
Inhomogeneous liver fibrosis distribution revealed by macromolecular proton fraction quantification based on spin-lock MRI
Introduction
Accurate assessment of fibrosis stages is critical for the management of chronic liver diseases. Liver biopsy is the current gold standard for the diagnosis of liver fibrosis. However, biopsy is subject to sampling error, intraobserver and interobserver variability, and risk of complications (1). Liver stiffness measurement by ultrasound elastography such as vibration-controlled transient elastography (FibroScan®) is increasingly used for non-invasive assessment of liver fibrosis with reasonable accuracy in detecting advanced liver fibrosis. However, it remains challenging to use non-invasive methods to detect early-stage liver fibrosis (2-4).
Liver fibrosis is characterized by deposition of collagen-rich fibrotic tissues in the extracellular region. Recently, a novel method termed Macromolecular Proton Fraction quantification based on Spin-Lock (MPF-SL) was developed to measure relative macromolecule content in the liver, which shows a potential for the assessment of liver fibrosis (5,6).
We report a patient with non-alcoholic fatty liver disease (NAFLD) who underwent liver biopsy, transient elastography, and MPF-SL. Liver biopsy and transient elastography produced contradictory results. MPF-SL results of this subject showed heterogeneous spatial distribution of fibrosis in her liver.
Case presentation
A 70-year-old woman with type 2 diabetes, hypertension and dyslipidaemia underwent liver biopsy for the evaluation of NAFLD as abdominal ultrasonography showed coarse liver echotexture. There were no clinical or radiological signs of portal hypertension such as splenomegaly, ascites or recanalization of the umbilical vein. Liver histology was performed within six months of MRI exam and confirmed the presence of non-alcoholic steatohepatitis (NASH) with F3 fibrosis, S2 steatosis, and a NASH activity score of 4 by the NASH Clinical Research Network system. Percutaneous liver biopsy was taken from the right lobe of the liver using a 16G Temno needle. It consisted of two cores of tan color tissue measuring 1.0 cm and 1.6 cm in length. About 35% of hepatocytes exhibited macrovesicular steatosis. Periportal fibrosis and bridging fibrosis with focal nodule formation was seen. Foci of spotty necrosis and a few balloon degenerations were noted. There were seven portal tracts present with periportal inflammatory infiltrates seen. No interface hepatitis and abnormal cellular deposits were observed. The blood test was performed one month before the MRI scan, and the results include alanine aminotransferase (ALT) 22 U/L, aspartate aminotransferase (AST) 23 U/L, albumin 40 g/L, total bilirubin 4 µmol/L, platelet count 273×109/L, INR 0.87, AST/ALT ratio 1.05, gamma-glutamyl transpeptidase 27 U/L, alpha-fetoprotein 2 IU/mL, total cholesterol 3.2 mmol/L, and triglycerides 2.5 mmol/L. The patient tested negative for viral hepatitis serology (hepatitis B surface antigen and anti-hepatitis C virus antibody) and autoimmune markers (anti-nuclear antibody, anti-mitochondrial antibody and anti-smooth muscle antibody). Figure 1 shows T2-weighted images at different slices from the subject. There was no visualized abnormality in spleen, pancreas, kidneys and adrenals.
In contradiction to the biopsy results, the liver stiffness measurement by transient elastography (FibroScan®) was 5.5 kPa, suggesting the absence of liver fibrosis or cirrhosis. In previous studies, a liver stiffness of <8 kPa had >90% negative predictive value in excluding advanced liver fibrosis (7,8). The interquartile range of 10 liver stiffness measurements was 0.5 kPa, supportive of a reliable examination. The controlled attenuation parameter was 297 dB/m, in keeping with the diagnosis of NAFLD.
