
Patients-associated compound etiology may have more severe acute pancreatitis: a retrospective cohort study
Introduction
Acute pancreatitis (AP) is one of the most common reasons for hospitalization for gastrointestinal diseases; it can have myriad clinical manifestation and prognosis (1), with morbidity that has increased by at least 20% (2). Although most AP cases are mild and self-limiting, the severe AP (SAP) mortality rate can reach 30%, which underlies the urgent need to find proper treatment (3,4). One of the aims of intensive AP treatment in the acute reaction stage is to address the etiology.
A meta-analysis of the aetiology of AP has shown (5) that biliary origin and alcohol are the two most frequent etiologies of AP worldwide. Biliary origin accounts for 42% of the etiology and occurs twice as frequently as the second most common cause. Hypertriglyceridemia (HTG) is also a common cause of AP, accounting for 2–5% of all cases of AP (2). Because of a high-fat diet and unhealthy lifestyle, the incidence of HTG has increased in recent years. Data have shown that the prevalence of HTG acute pancreatitis (HTG-AP) has increased in recent years and has even taken the place of alcohol as the second most common cause (6-8). Less frequent etiologies of AP include drugs, post-endoscopic retrograde cholangiopancreatography (ERCP), trauma, hypercalcemia, infections, HIV, neoplasms and idiopathic (no cause diagnosed) etiologies. There is no clear incidence of these miscellaneous causes (5).
Determining the exact etiology of AP may be challenging in some cases (9). There is still room for discussion regarding the diagnostic criteria and classification of the etiology of AP. The diagnostic criteria for AP etiology uniformity, which is not conducive to early treatment of the cause of disease. More importantly, the concept of “a single cause results in AP” has been deeply ingrained into clinical practice and academic research. However, some cases of AP in practice often simultaneously meet two or more diagnostic criteria, which is ignored.
In the early phase of AP, it is important to recognize the risk factors for a severe course and further identify potentially critical patients to deliver targeted therapies, thereby reducing the risk of disease aggravation. Studies (9-13) have reported the effect of etiology on AP outcomes. However, the description of the study subjects was single-etiologic AP in the overwhelming majority of studies. Few studies (10,13) have mentioned the situation in which patients with AP have two or more causes simultaneously. More importantly, the influence of complex types of etiology on the disease course was not fully evaluated.
In this regard, we conducted this study to explore the impact of the category of etiology on the clinical outcomes of AP. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1157/rc).
Methods
Patient selection
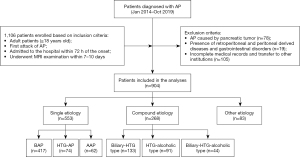
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Chengdu Third People’s Hospital (No. 2021S79) and individual consent for this retrospective analysis was waived. Patients who were admitted to the Chengdu Third People’s Hospital and diagnosed with AP from January 2014 to October 2019 were included in this retrospective study. We identified potential patients using discharge diagnoses with AP [ICD-10 code for acute pancreatitis (K85)]. AP diagnosis was based on the revised Atlanta classification (RAC) (4). The inclusion criteria were as follows: (I) adult patients (age greater than or equal to 18 years old); (II) first attack of AP; (III) admitted to the hospital within 72 h of the onset of symptoms and without any medical treatment; and (IV) underwent magnetic resonance (MR) imaging (MRI) examination within 7–10 days after the onset. The exclusion criteria were as follows: (I) AP caused by pancreatic tumors; (II) other diseases of retroperitoneal and peritoneal origin and gastrointestinal diseases; (III) incomplete medical charts; and (IV) transfer to other institutions. The flowchart of patient selection was shown in Figure 1.
Data collection
Electronic medical records were reviewed for general information, laboratory data, MRI and clinical outcomes. All hematological and biochemical data were routinely obtained within 1 h of initial presentation. MRI was reviewed to identify local complications of AP.
Classification and diagnosis of AP etiology
The etiology of AP was analyzed by the criteria shown in Table 1. The etiology of AP was accurately classified into two main categories: single-etiology category and compound-etiology category. The single-etiology category refers to the onset of AP caused by only one cause. The compound-etiology category refers to the type of AP with two or more causes simultaneously, which mainly include the dual-etiology category and triple-etiology category. Dual-etiology categories include the biliary-HTG type and HTG-alcoholic type. The triple-etiology category is mainly biliary-HTG-alcoholic type.
