
The value of noncontrast MRI in evaluating breast imaging reporting and data system category 0 lesions on digital mammograms
Introduction
The International Agency for Research on Cancer (IARC) recently demonstrated that female breast cancer has the highest incidence and prevalence of any cancer. In 2020, 2.3 million women were diagnosed with breast neoplasms, exceeding the incidence of lung cancer for the first time in recorded history. Breast neoplasms has a mortality rate of around 6.9% (1), a number that has improved through aggressive research on diagnostic and treatment methods over the years (2). Breast magnetic resonance imaging (MRI) is currently used during diagnosis, staging, and treatment assessment in breast cancer because it provides a longitudinal, noninvasive, and comprehensive evaluation of the disease. The breast imaging reporting and data system (BI-RADS) algorithm for mammography was developed specifically for imaging in 1992; its results provide a reliable and consistent method to categorize digital mammogram (DM) findings (3). The category of BI-RADS 0 is reported when there are lesions that need further investigation for complete analysis (4). However, there are relatively few studies on this category, and further research is needed. MRI is a functional technique that was first introduced by Heywang et al. (5) and Kaiser et al. (6) independently in the 1980s. Since then, breast MRI has been widely used for various clinical diseases, especially for the evaluation of benign and malignant tumors. Breast MRI has the best diagnostic performance for distinguishing benign from malignant among lesions that are classified as BI-RADS 0 with DM (7). MRI of the breast has the highest sensitivity for distinguishing benign from malignant lesions among current clinical imaging modalities and is indispensable for breast imaging practice (8). The use of breast MRI has been shown to greatly enhance the diagnostic sensitivity of DM from 32% to 84% (9). However, early studies included only short tau inversion recovery (STIR) sequences. Compared to T1-weighted and T2-weighted imaging, STIR provides greater resolution by suppression of adipose tissue in breast MRI (10). Diffusion-weighted imaging (DWI) is a routine clinical breast MRI sequence that uses motion-sensitizing gradients to measure water diffusivity in tissue, which reflects microstructural characteristics. It is a useful tool for detecting and characterizing breast neoplasms. However, conventional DWI provides limited spatial resolution for breast imaging because of the combined need for a large field of view (FOV) and restricted matrix sizes. To improve the image quality of breast DWI, a variety of techniques have been explored (11,12). STIR combined with high b value DWI (STIR-DWI) has emerged, which combines good spatial resolution and histological resolution to improve the image quality of breast neoplasms (13).
To date, existing research and practice shows that dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is the most sensitive technique in breast MRI. However, DCE-MRI has a few critical disadvantages, including variable specificity, the need for contrast agent administration (with the well-known associated risks, such as adverse reactions, brain deposition, and nephrogenic systemic fibrosis in patients with terminal renal insufficiency), long exam times, and high costs (14). To better distinguish benign from malignant BI-RADS category 0 lesions on DMs, the selection of breast MRI sequences needs to be further explored. The purpose of this study was thus to compare the diagnostic accuracy of STIR, STIR-DWI, and DCE-MRI in detecting the benign and malignant lesions of the nonpalpable BI-RADS category 0 lesions on DMs. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-968/rc).
Methods
Patients
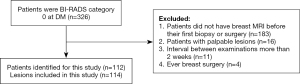
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval was granted by the Institutional Review Board of the First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Traditional Chinese Medicine). It was determined that as the design of this study was retrospective in nature informed consent was not needed. From January 2014 to June 2019, the data of 127 patients were selected using convenience sampling. The inclusion criteria were as follows: (I) patients must have received a breast MRI scan and DM at the First Affiliated Hospital of Zhejiang Chinese Medical University within 1 month before their first biopsy or surgery, with breast lesions being detected on DMs and/or MRI; (II) patients could not have received any preoperative treatment; and (III) patients had nonpalpable BI-RADS category 0 according to the DM. Patients with the following characteristics were excluded from the study: those with palpable lesions, patients with a history of breast cancer, and those with proven benign disease (Figure 1).
Digital mammography
All DMs were acquired with a Mammomat Inspiration mammography machine (Siemens, Erlangen, Germany). Imaging was conducted in the mediolateral oblique (MLO) and craniocaudal (CC) position using automatic exposure control.
