
Differential diagnosis of B-mode ultrasound Breast Imaging Reporting and Data System category 3–4a lesions in conjunction with shear-wave elastography using conservative and aggressive approaches
Introduction
Breast ultrasound (US) is used as a common screening tool to detect and diagnose breast lesions. Breast lesions can be classified according to the US Breast Imaging Reporting and Data System (BI-RADS), which recommends biopsy of breast lesions with a probability of malignancy of more than 2% (1). However, the high false-positive rates (46.0–85.7%) of US BI-RADS leads to excessive biopsies of numerous benign lesions (2-5). Therefore, it is clinically important to develop an imaging modality that reduces unnecessary biopsies of benign lesions and improves diagnostic efficacy.
In addition to becoming an adjunctive diagnostic tool of breast lesions, US elastography has been adopted by the BI-RADS-US lexicon in the second edition (6). Shear-wave elastography (SWE) is a promising elastographic technique that can provide quantitative parameters to depict tissue stiffness, increase confidence in breast lesion characterization, and provide fine intra- and inter-observer reproducibility (7-10). Previous studies have shown that most malignant lesions are much stiffer than benign lesions (3,11,12).
Previous studies have shown that SWE has advantages in distinguishing benign tumors from malignant tumors, and these studies have indicated that SWE features, including mean elasticity and maximum elasticity (Emax), could improve the specificity of B-mode US from 43.1–61.0% to 65.7–87.7% without resulting in a loss of sensitivity (12-15). However, the most valuable SWE feature and cutoff value have not yet been unified. Some studies have demonstrated that Young’s modulus Emax, which correlated with Emax measured in kilopascals, had the best diagnostic performance compared with other quantitative parameters when combined with the conventional US BI-RADS (11,16-18).
According to the latest BI-RADS edition, the malignant probability of BI-RADS 3 is less than or equal to 2%, while the probability of BI-RADS 4a is more than 2% with a less than or equal to 10% chance of being malignant (6). Applying SWE could reduce the unnecessary short-term follow-up or biopsy for BI-RADS category 3–4a lesions. Berg et al. (11) showed that SWE features changed the treatment of BI-RADS 3 and 4a lesions, but did not influence that of BI-RADS category 2 or BI-RADS category 4c or 5 lesions. They found that BI-RADS 4b lesions and above with suspicious morphology can achieve higher diagnostic accuracy, which remained unchanged when SWE was added to assist treatment decision-making, and biopsy can still be suggested because of patients’ requests, breast surgeons’ palpation, or suspicious criteria assessed by magnetic resonance imaging (MRI) or mammography.
In previous studies, conservative and aggressive approaches have rarely been used to judge the value of SWE Emax to assist the diagnosis of the special BI-RADS category 3–4a group patients. In addition, most of the existing studies have confirmed that Emax-assisted B-mode US is valuable in the diagnosis of breast lesions, of which the optimal thresholds used mostly came from their samples. Therefore, our study aimed to verify the clinical benefit of adding SWE Emax to B-mode US with conservative and aggressive approaches to differentiate benign and malignant BI-RADS category 3 and 4a lesions, to explore which diagnostic method was more beneficial. We present the following article in accordance with the Standards for Reporting Diagnostic accuracy studies (STARD) reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-916/rc).
Methods
Patients
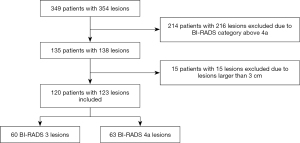
The study was conducted in accordance with the Declaration of (as revised in 2013). The Institutional Review Board of Fudan University Shanghai Cancer Center (FUSCC) approved the study (No. 2107238-18), and informed consent was provided by all participants. In this prospective study, from January 2019 to December 2019, a total of 349 consecutive women inpatients with 354 breast lesions classified as BI-RADS 3 to BI-RADS 6 underwent resection of lesions based on the patients’ requests or when suspicious features were identified by MRI or mammography. These patients underwent B-mode US and SWE examinations before undergoing surgical resection. The following patients were excluded: 214 patients with 216 lesions, including those breast lesions categorized as BI-RADS category 4b, 4c, or 5 on the US and BI-RADS category 6 as confirmed by pathology; and 15 lesions larger than 3 cm because they did not show changes in the Young’s modulus value of surrounding tissue. Finally, a total of 120 patients with 123 breast lesions classified as BI-RADS category 3 or 4a based on B-mode US were enrolled to undergo analysis with SWE (Figure 1). Among the 120 patients, 4 patients with breast cancer had a family history of breast cancer, and 1 patient with benign breast lesions had a family history of breast cancer.