Table 1
| Category | Biliary origin confirmation procedure shown in Figure 1 | S-TG ≥1,000 mg/dL or ≥500 mg/dL accompanied by lactescent serum | Prolonged excessive alcohol consumption |
|---|---|---|---|
| Single etiology | |||
| Biliary | √ | x | x |
| HTG | x | √ | x |
| Alcoholic | x | x | √ |
| Compound etiology | |||
| Biliary-HTG | √ | √ | x |
| HTG-alcoholic | x | √ | √ |
| Biliary-HTG-alcoholic | √ | √ | √ |
AP, acute pancreatitis; TG, triglyceride; S-TG, serum triglyceride; HTG, hypertriglyceridemia.
Based on current diagnostic criteria, we suggest that timeliness should be used as one of the diagnostic markers for AP. This means that some diagnostic indicators, especially laboratory data, need to be acquired within 72 h after onset. AP with biliary origin was confirmed by the procedure shown in Figure 2, which includes the confirmation of biliary sludge or gallstones using radiological imaging or the presence of elevated serum levels of alanine aminotransferase (>60 U/L) and a body mass index (BMI) <30 kg/m2 (14). HTG-AP was confirmed by serum triglyceride (S-TG) ≥1,000 mg/dL or ≥500 mg/dL accompanied by lactescent serum (15,16). The confirmation of acute alcoholic pancreatitis (AAP) rests on a history of prolonged excessive alcohol consumption (four to five drinks daily for more than 5 years) (6).
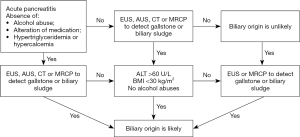
Less-frequent etiologies and idiopathic pancreatitis (unidentified etiology) fell into other etiology categories.
Clinical outcome measures
The clinical outcomes were measured by the differences in any organ failure (OF), persistent organ failure (POF), need for intensive care unit (ICU), length of hospital stay, local complications including pancreatic necrosis (PN), acute necrotic collection (ANC), infective necrosis (IN) and AP-associated gastrointestinal abnormalities. The primary outcome was POF.
According to the modified Marshall scoring system (4), OF was defined as a score greater than or equal to two for an organ system (cardiovascular, renal, and respiratory system), and OF exceeding 48 h was labeled POF. The predictive indicators were the MR severity index (MRSI) based on the computed tomography (CT) severity index (17) and C-reactive protein (CRP), which can indicate severity in the early stage of AP.
MRI has the advantage of evaluating the local complications of AP over CT (18-21). On MRI, PN manifested as hypointense on T1-weighted imaging, hyperintense on T2-weighted imaging and nonenhancing areas of pancreatic parenchyma. According to Balthazar’s criteria and grade points (22), the extent of PN could be further quantified as less than 30% (mild), 30–50% (moderate), and more than 50% (severe) of the pancreatic gland. ANC refers to a heterogeneous and nonliquefied peripancreatic collection containing variable amounts of both fluid and necrosis, manifesting as a heterogeneous signal. IN was determined when gas bubbles appeared in necrosis areas on MRI or a positive culture of necrosis areas obtained by image-guided fine-needle aspiration, drainage or necrosectomy (4,23). In this study, the gastrointestinal tract that we observed ranged from the stomach to the descending colon. It could manifest as edema of the gastrointestinal wall, gastrointestinal wall thickening and luminal dilatation. AP-associated gastrointestinal abnormality was defined as a gastrointestinal tract within the scope of observation that satisfied any of the above conditions, regardless of the extension or location.
Statistical analysis
Continuous variables are expressed as medians or means ± standard derivations. Categorical variables are expressed as frequencies and percentages. Continuous variables were analyzed by the Kruskal-Wallis H test and Nemenyi test (for pairwise comparisons). Categorical variables were analyzed by the chi-square test and partition of χ2.
A multivariate logistic regression model was used to determine whether the etiology category was independently associated with POF, which was reported as the odds ratio (OR) with 95% confidence interval (95% CI). The models were adjusted for relevant risk factors for POF. Variables with a P value <0.1 in the univariate analysis as the relevant risk factors for POF were included in the multivariate model.
All statistical analyses were conducted using SPSS statistical software (version 19.0, Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Results
A total of 904 patients meeting the conditions were included in our study. The enrolled patients comprised 553 patients in the single-etiology category [417 cases of biliary AP (BAP), 74 HTG-AP and 62 AAP], 224 patients in the dual-etiology category (133 cases of biliary-HTG type and 91 HTG-alcoholic type) and 44 of the biliary-HTG-alcoholic type. There were 83 patients in the other etiology category.
