade, n (%) | 0.317 | <0.001 | |||||
| Grade 0 | 0 (0.0) | 0 (0.0) | 2 (3.7) | 6 (11.1) | |||
| Grade 1 | 5 (38.4) | 6 (46.1) | 29 (53.7) | 39 (72.2) | |||
| Grade 2 | 8 (61.5) | 7 (53.8) | 23 (42.5) | 9 (16.6) | |||
LDL, low density lipoprotein; HDL, high density lipoprotein; CHOL, cholesterol; TG, triglyceride; PB, plaque burden, ER, enhancement ratio.
At baseline, the comparisons of plaque characteristics between patients with and without recurrence showed no significant difference. At follow-up, the changes in stenosis-area (P=0.002), plaque thickness (P=0.003), PB (P=0.005), and ER (P=0.022) all showed significant differences between patients with and without recurrence. Specifically, 9 of 13 cases (69.2%) in the recurrence group showed an increase in PB, in comparison to 22.2% of those in the nonrecurrence group (P=0.003). The ER decreased by 38.35% in the nonrecurrence group but only decreased by 3.34% in the recurrence group (P=0.022). Detailed comparisons of the baseline and changes in plaque characteristics between cases with or without recurrence are presented in Table 3. Figures 2,3 show 2 representative cases from the recurrence and nonrecurrence groups, respectively.
Table 3
| Characteristics | Patients with recurrence (n=13) | Patients without recurrence (n=54) | P value |
|---|---|---|---|
| Baseline characteristics | |||
| Stenosis (diameter) (%)* | 70.54±27.26 | 59.20±26.19 | 0.169 |
| Stenosis (area) (%)* | 68.89±24.99 | 69.22±23.84 | 0.965 |
| Plaque thickness, mm* | 1.28±0.34 | 1.37±0.37 | 0.410 |
| PB (%)* | 87.21±9.96 | 85.70±10.68 | 0.644 |
| ER (%)* | 99.20±56.45 | 87.03±65.88 | 0.542 |
| Enhancement grade, n (%) | 0.583 | ||
| Grade 0 | 0 (0.0) | 0 (0.0) | |
| Grade 1 | 5 (38.4) | 29 (53.7) | |
| Grade 2 | 8 (61.5) | 23 (42.5) | |
| Change in plaque characteristics | |||
| Change in stenosis (diameter) (%)† | 0.00 (−64.77, 9.54) | 7.42 (−3.44, 26.87) | 0.116 |
| Change in stenosis (area) (%)† | −1.60 (−24.08, 5.10) | 14.24 (0.00, 29.92) | 0.002 |
| Change in plaque thickness (%)† | −8.33 (−33.33, 0.00) | 6.70 (0.00, 15.49) | 0.003 |
| Change in PB (%)† | −2.47 (−9.26, 0.05) | 3.75 (0.00, 8.91) | 0.005 |
| Progression of PB, n (%) | 9 (69.2) | 12 (22.2) | 0.003 |
| Change in ER (%)† | 3.34 (−35.78, 32.58) | 38.35 (8.85, 68.56) | 0.022 |
| Change in enhancement grade, n (%) | 0.090 | ||
| Stable | 12 (92.3) | 36 (66.6) | |
| Increase | 0 (0.0) | 0 (0.0) | |
| Decrease | 1 (7.6) | 18 (33.3) |
*, data are expressed as mean ± standard deviation; †, data are expressed as median (IQR), IQR presented as the 25th and 75th percentile. PB, plaque burden; ER, enhancement ratio; IQR, interquartile range.
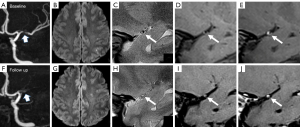
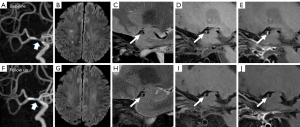
Association between changes in plaque characteristics and stroke recurrence
The changes in PB were significantly correlated with those in stenosis (area) and plaque thickness (r=0.685 and 0.378, respectively, both P<0.01). In other words, patients with an increase in PB after treatment were more likely to have an increase in stenosis (area) and plaque thickness. The VIF between change in PB and change in plaque thickness and between change in PB and change in stenosis (area) were 1.166 and 1.885, respectively, indicating a moderate collinearity, which was unlikely to affect the reliability of the following regression analysis.