US and SWE examination
For all breast lesion patients, imaging was performed to obtain B-mode US images and SWE images in two orthogonal planes with the US system (Aixplorer V10, SuperSonic Imagine, Aix-en-Provence, France) equipped with an SL15-4 MHz linear array transducer by two radiologists with 4–10 years of experience in breast US and SWE. The US characterizations were assessed based on the US BI-RADS lexicon by two radiologists who were blinded to pathology. The maximum diameter measured in both perpendicular planes on the US was defined as the final size of the lesion.
After B-mode US, participants were asked to hold their breath for at least 3–5 seconds to obtain stable images during the image acquisition process. A semitransparent color map of tissue stiffness overlaid on the B-mode US image indicated regions with the lowest stiffness to the highest stiffness (0–180 kPa) in a range from dark blue to red. The built-in quantification instrument—the region of interest (ROI) (Q-box; SuperSonic Imagine) of the system—had various sizes that were used to include the whole lesions and the adjacent tissue to quantify elasticity for the lesions. Two SWE acquisitions of two frozen frames were acquired with the default maximum color scale of 180 kPa by the same radiologist with no pressure applied. We manually delineated lesions on B-mode US and elastography. The contours of the lesions outlined on elastography included areas of relatively harder breast glandular tissue that surrounded the lesions. The quantitative elasticity values, including the Emax and other elasticity values, were automatically calculated. On the elastic image, the higher Emax of the two sets of SWE parameters was used as the Emax of the lesion. All elastic images and B-mode US images were recorded for review and data analysis. The average Emax of two breast nodule images was calculated and recorded as the final average maximum Young’s modulus.
Combined US and SWE with conservative and aggressive approaches
The conservative approach was defined as follows: combined with B-mode US, the final US BI-RADS grade of the lesion was downgraded with Emax of 30 kPa or less and upgraded with Emax of 160 kPa or more (11).
The aggressive approach was defined as follows: combined with B-mode US, the final US BI-RADS grade of the lesion was downgraded with Emax of 80 kPa or less and upgraded with Emax of 160 kPa or more (11).
Statistical analysis
Pathologic diagnosis was used as the reference standard. All statistical analyses were performed using the software SPSS 22.0 (IBM Corp., Armonk, NY, USA) and MedCalc (MedCalc Software, Mariakerke, Belgium). All the count data were described as the mean ± standard deviation (SD). The significant differences among different groups were analyzed with the independent-samples t-test. The diagnostic performance of US and US combined Emax were evaluated with receiver operating characteristic (ROC) curves.
Results
General information about patients and lesions
In our study cohort, the mean age of patients was 45.9±11.8 (range, 19 to 76) years, and the mean size of the lesion was 14.8±4.8 (range, 5 to 27) mm. Table 1 shows the histologic results of 123 lesions, including 60 category 3 lesions and 63 category 4a lesions, which were confirmed by surgery.
Table 1
| Histologic features | No. of lesions |
|---|---|
| Fibroadenoma | 68 |
| Intraductal papilloma | 10 |
| Adenosis | 17 |
| Benign phyllodes tumor | 5 |
| Mastitis | 2 |
| Borderline phyllodes tumor | 3 |
| Ductal carcinoma in situ | 6 |
| Invasive ductal carcinoma | 11 |
| Solid papillary carcinoma | 1 |
| Total | 123 |
The sizes of 102 benign lesions, 3 borderline phyllodes tumors, and 18 malignant lesions were 14.9±4.6 (range, 5 to 27), 23.0±1.0 (range, 22 to 24), and 12.7±4.7 (range, 6 to 22) mm, respectively. The mean ages of malignant and benign groups were 54.7±10.3 and 44.4±11.3 years, respectively, and there was no significant statistical difference (P=0.970).