Baseline characteristics of AP patients stratified by etiology category
The patients’ baseline characteristics are shown in Table 2. Compared with the single-etiology category and other etiology category, the patients in the compound-etiology category were younger (51.13±13.82 vs. 52.47±9.47 vs. 47.80±12.18, P<0.001), more predominantly males (49.2% vs. 47.0% vs. 58.6%, P=0.027), were more likely to be obese (BMI ≥30 kg/m2) (25.9% vs. 12.1% vs. 51.1%, P<0.001) and more likely to have pre-existing diabetes (17.9% vs. 7.2% vs. 26.5%, P<0.001).
Table 2
| Variables | “Single etiology” category | “Compound etiology” category | “Other” category (n=83) | P value# | P value& | P value* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biliary (n=417) | HTG (n=74) | Alcohol (n=62) | Biliary-HTG type (n=133) | HTG-alcohol type (n=91) | Biliary-HTG-alcohol type (n=44) | ||||||
| Age (years), mean ± SD | 54.36±13.03 | 42.35±11.07 | 39.87±11.32 | 51.47±11.98 | 41.95±10.91 | 48.84±10.82 | 52.47±9.47 | <0.001 | <0.001 | <0.001 | |
| Sex (male), n (%) | 174 (41.7) | 45 (60.8) | 53 (85.5) | 65 (48.9) | 64 (70.3) | 28 (63.6) | 39 (47.0) | <0.001 | 0.005 | 0.027 | |
| BMI ≥30 kg/m2, n (%) | 103 (24.7) | 29 (39.2) | 11 (17.7) | 59 (44.4) | 48 (52.7) | 30 (68.2) | 10 (12.1) | 0.010 | 0.022 | <0.001 | |
| Pre-existing diabetic status, n (%) | 78 (18.7) | 15 (20.3) | 6 (9.7) | 35 (26.3) | 21 (23.1) | 15 (34.1) | 6 (7.2) | 0.190 | 0.396 | <0.001 | |
#, compared in single etiology category; &, compared in compound etiology category; *, compared in the three etiology categories. AP, acute pancreatitis; BMI, body mass index; HTG, hypertriglyceridemia; SD, standard deviation.
These indices were further compared in the single- and compound-etiology categories. In the single-etiology category, patients with HTG-AP and AAP were more likely to be younger and male (P<0.001). Obesity (BMI ≥30 kg/m2) was more common in HTG-AP than in BAP and AAP (39.2% vs. 24.7% vs. 17.7%, P=0.010). In the compound-etiology category, the patients were younger and more often males (41.95±10.91 years, 70.3%, respectively, P<0.001) with the HTG-alcohol type and had a higher proportion of obesity (68.2%, P=0.022) with the biliary-HTG-alcohol type. There were no significant differences for pre-existing diabetes in the three etiology subgroups of the single-etiology and compound-etiology categories (P=0.190 and 0.396, respectively).
Clinical outcomes of AP patients stratified by etiology category
The other etiology category was not included in the comparison of outcomes due to the small sample size.
The evaluation of clinical outcomes is shown in Tables 3,4. Compared with the single-etiology category, there was an increased incidence of any OF, POF and AP-associated gastrointestinal abnormalities (all P<0.001) and a higher MRSI score and CRP (all P<0.05) in the dual-etiology and triple-etiology categories. There was a significant increase in ICU need, the median length of hospital stay, and the prevalence of PN and ANC as the complexity of etiology type increased (all P<0.01). For the extent of PN, the proportion of PN >50% in the triple-etiology category was considerably higher than that in the single-etiology category (41.2% vs. 9.5%, P=0.019). Direct comparison of patients with single etiology and compound etiology showed that the latter resulted in an increased incidence of IN (5.6% vs. 9.7%, P=0.030).