Multivariable logistic regression showed that change in PB was the only significant plaque marker associated with recurrent stroke events (OR =1.112 per 1% increase, 95% CI: 1.010 to 1.224, P=0.031; Table 4), after adjusting for age, gender, and laboratory indicators. When progression of PB was used instead of change in PB, such an association did not attenuate (OR =6.084, 95% CI: 1.513 to 24.470, P=0.011; Table 4).
Table 4
| Variables | OR (95% CI) | P value |
|---|---|---|
| Change in PB | 1.112 (1.010–1.224) | 0.031 |
| Change in stenosis (area) | 1.001 (0.981–1.022) | 0.892 |
| Change in ER | 1.004 (0.997–1.011) | 0.319 |
| Change in plaque thickness | 1.026 (0.998–1.055) | 0.068 |
| Progression of PB# | 6.084 (1.513–24.470) | 0.011 |
| Change in stenosis (area)# | 1.005 (0.987–1.022) | 0.605 |
| Change in ER # | 1.002 (0.993–1.011) | 0.719 |
| Change in plaque thickness# | 1.027 (0.999–1.056) | 0.063 |
#, multivariable logistic regression analysis used progression of PB instead of change in PB while keeping the other 3 variables unchanged. OR, odds ratio; CI, confidence interval; PB, plaque burden; ER, enhancement ratio.
Discussion
Our study observed a significant decrease in stenosis (area), PB, and plaque enhancement in cases without recurrent ischemic stroke after standard medical treatment. In contrast, cases with recurrence did not show marked changes in plaque features. The progression of PB was independently associated with stroke recurrence.
Traditionally, severe stenosis has been considered an indicator of ischemic stroke (17,18). However, a disconnection between stenosis severity and the presence of ischemic stroke has been increasingly observed. Dieleman et al. (19) found that luminal stenosis is insufficient to evaluate stroke risk because it provides limited pathological information about the vessel wall, while plaque characteristics such as plaque distribution, active inflammation, and plaque rupture may have a closer relationship with stroke recurrence. A previous clinical trial found that approximately 30% of symptomatic ICADs are caused by low-grade MCA stenosis (<50%) (20). This proportion is even higher in symptomatic patients with subcortical infarction (21). Our previous study also found that significant enhancement and superior distribution of MCA plaque were significantly related to a recent ischemic stroke, even in patients with low-grade MCA stenosis (22). Stenosis degree based on luminal modality might underestimate atheroma burden due to compensatory positive remodeling of vessel walls and weaken risk prediction capacity for stroke recurrence. In this study, we used 2 methods to evaluate the stenosis. We found that the stenosis (area) measured on VWMRI was associated with stroke recurrence, while stenosis (diameter) measured on TOF-MRA failed to predict recurrent events. Therefore, VWMRI may provide additional value over stenosis degree measured on TOF-MRA alone.
Plaque enhancement is an attractive marker of plaque vulnerability in patients with ICAD. Systematic reviews by Gupta et al. (23) and Lee et al. (24) found that plaque enhancement had a strong correlation with recent ischemic events, independent of stenosis degree. Kim et al. (12) and Song et al. (13) reported that baseline plaque enhancement could predict stroke recurrence after follow-up, but they failed to explore the dynamic change in enhancement at the time of stroke recurrence. Zhang et al. (25) found that stable or increased enhancement of MCA plaque was related to recurrent stroke events at follow-up. In contrast, a prospective study by Shi et al. (14) found that neither ER at baseline nor its percentage change after medical treatment were related to stroke recurrence. Our study showed that baseline enhancement did not differ between patients with or without recurrence. However, persistent (especially grade 2) plaque enhancement after treatment was related to recurrent events. The dynamic change in plaque enhancement rather than its baseline status might be a more important indicator for recurrent events.
A study by Ran et al. (26) found that higher PB of MCA was associated with recurrent ischemic stroke; however, their study used a cross-sectional design. A more recent prospective study from Shi et al. (14) found that progression of PB was independently associated with recurrent ischemic cerebrovascular events. Their analysis was performed on 2D wall imaging. We used 3D imaging acquisitions covering both carotid artery bifurcation and intracranial arteries, which might not only improve the characterization of plaque features, but also help rule out culprit plaque from carotid artery. We found that change in PB was significantly correlated with that in plaque thickness and stenosis (area). However, only change in PB was found to be an independent marker for predicting stroke recurrence. A possible explanation may be that PB is a more comprehensive imaging marker which embodies both stenosis degree and remodeling pattern. Therefore, the percentage change in PB might better elucidate the dynamic evolution of plaques after medical treatment.