MRI protocol
MRI imaging was conducted using a 3Tesla MRI system (Magnetom Verio, Siemens, Erlangen, Germany) along with a 16-channel breast coil. Both breasts were imaged with the patient in the prone position. The 3 sequences for imaging are described here. (I) The STIR sequence used the following parameters: repetition time (TR)/echo time (TE)/inversion time (TI) =4,000/70/230 ms, FOV =360×360 mm, matrix =448×448, slice thickness =4 mm, number of excitation (NEX) 2, voxel size =1.1×0.8×4.0 mm3, and sequence duration =2 min 48 s. (II) DWI was obtained using parallel imaging, a multisegment acquisition, and 3 diffusion directions under the following parameters: TR/TE =8,400/84 ms, FOV =360×360 mm, matrix =220×220, slice thickness =4 mm, NEX 3, b0=50 s/mm2, b1=400 s/mm2, b2=800 s/mm2, voxel size =1.8×1.6×4.0 mm3, and sequence duration =2 min 56 s. The 3 diffusion gradients were applied consecutively, and apparent diffusion coefficient (ADC) values were calculated automatically by the MRI system software from the DWI images. (III) DCE-MRI with volumetric interpolated breath-hold examination (VIBE) under the following parameters: VIBE slice thickness of the sequence interpolated to 1 mm, TR/TE =4.51/1.61 ms, flip angle =10°, FOV =340×340 mm, matrix =448×448, slice thickness =1 mm, and NEX 1. The single scanning time was 60 s. After injection of the contrast agent, 5 scans were completed, with a scanning time of 6 phases. The contrast used was Gadobenate dimeglumine (BeiLu Pharmaceutical, Beijing, China) administered intravenously at 0.1 mmol/kg with a flow rate of 2.0 mL/s. After that, 10 mL of normal saline was flushed. Contrast and saline are injected using an automated device. The STIR, DWI, and DCE-MRI sequences of patients were examined independently. Before breast MRI examinations, we routinely asked about the menstrual cycle in premenopausal women. We recommend that patients undergo breast MRI 1 week after menstruation. The purpose is to reduce the effect of hormone levels on image quality and to make the lesions appear more clearly.
Image interpretation
Images were read by 2 radiologists with 10 and 27 years of imaging experience specific to breast imaging. The 2 radiologists evaluated images separately, and when their opinions differed, they negotiated a decision. They independently and randomly reviewed and assessed different MRI sequences using a dedicated workstation [picture archiving and communications system (PACS)] and were blinded to the patient’s chart. Different MRI sequences including STIR, a combination of STIR-DWI and ADC maps, and DCE-MRI were independently and randomly reviewed by the 2 radiologists using BI-RADS assessment. The guidelines for determining malignant vs. benign lesions were supported by the morphology and enhancement characteristics of the lesion in accordance with the American College of Radiology (ACR) BI-RADS lexicon. The radiologists distinguished lesions using the stated criteria in combination with the anatomic and morphologic characteristics on imaging. Lesions were characterized by their shape, margin characteristics, enhancement pattern, and degree of enhancement. Further information from the physical exam was also included in this study. The BI-RADS assessment was used to rank the severity of select features (15). Afterward, the ADC value was calculated by referencing the DWI, STIR, and subtracted DCE-MRI sections. Manual region of interest (ROI) selection was then used to circumscribe the entire mass. The average ADC of each ROI was calculated 3 separate times, and the lowest ADC was selected. Lesions which were too small or had no ADC did not receive an ADC value. According to BI-RADS assessment categories in the 5th edition of the ACR BI-RADS Atlas, BI-RADS category 0 represents an incomplete image that needs additional imaging evaluation (16). In this study, the final BI-RADS assessments for MRI were recorded. A 7-point BI-RADS scale was used to determine the final assessment. Scores of 1, 2, and 3 represented negative, benign, and likely benign lesions, respectively. Those with scores of 4a, 4b, and 4c represented lesions which had a low, moderate, or high suspicion for malignancy, respectively. Finally, a score of 5 represented the greatest risk of cancer (17). BI-RADS category 0 lesions were followed up with fine-needle aspiration for core biopsy. Those that could not undergo fine-needle aspiration were examined using open excision under guidance from the Mastology Department at the First Affiliated Hospital of Zhejiang Chinese Medical University. The pathological tissues collected in this study were all obtained by surgery, including Mammotome (minimally invasive atherectomy under the guidance of a B-ultrasound) and mass resection as opposed to only using fine-needle biopsies. All samples were examined by designated breast pathologists with at least 6 years of experience. The substance P immunohistochemical method was used to detect the expression of estrogen receptor (ER) progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) in all samples.