Table 3
| Variables | “Single etiology” category | “Compound etiology” category | P value | |
|---|---|---|---|---|
| Dual-etiology | Triple-etiology | |||
| Any organ failure, n (%) | 152 (27.5) | 102 (45.5)a | 28 (63.6)a | <0.001 |
| Persistent organ failure, n (%) | 63 (11.4) | 53 (23.7)a | 18 (40.9)a | <0.001 |
| ICU admission, n (%) | 51 (9.2) | 40 (17.9) | 16 (36.4) | <0.001b |
| Hospital days, median [range] | 9 [4–62] | 12.5 [5–58] | 16 [10–74] | <0.001#; <0.001*; 0.005& |
| MRSI, median [range] | 3 [1–10] | 4 [1–10]a | 6.5 [2–10]a | 0.024#; <0.001*; 0.063& |
| Local complications | ||||
| Pancreatic necrosis, n (%) | 42 (7.6) | 43 (19.2) | 17 (38.6) | <0.001b |
| <30% | 26 (61.9) | 23 (53.5) | 6 (35.3) | 0.019c |
| 30–50% | 12 (28.6) | 9 (20.9) | 4 (23.5) | |
| >50% | 4 (9.5) | 11 (25.6) | 7 (41.2)a | |
| Acute necrotic collection, n (%) | 66 (11.9) | 57 (25.4) | 21 (47.7) | <0.001b |
| Infective necrosis, n (%) | 31 (5.6) | 21 (9.4) | 5 (11.4) | 0.030d |
| AP-associated gastrointestinal abnormalities, n (%) | 206 (37.3) | 144 (64.3)a | 35 (79.5)a | <0.001 |
| CRP, 48 h (mg/L), median [range] | 8 [3–41] | 25 [6–207]a | 33 [10–252]a | <0.001#; <0.001*; 0.139& |
a, compared with single etiology category; b, all pairwise comparisons were significant; c, pancreatic necrosis with ≤50% vs. with >50%; d, compound-etiology vs. single-etiology; #, single etiology category vs. dual-etiology category; *, single etiology category vs. triple-etiology category; &, dual-etiology category. vs. triple-etiology category. AP, acute pancreatitis; ICU, intensive care unit; MRSI, magnetic resonance severity index; CRP, C-reactive protein.
Table 4
| Variables | “Single etiology” category | “Compound etiology” category | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BAP | HTG-AP | AAP | P value | Biliary-HTG type | HTG-alcohol type | Biliary-HTG-alcohol type | P value | ||
| Any organ failure, n (%) | 118 (28.3)a | 28 (37.8)a | 6 (9.7) | 0.001 | 66 (49.6) | 36 (39.6) | 28 (63.6)d | 0.030 | |
| Persistent organ failure, n (%) | 48 (11.5) | 13 (17.6)a | 2 (3.2) | 0.032 | 36 (27.1) | 17 (18.7) | 18 (40.9)d | 0.023 | |
| ICU admission, n (%) | 39 (9.4) | 9 (12.2) | 3 (4.8) | 0.334 | 25 (18.8)e | 15 (16.5)e | 16 (36.4) | 0.020 | |
| Hospital days, median [range] | 9 [5–62] | 9.5 [4–43] | 7 [5–29] | 0.182 | 13 [5–58]e | 11 [6–44]e | 16 [10–74] | 0.061#; 0.001*; <0.001& | |
| MRSI, median [range] | 3 [1–10]a | 4 [1–10]a | 2 [1–8] | 0.139#; 0.008*; <0.001& | 5 [1–10]e | 4 [1–10]e | 6.5 [2–10] | <0.001#; 0.019*; <0.001& | |
| Local complications | |||||||||
| Pancreatic necrosis, n (%) | 32 (7.7) | 8 (10.8) | 2 (3.2) | 0.124 | 26 (19.5)e | 17 (18.7)e | 17 (38.6) | 0.018 | |
| <30% | 21 (65.6) | 5 (62.5) | 0 (0.0) | NS | 13 (50.0) | 10 (58.8) | 6 (35.3) | 0.480 | |
| 30–50% | 8 (25.0) | 2 (25.0) | 2 (100.0) | 6 (23.1) | 3 (17.7) | 4 (23.5) | |||
| >50% | 3 (9.4) | 1 (12.5) | 0 (0.0) | 7 (26.9) | 4 (23.5) | 7 (41.2) | |||
| Acute necrotic collection, n (%) | 46 (11.0)b | 16 (21.6) | 4 (6.5)b | 0.013 | 37 (27.8)e | 20 (22.0)e | 21 (47.7) | 0.008 | |
| Infective necrosis, n (%) | 27 (6.5) | 4 (5.4) | 0 (0.0) | NS | 14 (10.5) | 7 (7.7) | 5 (11.4) | 0.718 | |
| AP-associated gastrointestinal abnormalities, n (%) | 136 (32.6) | 40 (54.1)c | 30 (48.4)c | <0.001 | 77 (57.9) | 67 (73.6)f | 35 (79.5)f | 0.007 | |
| CRP, 48 h (mg/L), median [range] | 15 [7–37] | 17 [3–41] | 6 [4–29] | 0.271 | 30 [6–181] | 19 [10–207] | 33 [10–252] | 0.098 | |
a, compared with AAP; b, compared with HTG-AP; c, compared with ABP; d, compared with HTG-alcohol type; e, compared with biliary-HTG-alcohol type; f, compared with biliary-HTG type; #, single etiology category vs. dual-etiology category; *, single etiology category vs. triple-etiology category; &, dual-etiology category. vs. triple-etiology category. AP, acute pancreatitis; ICU, intensive care unit; MRSI, Magnetic resonance severity index; CRP, C-reactive protein; HTG, hypertriglyceridemia; BAP, biliary acute pancreatitis; AAP, acute alcoholic pancreatitis.