Conventional US evaluation
Among the 123 lesions, 60 were classified as BI-RADS category 3, of which 58 (96.7%, 58/60) were benign, including 47 fibroadenomas, 9 breast adenosis, 1 benign phyllodes tumor, and 1 intraductal papilloma. There were 2 invasive ductal carcinomas, and the malignancy rate was 3.3%. Of the 63 BI-RADS category 4a lesions, 44 (69.8%, 44/63) were benign, which included 21 fibroadenomas, 9 intraductal papillomas, 2 breast mastitides, 8 breast adenosis, and 4 benign phyllodes tumors. Three (4.8%, 3/63) were borderline phyllodes tumors, and 16 (25.4%, 16/63) were malignant tumors, which included 9 invasive ductal carcinomas, 6 ductal carcinomas in situ, and 1 solid papillary carcinoma.
The BI-RADS category 3 lesions were classified as benign and the BI-RADS 4a lesions were classified as malignant according to the standard. The US BI-RADS category had a sensitivity of 88.9%, specificity of 55.2%, positive predictive value (PPV) of 25.4%, negative predictive value (NPV) of 96.7%, and an accuracy of 60.2% (Table 2).
Table 2
| Different methods | Sen (%) | Spe (%) | PPV (%) | NPV (%) | Accuracy (%) | AUC | SE | 95% CI |
|---|---|---|---|---|---|---|---|---|
| US | 88.9 | 55.2 | 25.4 | 96.7 | 60.2 | 0.721 | 0.0452 | 0.633–0.798 |
| Conservative approach | 94.4 | 60.0 | 28.8 | 98.4 | 65.0 | 0.772 | 0.0367 | 0.688–0.843 |
| Aggressive approach | 66.7 | 72.4 | 29.3 | 92.7 | 71.5 | 0.695 | 0.0612 | 0.606–0.775 |
US, ultrasound; SWE, shear-wave elastography; Sen, sensitivity; Spe, specificity; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the receiver operating characteristic curve; SE, standard error; CI, confidence interval.
SWE quantitative evaluation
The average elasticity values of the benign, malignant, and borderline phyllodes tumor groups were 73.5±56.3 (range, 65.3 to 300.0), 133.9±67.3 (range, 65.3 to 300.0), and 171.0±115.3 (range, 77.9 to 300.0) kPa, respectively. The average Emax in malignant tumors and borderline phyllodes tumor groups was significantly higher than that in benign lesions (P<0.001 and P=0.006, respectively; Table 3; Figures 2-4). The Emax of malignant lesions was significantly higher than that of adenosis [82.2±69.7 (range, 9.0 to 225.0) kPa; P=0.010] and fibroadenoma [64.5±50.8 (range, 10.5 to 234.3) kPa; P=0.000]. The Emax of borderline phyllodes tumors was higher than that of benign phyllodes tumors (80.1±26.8 kPa; P=0.036), adenosis (P=0.017), and fibroadenoma (P=0.003). The Emax of intraductal papillomas [113.4±58.6 (range, 34.3 to 206.4) kPa] was higher than that of fibroadenoma (P=0.015), but no differences were found between breast cancers and borderline phyllodes tumors (P=0.312), mastitis [91.0±5.4 (range, 87.2 to 94.8) kPa; P=0.329], and intraductal papillomas (P=0.379).
Table 3
| Histologic features | Number of lesions | Emax (kPa), mean ± SD [range] | Size (mm), mean ± SD [range] | Age (years), mean ± SD [range] |
|---|---|---|---|---|
| Fibroadenoma | 68 | 64.5±50.8 [10.5–234.3] | 14.9±4.1 [7–25] | 42.6±11.2 [19–65] |
| Intraductal papilloma | 10 | 113.4±58.6 [34.3–206.4] | 14.7±4.4 [9–21] | 51.6±15.0 [36–76] |
| Adenosis | 17 | 82.2±69.7 [9.0–225.0] | 13.8±6.0 [5–27] | 48.5±8.8 [31–69] |
| Benign phyllodes tumor | 5 | 80.1±59.9 [33.5–148.2] | 20.2±2.6 [17–23] | 41.0±4.6 [38–49] |
| Mastitis | 2 | 113.4±58.6 [87.2–94.8] | 13.5±5.0 [10–17] | 41.5±0.7 [41–42] |
| Borderline phyllodes tumor | 3 | 171.0±115.3 [77.9–300.0] | 23.0±1.0 [22–24] | 43.3±18.0 [25–61] |
| Breast cancer | 18 | 133.9±67.3 [65.3–300.0] | 12.7±4.7 [6–22] | 54.7±10.3 [38–69] |
| Total | 123 | – | – | – |
Emax, maximum elasticity; SD, standard deviation.