There were several limitations to this study. First, the sample size was relatively small. Since most of the existing studies have been single-center and with small sample sizes, there is a need for future multicenter, prospective studies with larger cohorts to validate the added value of specific imaging markers of high-risk intracranial plaques for predicting stroke recurrence. Second, the plaque features assessed in this study were limited. Other plaque characteristics on VWMRI, such as plaque morphology (concentric or eccentric) and intraplaque hemorrhage, warrant investigation in further study. Third, this retrospective study had a large variance in follow-up intervals, although no significant difference was found between the groups with or without recurrence.
Conclusions
Patients with ICAD who benefit from medical treatment strategies show obvious decrease in stenosis (area), PB, and plaque enhancement. The progression of PB may serve as an independent imaging marker for predicting a recurrent ischemic stroke event. The VWMRI may provide valuable information for risk stratification of stroke recurrence.
Acknowledgments
We thank Ting Xu from the Department of Laboratory Medicine of the First Affiliated Hospital of Nanjing Medical University for her help with data collection and Min-Lin Zhou from the National Clinical Research Center of Kidney Diseases, Jinling Hospital, for reviewing all the statistics.
Funding: This work was supported by the National Natural Science Foundation of China (No. 82171907 to SSL).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-210/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-210/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2021-SRFA-111), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117-71. [Crossref] [PubMed]
- Banerjee C, Chimowitz MI. Stroke Caused by Atherosclerosis of the Major Intracranial Arteries. Circ Res 2017;120:502-13. [Crossref] [PubMed]
- Mazighi M, Tanasescu R, Ducrocq X, Vicaut E, Bracard S, Houdart E, Woimant F. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology 2006;66:1187-91. [Crossref] [PubMed]
- Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014;383:333-41. [Crossref] [PubMed]
- Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TN. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke 2013;44:287-92. [Crossref] [PubMed]
- Teng Z, Peng W, Zhan Q, Zhang X, Liu Q, Chen S, Tian X, Chen L, Brown AJ, Graves MJ, Gillard JH, Lu J. An assessment on the incremental value of high-resolution magnetic resonance imaging to identify culprit plaques in atherosclerotic disease of the middle cerebral artery. Eur Radiol 2016;26:2206-14. [Crossref] [PubMed]
- Mandell DM, Mossa-Basha M, Qiao Y, Hess CP, Hui F, Matouk C, Johnson MH, Daemen MJ, Vossough A, Edjlali M, Saloner D, Ansari SA, Wasserman BA, Mikulis DJVessel Wall Imaging Study Group of the American Society of Neuroradiology. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2017;38:218-29. [Crossref] [PubMed]
- Kwee RM, Qiao Y, Liu L, Zeiler SR, Wasserman BA. Temporal course and implications of intracranial atherosclerotic plaque enhancement on high-resolution vessel wall MRI. Neuroradiology 2019;61:651-7. [Crossref] [PubMed]
- Schindler A, Schinner R, Altaf N, Hosseini AA, Simpson RJ, Esposito-Bauer L, Singh N, Kwee RM, Kurosaki Y, Yamagata S, Yoshida K, Miyamoto S, Maggisano R, Moody AR, Poppert H, Kooi ME, Auer DP, Bonati LH, Saam T. Prediction of Stroke Risk by Detection of Hemorrhage in Carotid Plaques: Meta-Analysis of Individual Patient Data. JACC Cardiovasc Imaging 2020;13:395-406. [Crossref] [PubMed]
- Zhang DF, Chen YC, Chen H, Zhang WD, Sun J, Mao CN, Su W, Wang P, Yin X. A High-Resolution MRI Study of Relationship between Remodeling Patterns and Ischemic Stroke in Patients with Atherosclerotic Middle Cerebral Artery Stenosis. Front Aging Neurosci 2017;9:140. [Crossref] [PubMed]