The comparison of these outcomes among patients with a single etiology showed an increased incidence of any OF, POF and ANC and a higher MRSI score in HTG-AP (all P<0.05). In addition, the incidence of AP-associated gastrointestinal abnormalities in HTG-AP and AAP was higher than that in BAP (54.1%, 48.4% vs. 32.6%, P<0.001). No significant differences were noted in ICU need, median length of hospital stay, CRP or the incidence of PN and IN among the three single-etiology subgroups.
Further comparison of these outcomes among patients with compound etiology showed that biliary-HTG-alcoholic type had a significantly higher incidence of any OF, POF, PN, and ANC and a higher ICU need, median length of hospital stay and MRSI score (all P<0.05). In terms of the extent of PN, there was no significant difference among the three compound-etiology subgroups. The incidence of AP-associated gastrointestinal abnormalities in the HTG-alcoholic and biliary-HTG-alcoholic types was higher than that in the biliary-HTG type (73.6% and 79.5% vs. 57.9%, P=0.007). No significant difference in IN was found among the three groups.
Multivariate analysis showing the association of risk factors with POF
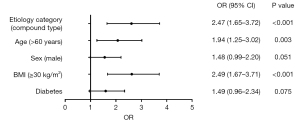
A univariate analysis was performed to analyze the association of risk factors with POF. Multivariate analysis, adjusting for risk factors such as advanced age (>60 years), male sex, obesity (BMI ≥30 kg/m2) and pre-existing diabetes, showed that advanced age (>60 years) (OR: 1.94, 95% CI: 1.25–3.02, P=0.003), obesity (BMI ≥30 kg/m2) (OR: 2.49, 95% CI: 1.67–3.71, P<0.001) and compound etiology (OR: 2.47, 95% CI: 1.65–3.72, P<0.001) were independently associated with POF (Figure 3).
Discussion
The etiologies of AP are currently mainly cholelithiasis, HTG and alcohol and relatively rare causes, such as hypercalcemia, infections, drugs, post-ERCP and others. The three main etiologies are related to metabolic factors and are interrelated and influence each other. In previous studies (11-13), the overwhelming majority of the description of etiological characteristics of patients with AP enrolled is single etiology and/or idiopathic cause. Enough importance is not attached to the compound-etiology of pancreatitis in clinical practices. In this study, we have classified patients with AP into a single etiological category and a compound etiological category and further evaluated and compared the clinical features and outcomes of AP patients with different categories, which has generated new findings that improve our understanding of AP etiology. We found that the clinical outcomes in AP patients with compound etiology were significantly different from those in patients with single etiology.
The clinical guidelines for the diagnosis criteria of AP etiology still have certain limitations, which leaves room for discussion. In this study, we add timeliness as an indicator to the existing criteria for determining AP etiology. From clinical experience, the laboratory indicators within 72 h of onset reflect the actual condition of the body when AP occurred; thus, they have the most diagnostic value. Another reason is that during the acute response phase, especially within 72 h of onset, if patients receive prompt and appropriate treatment, then the incidence and complications of late infections could be very low, thereby significantly improving the survival rate (10). S-TG ≥1,000 or ≥500 mg/dL accompanied by lactescent serum indicates an episode of AP with an HTG origin, which is widely accepted and used (16,24). However, there is a question of whether HTG is a “cause” or “result” of AP. A mild-to-moderate increase in S-TG levels can be present in up to one-third of all cases of AP, regardless of etiology (25,26). S-TG concentrations can rapidly decrease during fasting after AP diagnosis. Thus, we recommend that the key to this problem be to consider two aspects. One is the timeliness, where the values measured within 72 h of onset more likely reflect the actual condition. The second is the S-TG level, which if less than 500 mg/dL within 72 h of onset is more likely to be a “result”. In contrast, a value of more than 500 mg/dL is more likely to be the “cause”. After more than 72 h, S-TG ≥500 mg/dL is most likely due to lipid metabolism that is affected after AP occurs; thus, it is more of a “result”. Clinical usefulness and accuracy of “timeliness” in the future through big data. In addition, the alcoholic origin is an etiology that can be extremely confusing to clinicians. Its diagnostic criteria only depend on the patient’s complaint, and the current so-called daily alcohol consumption and duration standards are wrought with errors and uncertainties, which require further research.