The patient was subsequently referred for an MRI examination on a Philips 3T scanner (Achieva TX, Philips Healthcare, Best, Netherlands) with a 32-channel cardiac coil (Invivo Corp, Gainesville, FL, USA). The MRI scan was arranged around five months after transient elastography examination. Imaging protocols included the newly developed MPF-SL technique (5) and gradient echo acquisitions for quantification of liver fat fraction and liver iron content using the method described in (9). The MPF-SL acquisition parameters included: field of view 380×278 mm, resolution: 2×2 mm, slice thickness 7 mm, Repetition Time (TR) / Echo Time (TE) 2000/17 ms, frequency of spin-lock 100 Hz and 400 Hz, frequency offset 1,000 Hz and 4,000 Hz, and time of spin-lock 50 ms. Double inversion recovery (DIR) combined with single shot fast spin echo acquisition was used for suppression of blood signal (10). Spectral pre-saturation with inversion recovery (SPIR) was used for fat suppression. Vendor-provided dual transmit and RF shim were applied to reduce the B1 inhomogeneity. The imaging data acquisition of each slice of MPF-SL is 10 seconds. The relaxation rate map related to Macromolecular Proton Fraction (MPF) (Rmpfsl) and MPF were calculated using the same method as described in our previous paper (5,6). Total 5 slices of MPF were acquired. The data sets were processed using MATLAB R2021a (MathWorks, USA).
MRI results showed that the subject had a liver proton density fat fraction (PDFF) of 10% and iron content of 15 µmol/g (dry tissue weight). The Rmpfsl maps of the subject showed that there were obvious variations of the liver Rmpfsl at different slice locations (Figure 2). The estimated MPF values were listed in the Table 1. This subject was invited to repeat the MRI exam two months after the first MRI exam. The results show similar heterogeneity of MRI measurement in the liver with high MPF in the same region.
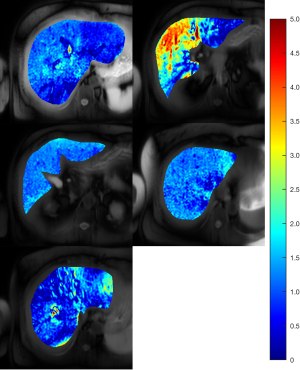
Table 1
| Slice | MPF (%) |
|---|---|
| 1 | 4.98 |
| 2 | 9.88 |
| 3 | 5.59 |
| 4 | 4.95 |
| 5 | 3.40 |
MPF, macromolecular proton fraction.
This study was approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee (The Joint CUHK-NTEC CREC). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Generally, fibrosis formation and collagen deposition affect the entire liver. For specific diseases, fibrous material tends to form regionally depending on the nature and location of injury (1,11), which can bring challenges to accurate diagnosis using liver biopsy or ultrasound elastography. Recent advances in quantitative MRI such as MPF-SL may be beneficial for these cases as it can provide information about fibrosis distribution in the liver.
Recently, other liver fibrosis studies also observed the spatial inhomogeneous distribution. Dhall et al. (12) found a quarter of patients with congestive hepatopathy secondary to heart failure had heterogeneous liver biopsy results. Reiter et al. (13) demonstrated heterogeneous distribution of fibrosis in patients with primary sclerosing cholangitis using magnetic resonance elastography. While the stiffness map may highlight focal liver lesions (14), liver stiffness measurement sometimes may be confounded by acute inflammation, biliary obstruction, and congestion. Future studies on both MPF-SL technology development and clinical studies may help better understand liver fibrosis distribution in different groups of patients.
Conclusions
This case illustrates that the discrepancies between liver biopsy and transient elastography may be due to heterogeneous fibrosis distribution in the liver. Multi-slice MPF-SL may be beneficial for assessing liver fibrosis by providing spatial distribution of liver fibrosis and avoiding the focal sampling error. Further clinical evaluation of MPF-SL on patient cohorts for assessment of liver fibrosis is needed.
Acknowledgments
The authors thank Dr. Anthony Chan for the histological assessment of the liver biopsy samples.