Statistical analysis
Statistical analysis was conducted using SPSS Version 25.0 (IBM, Armonk, NY, USA) and MedCalc Version 15.2.2 (MedCalc, Inc., Mariakerke, Belgium). The level of significance was set at P≤0.05, and a 95% CI was used to determine the accuracy of diagnostic MRI. Quantitative findings are presented in tables and graphs. The χ2 test was used to determine the diagnostic performances of MRI for BI-RADS category 0 lesions on DMs. The receiver operating characteristic (ROC) curve was also used to delineate between benign and malignant lesions using ADC values. ADC values were generated. The cutoff value for all 3 imaging sequences (STIR, STIR-DWI, DCE-MRI) was 3, and the areas under the curve (AUCs) for the 3 sequences were calculated and compared using of DeLong’s test.
Results
The data of from 112 patients comprising 114 lesions were selected for analysis in this study (Figure 1). The age of the patients ranged from 22 to 80 years with the median of 47 years. Among the 112 patients, 65.2% (n=73) were premenopausal and 34.8% (n=39) were postmenopausal. Of the 114 lesions, 48 (42.1%) were determined to be benign and 66 (57.9%) were determined to be malignant. The specific diagnosis established through histological examination of samples included the following: invasive carcinoma (n=55), fibroadenomas (n=24), lobular hyperplasia (n=10), intraductal papilloma (n=8), granulomatous mastitis (n=5), ductal carcinoma in situ (n=5), mucinous carcinoma (n=4), diffuse large B cell lymphoma (n=1), phyllode tumor of the breast (n=1), and adenomyoepithelioma (n=1). Representative cases are shown in Figures 2-4. The size of the lesion was defined as the maximum dimension on the MRI and ranged from 5 to 63 mm with a median of 19 mm (Table 1).
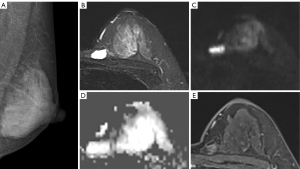
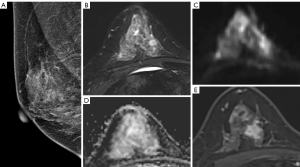
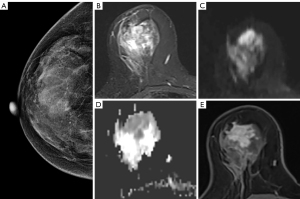
Table 1
| Observations | Value |
|---|---|
| Patient demographic characteristics (n=112) | |
| Age (years), mean ± SD | 48.0±29 |
| Menopausal status | |
| Premenopausal | 73 |
| Postmenopausal | 39 |
| Mass characteristics (n=114) | |
| Size (mm), mean ± SD | 22.3±15.09 |
| Pathology | |
| Invasive carcinoma of no special type | 55 |
| Fibroadenoma | 24 |
| Lobular hyperplasia | 10 |
| Intraductal papilloma | 8 |
| Granulomatous mastitis | 5 |
| Ductal carcinoma in situ | 5 |
| Mucinous carcinoma | 4 |
| Diffuse large B cell lymphoma | 1 |
| Phyllode tumor of the breast | 1 |
| Adenomyoepithelioma | 1 |
Values are presented as number or mean ± SD.
Final assessments of breast STIR, STIR-DWI, and DCE-MRI for nonpalpable BI-RADS category 0 lesions in DMs are summarized in Table 2. The BI-RADS classifications made were the clinical BI-RADS classifications of the 2 study radiologists. Final STIR assessments categorized 76 lesions as BI-RADS 1–3 (malignant, 32), 36 lesions as BI-RADS 4 (malignant, 32), and 2 lesions as BI-RADS 5 (malignant, 1). Final STIR-DWI assessments categorized 44 lesions as BI-RADS 1–3 (malignant, 9), 39 lesions as BI-RADS 4 (malignant, 27), and 31 lesions as BI-RADS 5 (malignant, 29). Final DCE-MRI assessments categorized 30 lesions as BI-RADS 1–3 (malignant, 3), 43 lesions as BI-RADS 4 (malignant, 23), and 41 lesions as BI-RADS 5 (malignant, 39). Chi-square test analysis of the malignant percentage in relation to lesions categorized as BI-RADS 1–3 showed a statistically significant difference (χ2=13.064; df=2; P=0.001), resulting from the much greater presence of STIR (42.1%) compared to STIR-DWI (20.5%) and DCE-MRI (10%). There was no statistically significant difference between STIR-DWI and DCE-MRI, but there was a significant difference between STIR and STIR-DWI. Chi-square test analysis of the malignant percentage in relation to lesions categorized as BI-RADS 4 showed a statistically significant difference (χ2=11.585; df=2; P=0.003), resulting from the greater presence of STIR (88.9%) compared to STIR-DWI (69.2%) and DCE-MRI (53.5%). There was no difference between STIR-DWI and DCE-MRI, but there was a significant difference between STIR and DCE-MRI. Chi-square test analysis of the malignant percentage in relation to lesions categorized as BI-RADS 5 showed a statistically significant difference (χ2=6.171; df=2; P=0.046), resulting from the greater presence of STIR (50%) compared to STIR-DWI (93.5%) and DCE-MRI (95.1%). There was no difference between STIR-DWI and DCE-MRI, but there was a significant difference between STIR and DCE-MRI.