Many studies have investigated the effect of etiology on AP outcomes, although their conclusions are not completely consistent. Kiss et al. (11) and Wang et al. (12) compared HTG-AP and non-HTG-AP patients’ clinical characteristics and outcome and showed that HTG-AP is positively related to the severity of AP. However, they did not further classify the non-HTG-AP group according to etiology. The classification can include all etiologies (such as “other” and “idiopathic”) except HTG-AP, which is rather heterogeneous. Bálint et al. (9) conducted a meta-analysis to elucidate clinical data on how various etiologies (biliary, HTG, alcohol abuse, post-ERCP) affect the course of AP. In contrast to the abovementioned two studies, Bálint et al. analyzed well-defined and clear etiologies, but the description of the study subjects was single-etiologic AP. Few studies have mentioned the complex etiology type of AP. Mao (10) proposed that a portion of APs belong to complex AP in which the etiology meets the diagnostic criteria for BAP and HTG-AP simultaneously. Wang et al. (13) reported a new etiology type named biliary-hyperlipidemic-AP, which includes AP patients with both biliary and HTG etiologies. However, there is not just this type of complex etiology in clinical practice, and the influence of complex etiologies on the disease course has not been fully evaluated. In this study, we proposed the concept of compound etiology and evaluated the clinical data in detail, showing that patients with compound etiology have some unique characteristics.
In this study, 29.65% of the patients with AP belonged to the compound-etiology category. This high proportion deserves higher priority. Compared to patients with a single etiology, those with compound etiology were younger, significantly more often male, and had a higher burden of metabolic comorbidities, including obesity and pre-existing diabetes. Given the difference in clinical outcomes between patients with different etiological categories, clinicians should be aware of this high-risk group. It should be determined whether young patients with metabolic problems presenting with AP have multiple causes.
We found that the clinical outcomes and disease severity predictors at presentation were worse for patients with increasing complexity of etiology type, as shown by comparison of the incidences of any OF, POF, ICU need, PN, ANC and AP-associated gastrointestinal abnormalities, length of hospital stay, MRSI score and CRP value for those with single etiology, dual etiology and triple etiology. There was a trend towards increased AP severity in the dual-etiology category, while the severity markedly increased in the triple-etiology category. A meta-analysis showed that IN, like OF, was an independent risk factor for mortality in patients with AP (27). Of particular note, we found that the risk of IN is significantly higher when compound etiology is present. These features indicate that the compound-etiology category is different from AP belonging to the single-etiology category, which supports our more precise and rigorous classification of AP in clinical practice.
Here, we further studied the differences in clinical outcomes among each subgroup in the single- and compound-etiology categories. Similar to the results of previous studies (9,12), we found that AP with HTG has a higher rate of POF and local complications than AP with other etiologies, such as cholelithiasis or alcohol. More importantly, when other etiologies simultaneously accompany HTG-AP, particularly the biliary-HTG-alcoholic type, the clinical outcomes were significantly worse than those in HTG-AP without other etiologies. A study (28) reported that the presence of cholelithiasis or alcohol abuse increases the likelihood of developing AP in patients with HTG compared to patients with HTG alone, while our results provide new evidence for HTG and other etiologies together leading to the severity of AP. Similar to our findings, Cheng et al. (29) reported that BAP patients with TG elevation (576.99±134.51 mg/dL) had an increased risk of SAP and OF. Another study by Zeng et al. (30) also reported that BAP patients with S-TG levels >500 mg/dL were more likely to develop SAP. While the abovementioned studies agree with our results, the S-TG level of some patients enrolled in these two studies did not strictly meet the HTG etiologic criteria. In addition, we found that when alcoholic etiology is present, patients have a higher risk of developing AP-associated gastrointestinal abnormalities. Gastrointestinal dysfunction may be a stimulus for the development of OF and correlate with poor outcomes in AP patients; thus, it needs to be recognized early and the patients then provided with appropriate therapy (31).
In concordance with the RAC (4), POF is considered the primary outcome and a determinant of severity. When adjusting for baseline parameters of age, sex, BMI ≥30 kg/m2, and pre-existing diabetes, the compound-etiology category was independently associated with POF.