- Xiao J, Padrick MM, Jiang T, Xia S, Wu F, Guo Y, Gonzalez NR, Li S, Schlick KH, Dumitrascu OM, Maya MM, Diniz MA, Song SS, Lyden PD, Li D, Yang Q, Fan Z. Acute ischemic stroke versus transient ischemic attack: Differential plaque morphological features in symptomatic intracranial atherosclerotic lesions. Atherosclerosis 2021;319:72-8. [Crossref] [PubMed]
- Kim JM, Jung KH, Sohn CH, Moon J, Shin JH, Park J, Lee SH, Han MH, Roh JK. Intracranial plaque enhancement from high resolution vessel wall magnetic resonance imaging predicts stroke recurrence. Int J Stroke 2016;11:171-9. [Crossref] [PubMed]
- Song X, Zhao X, Liebeskind DS, Wang L, Xu W, Xu Y, Hou D, Zheng Z, Wu J. Incremental value of plaque enhancement in predicting stroke recurrence in symptomatic intracranial atherosclerosis. Neuroradiology 2020;62:1123-31. [Crossref] [PubMed]
- Shi Z, Li J, Zhao M, Zhang X, Degnan AJ, Mossa-Basha M, Saloner D, Lu J, Liu Q, Zhu C. Progression of Plaque Burden of Intracranial Atherosclerotic Plaque Predicts Recurrent Stroke/Transient Ischemic Attack: A Pilot Follow-Up Study Using Higher-Resolution MRI. J Magn Reson Imaging 2021;54:560-70. [Crossref] [PubMed]
- van der Kolk AG, Zwanenburg JJ, Brundel M, Biessels GJ, Visser F, Luijten PR, Hendrikse J. Intracranial vessel wall imaging at 7.0-T MRI. Stroke 2011;42:2478-84. [Crossref] [PubMed]
- Qiao Y, Zeiler SR, Mirbagheri S, Leigh R, Urrutia V, Wityk R, Wasserman BA. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 2014;271:534-42. [Crossref] [PubMed]
- Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013;12:1106-14. [Crossref] [PubMed]
- Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, Wang Y, Zou X, Leung TW, Cai Y, Bai Q, Wu Y, Wang C, Pan X, Luo B, Wong KSCICAS Study Group. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014;45:663-9. [Crossref] [PubMed]
- Dieleman N, van der Kolk AG, Zwanenburg JJ, Harteveld AA, Biessels GJ, Luijten PR, Hendrikse J. Imaging intracranial vessel wall pathology with magnetic resonance imaging: current prospects and future directions. Circulation 2014;130:192-201. [Crossref] [PubMed]
- Kim JT, Yoo SH, Kwon JH, Kwon SU, Kim JS. Subtyping of ischemic stroke based on vascular imaging: analysis of 1,167 acute, consecutive patients. J Clin Neurol 2006;2:225-30. [Crossref] [PubMed]
- Zhu T, Ren L, Zhang L, Shao Y, Wan L, Li Y, Liang D, Zheng H, Liu X, Zhang N. Comparison of plaque characteristics of small and large subcortical infarctions in the middle cerebral artery territory using high-resolution magnetic resonance vessel wall imaging. Quant Imaging Med Surg 2021;11:57-66. [Crossref] [PubMed]
- Lu SS, Ge S, Su CQ, Xie J, Shi HB, Hong XN. Plaque Distribution and Characteristics in Low-Grade Middle Cerebral Artery Stenosis and Its Clinical Relevance: A 3-Dimensional High-Resolution Magnetic Resonance Imaging Study. J Stroke Cerebrovasc Dis 2018;27:2243-9. [Crossref] [PubMed]
- Gupta A, Baradaran H, Al-Dasuqi K, Knight-Greenfield A, Giambrone AE, Delgado D, Wright D, Teng Z, Min JK, Navi BB, Iadecola C, Kamel H. Gadolinium Enhancement in Intracranial Atherosclerotic Plaque and Ischemic Stroke: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2016;5:003816. [Crossref] [PubMed]
- Lee HN, Ryu CW, Yun SJ. Vessel-Wall Magnetic Resonance Imaging of Intracranial Atherosclerotic Plaque and Ischemic Stroke: A Systematic Review and Meta-Analysis. Front Neurol 2018;9:1032. [Crossref] [PubMed]
- Zhang X, Chen L, Li S, Shi Z, Tian X, Peng W, Chen S, Zhan Q, Liu Q, Lu J. Enhancement Characteristics of Middle Cerebral Arterial Atherosclerotic Plaques Over Time and Their Correlation With Stroke Recurrence. J Magn Reson Imaging 2021;53:953-62. [Crossref] [PubMed]
- Ran Y, Wang Y, Zhu M, Wu X, Malhotra A, Lei X, Zhang F, Wang X, Xie S, Zhou J, Zhu J, Cheng J, Zhu C. Higher Plaque Burden of Middle Cerebral Artery Is Associated With Recurrent Ischemic Stroke: A Quantitative Magnetic Resonance Imaging Study. Stroke 2020;51:659-62. [Crossref] [PubMed]