Table 2
| Type of image | BI-RADS | Pathology | |||
|---|---|---|---|---|---|
| Total | Benign | Malignant | Malignant percentage (%) | ||
| STIR | 1–3 | 76 | 44 | 32 | 42.1 |
| 4 | 36 | 4 | 32 | 88.9 | |
| 5 | 2 | 1 | 1 | 50 | |
| STIR-DWI | 1–3 | 44 | 35 | 9 | 20.5 |
| 4 | 39 | 12 | 27 | 69.2 | |
| 5 | 31 | 2 | 29 | 93.5 | |
| DCE-MRI | 1–3 | 30 | 27 | 3 | 10 |
| 4 | 43 | 20 | 23 | 53.5 | |
| 5 | 41 | 2 | 39 | 95.1 | |
STIR, short tau inversion recovery; STIR-DWI, short tau inversion recovery diffusion-weighted imaging; DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; BI-RADS, breast imaging reporting and data system; DM, digital mammogram.
The results suggested that the ADC showed a strong ability to differentiate tumor subtypes (AUC =0.799; 95% CI: 0.714–0.884; sensitivity =66.7%; specificity =86.4%; cutoff =1.058×10−3 mm2/s). Figures 5,6 and Table 3 show the ROC for STIR, STIR-DWI, and DCE-MRI. The accuracies of STIR, STIR-DWI, and DCE-MRI as measured by AUC were 0.754 (95% CI: 0.664–0.830), 0.858 (95% CI: 0.781–0.917), and 0.884 (95% CI: 0.811–0.936), respectively. The sensitivities of STIR, STIR-DWI, and DCE-MRI were 51.5% (95% CI: 38.9–64.0%), 87.8% (95% CI: 77.5–94.6%), and 95.5% (95% CI: 87.3–99.1%), respectively. The specificities of STIR, STIR-DWI, and DCE-MRI were 91.7% (95% CI: 80.0–97.7%), 72.9% (95% CI: 58.2–84.7%), 56.3% (95% CI: 41.2–70.5%), respectively. The AUC of STIR was significantly inferior to STIR-DWI (95% CI: 0.0353–0.178; P=0.0034, DeLong’s test), and DCE-MRI (95% CI: 0.0504–0.210; P=0.0014, DeLong’s test). No significant differences in AUC were found between STIR-DWI and DCE-MRI (95% CI: −0.0400 to 0.0867; P=0.4698, DeLong’s test).
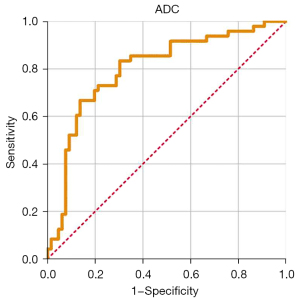
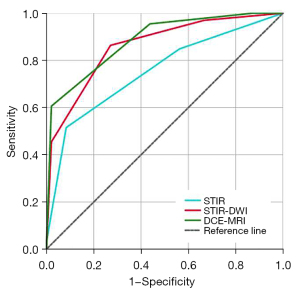
Table 3
| Type of image | AUC (95% CI) | Sensitivity (95% CI), % | Specificity (95% CI), % | Cutoff |
|---|---|---|---|---|
| STIR | 0.754 (0.664–0.830) | 51.5 (38.9–64.0) | 91.7 (80.0–97.7) | 3 |
| STIR-DWI | 0.858 (0.781–0.917) | 87.8 (77.5–94.6) | 72.9 (58.2–84.7) | 3 |
| DCE-MRI | 0.884 (0.811–0.936) | 95.5 (87.3–99.1) | 56.3 (41.2–70.5) | 3 |
ROC, receiver operating characteristic; STIR, short tau inversion recovery; STIR-DWI, short tau inversion recovery diffusion-weighted imaging; DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; AUC, the area under the ROC curve.
Discussion
Breast MRI has evolved from a screening tool to an additional step in the examination of patients with intermediate- and high-risk breast carcinoma (8,18). Breast MRI has demonstrated a higher sensitivity than has using a DM alone. In one study, the sensitivity of using a DM alone was 53%, while that for using breast MRI in conjunction with a DM was 94% (19). Research shows that the sensitivity of combination DM and MRI can range from 94% to 100% when detecting breast malignancies (20). Existing studies also show that STIR and DWI in combination can greatly improve the diagnostic accuracy of malignant lesions (21,22). Published reports show that nonenhanced MRI in combination with STIR and DWI can provide a greater sensitivity over traditional DMs in detecting breast carcinomas (23). In our study, DCE-MRI is the most sensitive test for breast cancer detection and STIR-DWI sequences are less sensitive but more specific than DCE-MRI. These findings are consistent with Pinker et al.’s existing literature (24).