This study has several potential limitations. First, due to the retrospective nature of this research, it may have data bias. Second, the presence of pre-existing diabetes mellitus is based on the main complaint of patients, which may underestimate its incidence owing to an underdiagnosis or missed diagnosis of DM before initial AP hospitalization. In addition, because of a fasting state, none of the patients in this study were prepared for the gastrointestinal tract investigations, which may have affected the observations of AP-associated gastrointestinal abnormalities. Last, due to small sample size, rare causes were not included in this study. We will work on expanding the sample size and conducting multicenter studies to refine the findings in the future
Conclusions
Our study results highlight the importance of determining AP etiology and the prevalence of the “compound-type” etiology Given the finding that the clinical features in AP patients with compound etiology were significantly different from those in patients with single etiology, the compound-etiology category should be recognized as a separate concept in AP etiology and deserve higher priority. It has worse clinical outcomes and is independently associated with POF. Therefore, clinicians should be aware of this high-risk group and more alert for the potentially higher risk of developing SAP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1157/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1157/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Chengdu Third People’s Hospital (No. 2021S79) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peng R, Zhang L, Zhang ZM, Wang ZQ, Liu GY, Zhang XM. Chest computed tomography semi-quantitative pleural effusion and pulmonary consolidation are early predictors of acute pancreatitis severity. Quant Imaging Med Surg 2020;10:451-63. [Crossref] [PubMed]
- Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med 2016;375:1972-81. [Crossref] [PubMed]
- Hammad AY, Ditillo M, Castanon L. Pancreatitis. Surg Clin North Am 2018;98:895-913. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SSAcute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Zilio MB, Eyff TF, Azeredo-Da-Silva ALF, Bersch VP, Osvaldt AB. A systematic review and meta-analysis of the aetiology of acute pancreatitis. HPB (Oxford) 2019;21:259-67. [Crossref] [PubMed]
- Zhu Y, Pan X, Zeng H, He W, Xia L, Liu P, Zhu Y, Chen Y, Lv N. A Study on the Etiology, Severity, and Mortality of 3260 Patients With Acute Pancreatitis According to the Revised Atlanta Classification in Jiangxi, China Over an 8-Year Period. Pancreas 2017;46:504-9. [Crossref] [PubMed]
- Huang YX, Jia L, Jiang SM, Wang SB, Li MX, Yang BH. Incidence and clinical features of hyperlipidemic acute pancreatitis from Guangdong, China: a retrospective multicenter study. Pancreas 2014;43:548-52. [Crossref] [PubMed]
- Yin G, Cang X, Yu G, Hu G, Ni J, Xiong J, Hu Y, Xing M, Chen C, Huang Y, Tang M, Zhao Y, Cheng G, Wan R, Wang S, Wang X. Different Clinical Presentations of Hyperlipidemic Acute Pancreatitis: A Retrospective Study. Pancreas 2015;44:1105-10. [Crossref] [PubMed]
- Bálint ER, Fűr G, Kiss L, Németh DI, Soós A, Hegyi P, Szakács Z, Tinusz B, Varjú P, Vincze Á, Erőss B, Czimmer J, Szepes Z, Varga G, Rakonczay Z Jr. Assessment of the course of acute pancreatitis in the light of aetiology: a systematic review and meta-analysis. Sci Rep 2020;10:17936. [Crossref] [PubMed]
- Mao E. Intensive management of severe acute pancreatitis. Ann Transl Med 2019;7:687. [Crossref] [PubMed]
- Kiss L, Fűr G, Mátrai P, Hegyi P, Ivány E, Cazacu IM, Szabó I, Habon T, Alizadeh H, Gyöngyi Z, Vigh É, Erőss B, Erős A, Ottoffy M, Czakó L, Rakonczay Z Jr. The effect of serum triglyceride concentration on the outcome of acute pancreatitis: systematic review and meta-analysis. Sci Rep 2018;8:14096. [Crossref] [PubMed]
- Wang Q, Wang G, Qiu Z, He X, Liu C. Elevated Serum Triglycerides in the Prognostic Assessment of Acute Pancreatitis: A Systematic Review and Meta-Analysis of Observational Studies. J Clin Gastroenterol 2017;51:586-93. [Crossref] [PubMed]