Funding: This study was supported by a grant from the Innovation and Technology Commission of the Hong Kong SAR (No. MRP/046/20X), a grant from the Hong Kong Health and Medical Research Fund (HMRF) (No. 06170166), Faculty Innovation Award from the Faculty of Medicine, the Chinese University of Hong Kong, and a grant from the Research Grants Council of the Hong Kong SAR (Project SEG No. CUHK02).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-302/coif). WC, WCWC and VWSW are shareholders of Illuminatio Medical Technology Limited and report this study was supported by a grant from the Innovation and Technology Commission of the Hong Kong SAR (Project No. MRP/046/20X), a grant from the Hong Kong Health and Medical Research Fund (HMRF) (No. 06170166), Faculty Innovation Award from the Faculty of Medicine, the Chinese University of Hong Kong, and a grant from the Research Grants Council of the Hong Kong SAR (Project SEG No. CUHK02). VWSW also served as a speaker and advisory board member for Echosens. BJ is an employee of Illuminatio Medical Technology Limited. BJ, JH, and WC report an USA patent of MPF-SL (US 11,280,867 B2) is granted. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee (The Joint CUHK-NTEC CREC). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol 2010;7:425-36. [Crossref] [PubMed]
- Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, Garra B, Myers RP, Wilson SR, Rubens D, Levine D. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2015;276:845-61. [Crossref] [PubMed]
- Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, Sung JJ, de Lédinghen V. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010;51:454-62. [Crossref] [PubMed]
- Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan(®)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease - Where do we stand? World J Gastroenterol 2016;22:7236-51. [Crossref] [PubMed]
- Hou J, Wong VW, Jiang B, Wang YX, Wong GL, Chan AW, Chu WC, Chen W. Macromolecular proton fraction mapping based on spin-lock magnetic resonance imaging. Magn Reson Med 2020;84:3157-71. [Crossref] [PubMed]
- Hou J, Wong VWS, Wong GLH, et al. Macromolecular proton fraction mapping based on spin-lock for the non-invasive diagnosis of early stage liver fibrosis. Int. Soc. Magn. Reson. Med., International Society for Magnetic Resonance in Medicine; 2021. Available online: https://cds.ismrm.org/protected/21MPresentations/abstracts/0314.html
- Wong VW, Irles M, Wong GL, Shili S, Chan AW, Merrouche W, Shu SS, Foucher J, Le Bail B, Chan WK, Chan HL, de Ledinghen V. Unified interpretation of liver stiffness measurement by M and XL probes in non-alcoholic fatty liver disease. Gut 2019;68:2057-64. [Crossref] [PubMed]
- Papatheodoridi M, Hiriart JB, Lupsor-Platon M, Bronte F, Boursier J, Elshaarawy O, et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J Hepatol 2021;74:1109-16. [Crossref] [PubMed]
- Paisant A, d'Assignies G, Bannier E, Bardou-Jacquet E, Gandon Y. MRI for the measurement of liver iron content, and for the diagnosis and follow-up of iron overload disorders. Presse Med 2017;46:e279-87. [Crossref] [PubMed]
- Chen W, Chan Q, Wáng YX. Breath-hold black blood quantitative T1rho imaging of liver using single shot fast spin echo acquisition. Quant Imaging Med Surg 2016;6:168-77. [Crossref] [PubMed]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209-18. [Crossref] [PubMed]
- Dhall D, Kim SA, Mc Phaul C, Kransdorf EP, Kobashigawa JA, Sundaram V, Mirocha J, Guindi M. Heterogeneity of Fibrosis in Liver Biopsies of Patients With Heart Failure Undergoing Heart Transplant Evaluation. Am J Surg Pathol 2018;42:1617-24. [Crossref] [PubMed]
- Reiter R, Shahryari M, Tzschätzsch H, Klatt D, Siegmund B, Hamm B, Braun J, Sack I, Asbach P. Spatial heterogeneity of hepatic fibrosis in primary sclerosing cholangitis vs. viral hepatitis assessed by MR elastography. Sci Rep 2021;11:9820. [Crossref] [PubMed]
- Venkatesh SK, Wells ML, Miller FH, Jhaveri KS, Silva AC, Taouli B, Ehman RL. Magnetic resonance elastography: beyond liver fibrosis-a case-based pictorial review. Abdom Radiol (NY) 2018;43:1590-611. [Crossref] [PubMed]