Gadolinium (Gd) contrast agents can accumulate in neural tissues and systemic organs for months and years, raising concerns about long term toxicity (25-27). Yet, as of now, no relationship between Gd and systemic toxicity has been established, and thus further study is needed.
In this study, all the patients we selected had a DM-rated BI-RADS category 0 classification. The radiologists interpreted the breast MRI using the ACR BI-RADS lexicon, and the final standard references were made during biopsy and/or surgery (28,29). The purpose of this study was to evaluate the diagnostic power of STIR, STIR-DWI, and DCE-MRI for BI-RADS category 0 in DMs. The principal finding of our study was that STIR-DWI and DCE-MRI showed a higher diagnostic accuracy than did STIR (P<0.01). The results suggest that STIR with DWI can offer a higher sensitivity when evaluating patients with a DM-rated BI-RADS category 0 classification while maintaining an optimal safety profile. In this study, 32 (48.5%) malignant lesions were missed by STIR, 9 (13.4%) malignant lesions were missed by STIR-DWI, while 3 (4.6%) malignant lesions were missed by DCE-MRI. There were 7 (11%) malignant lesions that were missed by STIR-DWI but detected by DCE-MRI. Due to their high ADC values for pathological characteristics, we classification their as STIR-DWI BI-RADS 2–3. Three malignant lesions were small, smooth, and had high ADC values.
There are several reasons for the enhanced performance of STIR-DWI and DCE-MRI compared to STIR. As a functional imaging modality, STIR-DWI is highly affected by changes in the tumor microenvironment. In addition, STIR-DWI shows tumors as a hyperdense lesion, similar to the behavior of contrast agents. DCE-MRI has its unique advantages in tumor detection sensitivity. However, in our study, 3 (4.6%) malignant lesions were missed by DCE-MRI, 1 was detected by STIR-DWI, and the other 2 were missed by STIR-DWI. Retrospective analysis revealed that 1 growth was a mucinous carcinoma (Figure 2). Due to its high ADC values for pathological characteristics, we classified it as DCE-MRI BI-RADS 3. We also misdiagnosed the other 2 lesions as DCE-MRI BI-RADS 3. The patients were young and the enhancement of the lesions was very similar to that of mastitis. In this study, the specificity of DCE-MRI was lower than usual, which might have occurred because the patients enrolled in this study were all BI-RADS category 0, and so they all had suspicious lesions. The degree of enhancement of some benign lesions was similar to that of malignant lesions, and there were some misdiagnosed cases. In this respect, STIR-DWI has some advantages to compensate for the shortcomings of DCE-MRI. By comparison, DMs do not offer functional information. Studies have demonstrated that STIR-DWI is also able to scan for morphological characteristics when detecting breast lesions (30,31). High-resolution imaging can be used in follow-up studies to augment the performance of the established model (32). For example, some high-resolution techniques for neural imaging may be applicable to breast imaging (33,34). The greater accuracy and applicability in the detection of benign and malignant lesions using STIR with DWI and DCE-MRI when compared to DM has also been reported in other studies (35-39). Our findings demonstrate a novel combination of STIR-DWI that can be used to determine the safety and efficacy of the modality in patients with a BI-RADS category 0 in DMs. Previously, DWI was limited by its poor resolution and was therefore used as an adjunct method for imaging breast lesions. This study showed that, although DCE-MRI was higher in malignant percentage than was STIR-DWI, there was no statistical difference between the methods. Another factor dictating the efficacy of treatment is the cost and time associated with examination (40,41), and omitting the need for contrast reduces both the cost and time investment (42,43).
While this study provides insights into the relative abilities of different breast MRI sequences, several shortcomings are worth noting. First, the patients were obtained from the inpatient roster as opposed to the general population. In the future, research should also evaluate the feasibility of prospective screening with a large population to obtain a definite conclusion. Second, it is difficult to ensure full blindness when given the inclusion criteria for this study, which selected for high-risk patients. As a consequence of this, the radiologists might have been biased to scrutinize the images due to knowledge regarding the patient population (44,45). Future studies should also examine the performance of DWI against ultrasound, digital breast tomosynthesis (DBT), and other multimodal imaging methods that have been suggested to be suitable for BI-RADS category 0 lesions in DM (46-48).