- Wang YH, Xu ZH, Zhou YH, Sun SL, Xu ZW, Qi X, Zhou WJ, Sheng HQ, Zhao B, Mao EQ. The clinical characteristic of biliary-hyperlipidemic etiologically complex type of acute pancreatitis: a retrospective study from a tertiary center in China. Eur Rev Med Pharmacol Sci 2021;25:1462-71. [PubMed]
- van Geenen EJ, van der Peet DL, Bhagirath P, Mulder CJ, Bruno MJ. Etiology and diagnosis of acute biliary pancreatitis. Nat Rev Gastroenterol Hepatol 2010;7:495-502. [Crossref] [PubMed]
- Chinese Pancreatic Surgery Association, Chinese Society of Surgery, Chinese Medical Association. Guidelines for diagnosis and treatment of acute pancreatitis in China (2021). Zhonghua Wai Ke Za Zhi. 2021;59:578-87. [PubMed]
- Zhang R, Deng L, Jin T, Zhu P, Shi N, Jiang K, Li L, Yang X, Guo J, Yang X, Liu T, Mukherjee R, Singh VK, Windsor JA, Sutton R, Huang W, Xia Q. Hypertriglyceridaemia-associated acute pancreatitis: diagnosis and impact on severity. HPB (Oxford) 2019;21:1240-9. [Crossref] [PubMed]
- Vriens PW, van de Linde P, Slotema ET, Warmerdam PE, Breslau PJ. Computed tomography severity index is an early prognostic tool for acute pancreatitis. J Am Coll Surg 2005;201:497-502. [Crossref] [PubMed]
- Xiao B, Xu HB, Jiang ZQ, Zhang J, Zhang XM. Current concepts for the diagnosis of acute pancreatitis by multiparametric magnetic resonance imaging. Quant Imaging Med Surg 2019;9:1973-85. [Crossref] [PubMed]
- Tang MY, Chen TW, Bollen TL, Wang YX, Xue HD, Jin ZY, Huang XH, Xiao B, Li XH, Ji YF, Zhang XM. MR imaging of hemorrhage associated with acute pancreatitis. Pancreatology 2018;18:363-9. [Crossref] [PubMed]
- Sternby H, Verdonk RC, Aguilar G, Dimova A, Ignatavicius P, Ilzarbe L, Koiva P, Lantto E, Loigom T, Penttilä A, Regnér S, Rosendahl J, Strahinova V, Zackrisson S, Zviniene K, Bollen TL. Significant inter-observer variation in the diagnosis of extrapancreatic necrosis and type of pancreatic collections in acute pancreatitis - An international multicenter evaluation of the revised Atlanta classification. Pancreatology 2016;16:791-7. [Crossref] [PubMed]
- Jiang ZQ, Xiao B, Zhang XM, Xu HB. Early-phase vascular involvement is associated with acute pancreatitis severity: a magnetic resonance imaging study. Quant Imaging Med Surg 2021;11:1909-20. [Crossref] [PubMed]
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002;223:603-13. [Crossref] [PubMed]
- Dellinger EP, Forsmark CE, Layer P, Lévy P, Maraví-Poma E, Petrov MS, Shimosegawa T, Siriwardena AK, Uomo G, Whitcomb DC, Windsor JAPancreatitis Across Nations Clinical Research and Education Alliance (PANCREA). Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg 2012;256:875-80. [Crossref] [PubMed]
- Adiamah A, Psaltis E, Crook M, Lobo DN. A systematic review of the epidemiology, pathophysiology and current management of hyperlipidaemic pancreatitis. Clin Nutr 2018;37:1810-22. [Crossref] [PubMed]
- Balachandra S, Virlos IT, King NK, Siriwardana HP, France MW, Siriwardena AK. Hyperlipidaemia and outcome in acute pancreatitis. Int J Clin Pract 2006;60:156-9. [Crossref] [PubMed]
- Anderson F, Thomson SR, Clarke DL, Buccimazza I. Dyslipidaemic pancreatitis clinical assessment and analysis of disease severity and outcomes. Pancreatology 2009;9:252-7. [Crossref] [PubMed]
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010;139:813-20. [Crossref] [PubMed]
- Amblee A, Mohananey D, Morkos M, Basu S, Abegunde AT, Ganesh M, Bhalerao N, George AM, Jain M, Fogelfeld L. Acute pancreatitis in patients with severe hypertriglyceridemia in a multi-ethnic minority population. Endocr Pract 2018;24:429-36. [Crossref] [PubMed]
- Cheng L, Luo Z, Xiang K, Ren J, Huang Z, Tang L, Tian F. Clinical significance of serum triglyceride elevation at early stage of acute biliary pancreatitis. BMC Gastroenterol 2015;15:19. [Crossref] [PubMed]
- Zeng Y, Zhang W, Lu Y, Huang C, Wang X. Impact of hypertriglyceridemia on the outcome of acute biliary pancreatitis. Am J Med Sci 2014;348:399-402. [Crossref] [PubMed]
- Liu H, Li W, Wang X, Li J, Yu W. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas 2008;36:192-6. [Crossref] [PubMed]