Conclusions
STIR-DWI was superior to STIR alone and comparable with DCE-MRI in terms of diagnostic performance in detecting nonpalpable BI-RADS category 0 lesions. Due to a high sensitivity and the potential to examine breast lesions regardless of density, STIR-DWI should be suggested as a front-line modality to follow up BI-RADS category 0 lesions. The findings of this study, along with a short acquisition time and low-cost of STIR-DWI, can improve the safety and efficacy of breast cancer screening, especially in nonpalpable BI-RADS category 0 lesions in DMs.
Acknowledgments
Funding: This work was supported by the Scientific Research Fund of National Health Commission-Zhejiang Provincial Major Science and Technology Project of Pharmaceutical Technology (No. WKJ-ZJ-2039), the Zhejiang Provincial Medical and Health Research Program (No. 2021KY224), the Chinese Medicine Science and Technology Project of Zhejiang Province (No. 2021ZB089), the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2020KY199), the Chinese Medicine Research Foundation Project of Zhejiang Province (No. 2018ZA037), the Zhejiang Traditional Chinese Medicine Science and Technology Project (No. 2018ZB048), the Zhejiang Provincial Medicine and Health Discipline Platform Project (No. 2018RC058), the Zhejiang Provincial Department of Health Platform Backbone Project (No. 2016RCA025), the Zhejiang Basic Public Welfare Research Project (No. LGF21H180003), and the Research Project of Zhejiang Chinese Medical University (No. 2022JKZKTS39).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-968/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-968/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was defined using a retrospective design. Approval was granted by the Institutional Review Board of the First Affiliated Hospital of Zhejiang Chinese Medical University. It was determined that, due to the nature of the retrospective study, informed consent was not needed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer. Nat Rev Dis Primers 2019;5:66. [Crossref] [PubMed]
- Lee JM, Ichikawa L, Valencia E, Miglioretti DL, Wernli K, Buist DSM, Kerlikowske K, Henderson LM, Sprague BL, Onega T, Rauscher GH, Lehman CD. Performance Benchmarks for Screening Breast MR Imaging in Community Practice. Radiology 2017;285:44-52. [Crossref] [PubMed]
- Zanello PA, Robim AF, Oliveira TM, Elias J Junior, Andrade JM, Monteiro CR, Sarmento Filho JM, Carrara HH, Muglia VF. Breast ultrasound diagnostic performance and outcomes for mass lesions using Breast Imaging Reporting and Data System category 0 mammogram. Clinics (Sao Paulo) 2011;66:443-8. [Crossref] [PubMed]
- Heywang SH, Hahn D, Schmidt H, Krischke I, Eiermann W, Bassermann R, Lissner J. MR imaging of the breast using gadolinium-DTPA. J Comput Assist Tomogr 1986;10:199-204. [Crossref] [PubMed]
- Kaiser WA, Zeitler E. MR imaging of the breast: fast imaging sequences with and without Gd-DTPA. Preliminary observations. Radiology 1989;170:681-6. [Crossref] [PubMed]
- Spak DA, Plaxco JS, Santiago L, Dryden MJ, Dogan BE. BI-RADS® fifth edition: A summary of changes. Diagn Interv Imaging 2017;98:179-90.
- Mann RM, Cho N, Moy L. Breast MRI: State of the Art. Radiology 2019;292:520-36. [Crossref] [PubMed]
- Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, Smith RA, Warner E, Yaffe M, Andrews KS, Russell CAAmerican Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57:75-89. [Crossref] [PubMed]
- Forrai G, Polgar C, Zana K, Riedl E, Fodor J, Nemeth G, Fornet B. The role of STIR MRI sequence in the evaluation of the breast following conservative surgery and radiotherapy. Neoplasma 2001;48:7-11. [PubMed]
- Partridge SC. Emerging Techniques Bring Diffusion-weighted Imaging of the Breast into Focus. Radiology 2020;297:313-5. [Crossref] [PubMed]
- Cho E, Baek HJ, Szczepankiewicz F, An HJ, Jung EJ, Lee HJ, Lee J, Gho SM. Clinical experience of tensor-valued diffusion encoding for microstructure imaging by diffusional variance decomposition in patients with breast cancer. Quant Imaging Med Surg 2022;12:2002-17. [Crossref] [PubMed]
- Kazama T, Nasu K, Kuroki Y, Nawano S, Ito H. Comparison of diffusion-weighted images using short inversion time inversion recovery or chemical shift selective pulse as fat suppression in patients with breast cancer. Jpn J Radiol 2009;27:163-7. [Crossref] [PubMed]
- Rizzo V, Moffa G, Kripa E, Caramanico C, Pediconi F, Galati F. Preoperative Staging in Breast Cancer: Intraindividual Comparison of Unenhanced MRI Combined With Digital Breast Tomosynthesis and Dynamic Contrast Enhanced-MRI. Front Oncol 2021;11:661945. [Crossref] [PubMed]
- Mahoney MC, Gatsonis C, Hanna L, DeMartini WB, Lehman C. Positive predictive value of BI-RADS MR imaging. Radiology 2012;264:51-8. [Crossref] [PubMed]
- Fujiwara K, Yamada T, Kanemaki Y, Okamoto S, Kojima Y, Tsugawa K, Nakajima Y. Grading System to Categorize Breast MRI in BI-RADS 5th Edition: A Multivariate Study of Breast Mass Descriptors in Terms of Probability of Malignancy. AJR Am J Roentgenol 2018;210:W118-27.
- Mercado CL. BI-RADS update. Radiol Clin North Am 2014;52:481-7. [Crossref] [PubMed]
- Vinnicombe S. How I report breast magnetic resonance imaging studies for breast cancer staging and screening. Cancer Imaging 2016;16:17. [Crossref] [PubMed]
- González-Huebra I, Elizalde A, García-Baizán A, Calvo M, Ezponda A, Martínez-Regueira F, Pina L. Is it worth to perform preoperative MRI for breast cancer after mammography, tomosynthesis and ultrasound? Magn Reson Imaging 2019;57:317-22. [Crossref] [PubMed]
- Freitas V, Scaranelo A, Menezes R, Kulkarni S, Hodgson D, Crystal P. Added cancer yield of breast magnetic resonance imaging screening in women with a prior history of chest radiation therapy. Cancer 2013;119:495-503. [Crossref] [PubMed]
- Belli P, Bufi E, Bonatesta A, Patrolecco F, Padovano F, Giuliani M, Rinaldi P, Bonomo L. Unenhanced breast magnetic resonance imaging: detection of breast cancer. Eur Rev Med Pharmacol Sci 2016;20:4220-9. [PubMed]
- Ouyang Z, Ouyang Y, Zhu M, Lu Y, Zhang Z, Shi J, Li X, Ren G. Diffusion-weighted imaging with fat suppression using short-tau inversion recovery: Clinical utility for diagnosis of breast lesions. Clin Radiol 2014;69:e337-44. [Crossref] [PubMed]
- Amornsiripanitch N, Bickelhaupt S, Shin HJ, Dang M, Rahbar H, Pinker K, Partridge SC. Diffusion-weighted MRI for Unenhanced Breast Cancer Screening. Radiology 2019;293:504-20. [Crossref] [PubMed]
- Pinker K, Moy L, Sutton EJ, Mann RM, Weber M, Thakur SB, Jochelson MS, Bago-Horvath Z, Morris EA, Baltzer PA, Helbich TH. Diffusion-Weighted Imaging With Apparent Diffusion Coefficient Mapping for Breast Cancer Detection as a Stand-Alone Parameter: Comparison With Dynamic Contrast-Enhanced and Multiparametric Magnetic Resonance Imaging. Invest Radiol 2018;53:587-95. [Crossref] [PubMed]
- Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruyama T, Kitajima K, Furui S. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology 2015;276:228-32. [Crossref] [PubMed]
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 2015;11:457-70. [Crossref] [PubMed]
- Le Fur M, Rotile NJ, Correcher C, Clavijo Jordan V, Ross AW, Catana C, Caravan P. Yttrium-86 Is a Positron Emitting Surrogate of Gadolinium for Noninvasive Quantification of Whole-Body Distribution of Gadolinium-Based Contrast Agents. Angew Chem Int Ed Engl 2020;59:1474-8. [Crossref] [PubMed]
- Liberman L, Menell JH. Breast imaging reporting and data system (BI-RADS). Radiol Clin North Am 2002;40:409-30. v. [Crossref] [PubMed]
- Shimauchi A, Machida Y, Maeda I, Fukuma E, Hoshi K, Tozaki M. Breast MRI as a Problem-solving Study in the Evaluation of BI-RADS Categories 3 and 4 Microcalcifications: Is it Worth Performing? Acad Radiol 2018;25:288-96. [Crossref] [PubMed]
- Barentsz MW, Taviani V, Chang JM, Ikeda DM, Miyake KK, Banerjee S, van den Bosch MA, Hargreaves BA, Daniel BL. Assessment of tumor morphology on diffusion-weighted (DWI) breast MRI: Diagnostic value of reduced field of view DWI. J Magn Reson Imaging 2015;42:1656-65. [Crossref] [PubMed]
- Whisenant JG, Romanoff J, Rahbar H, Kitsch AE, Harvey SM, Moy L, et al. Factors Affecting Image Quality and Lesion Evaluability in Breast Diffusion-weighted MRI: Observations from the ECOG-ACRIN Cancer Research Group Multisite Trial (A6702). J Breast Imaging 2020;3:44-56. [Crossref] [PubMed]
- Shin HJ, Park JY, Shin KC, Kim HH, Cha JH, Chae EY, Choi WJ. Characterization of tumor and adjacent peritumoral stroma in patients with breast cancer using high-resolution diffusion-weighted imaging: Correlation with pathologic biomarkers. Eur J Radiol 2016;85:1004-11. [Crossref] [PubMed]
- Springer E, Dymerska B, Cardoso PL, Robinson SD, Weisstanner C, Wiest R, Schmitt B, Trattnig S. Comparison of Routine Brain Imaging at 3 T and 7 T. Invest Radiol 2016;51:469-82. [Crossref] [PubMed]
- In MH, Posnansky O, Speck O. High-resolution distortion-free diffusion imaging using hybrid spin-warp and echo-planar PSF-encoding approach. Neuroimage 2017;148:20-30. [Crossref] [PubMed]
- Alonso Roca S, Delgado Laguna AB, Arantzeta Lexarreta J, Cajal Campo B, López Ruiz A. Screening in patients with increased risk of breast cancer (part 2). Where are we now? Actual MRI screening controversies. Radiologia 2020;62:417-33. (Engl Ed). [Crossref] [PubMed]
- Leithner D, Moy L, Morris EA, Marino MA, Helbich TH, Pinker K. Abbreviated MRI of the Breast: Does It Provide Value? J Magn Reson Imaging 2019;49:e85-e100. [Crossref] [PubMed]
- Radhakrishna S, Agarwal S, Parikh PM, Kaur K, Panwar S, Sharma S, Dey A, Saxena KK, Chandra M, Sud S. Role of magnetic resonance imaging in breast cancer management. South Asian J Cancer 2018;7:69-71. [Crossref] [PubMed]
- Lehman CD, Smith RA. The role of MRI in breast cancer screening. J Natl Compr Canc Netw 2009;7:1109-15. [Crossref] [PubMed]
- Bickelhaupt S, Tesdorff J, Laun FB, Kuder TA, Lederer W, Teiner S, Maier-Hein K, Daniel H, Stieber A, Delorme S, Schlemmer HP. Independent value of image fusion in unenhanced breast MRI using diffusion-weighted and morphological T2-weighted images for lesion characterization in patients with recently detected BI-RADS 4/5 x-ray mammography findings. Eur Radiol 2017;27:562-9. [Crossref] [PubMed]
- Chen SQ, Huang M, Shen YY, Liu CL, Xu CX. Abbreviated MRI Protocols for Detecting Breast Cancer in Women with Dense Breasts. Korean J Radiol 2017;18:470-5. [Crossref] [PubMed]
- Zhang X, Wang D, Liu Z, Wang Z, Li Q, Xu H, Zhang B, Liu T, Jin F. The diagnostic accuracy of magnetic resonance imaging in predicting pathologic complete response after neoadjuvant chemotherapy in patients with different molecular subtypes of breast cancer. Quant Imaging Med Surg 2020;10:197-210. [Crossref] [PubMed]
- Dorrius MD, Dijkstra H, Oudkerk M, Sijens PE. Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: a systematic review and meta-analysis. Eur Radiol 2014;24:2835-47. [Crossref] [PubMed]
- Baltzer PA, Benndorf M, Dietzel M, Gajda M, Camara O, Kaiser WA. Sensitivity and specificity of unenhanced MR mammography (DWI combined with T2-weighted TSE imaging, ueMRM) for the differentiation of mass lesions. Eur Radiol 2010;20:1101-10. [Crossref] [PubMed]
- Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM, Goss PE. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Cheng Y, Yan Y, Gong J, Yang N, Nie S. Trends in incidence and mortality of female breast cancer during transition in Central China. Cancer Manag Res 2018;10:6247-55. [Crossref] [PubMed]
- Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging 2019;50:377-90. [Crossref] [PubMed]
- Autier P, Boniol M. Mammography screening: A major issue in medicine. Eur J Cancer 2018;90:34-62. [Crossref] [PubMed]
- Roganovic D, Djilas D, Vujnovic S, Pavic D, Stojanov D. Breast MRI, digital mammography and breast tomosynthesis: comparison of three methods for early detection of breast cancer. Bosn J Basic Med Sci 2015;15:64-8. [Crossref] [PubMed]