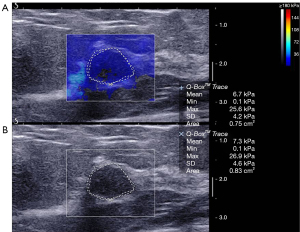
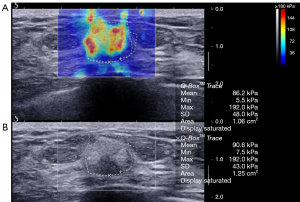
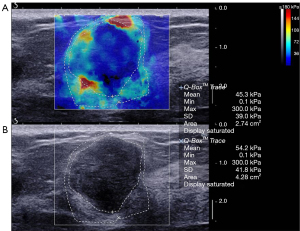
Diagnostic performance of combined US and SWE
Figure 5 shows the changes of the final BI-RADS assessment after applying the combination of B-mode US and SWE. When using the conservative approach in this study, adding the SWE Emax feature to B-mode US improved the diagnostic performance of breast lesion assessment (Table 2). Compared to the B-mode US, the sensitivity, specificity, PPV, NPV, accuracy, and area under the ROC curve (AUC) of the combined US and SWE method changed from 88.9% to 94.4%, 55.2% to 60.0%, 25.4% to 28.8%, 96.7% to 98.4%, 60.2% to 65.0%, and 0.721 to 0.772, respectively.
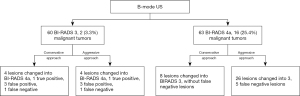
Using the conservative approach, none of the 17 cancers were downgraded. One cancer with Emax 300 kPa was upgraded from BI-RADS category 3 to 4a, which shown to have avoided misdiagnosis. Out of the 47 false-positive lesions diagnosed by US, 17.0% (8/47) were properly downgraded from category 4a to 3 and recommended to be followed up.
Using the aggressive approach, out of the 47 false-positive lesions diagnosed by US, 44.7% (21/47) were properly downgraded from category 4a to 3, which avoided unnecessary biopsies. Five out of 18 cancerous lesions (27.8%) were underestimated and downgraded from BI-RADS category 4a to 3 (Figure 6). Compared to the B-mode US, the specificity, PPV, and accuracy of the combined B-mode US and SWE method increased from 55.2% to 72.4%, 25.4% to 29.3%, and 60.2% to 71.5%, respectively. However, this was accompanied with a decrease in sensitivity from 88.9% to 66.7%, decrease in NPV from 96.7% to 92.7%, and decrease in AUC from 0.721 to 0.695 (Table 2).
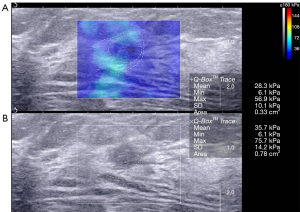
One cancer with an Emax of 75.8 kPa was diagnosed as BI-RADS category 3 using B-mode US alone and with the combination of US and SWE using the conservative approach or the aggressive approach.
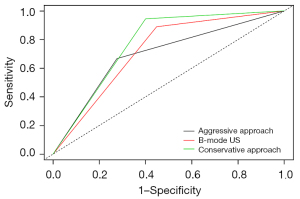
Figure 7 shows the diagnostic performances of B-mode US only and the combined B-mode US and SWE method using the conservative and aggressive approaches.
Discussion
As a complementary modality to B-mode US, SWE can quantify the elasticity of breast lesions. The addition of SWE to B-mode US yields better diagnostic performance than using B-mode US alone, especially when identifying the Emax in low suspicion masses (13,18,19). Previous studies have shown that SWE can downgrade the BI-RADS category of some lesions that have a low likelihood of malignancy, which can reduce unnecessary biopsies (20-22). We showed that a combination of B-mode US and SWE increased the specificity of B-mode US alone from 55.2% to 72.4% using the aggressive approach and to 60.0% using the conservative approach, which was consistent with previous studies (18). Adding the quantitative Emax value in SWE modified US BI-RADS category 4a to US BI-RADS category 3 for 8 lesions using the conservative approach and 21 lesions using the aggressive approach. This meant that biopsy could be avoided in 17.0% and 44.7% of 47 false-positive cases based on the conservative and aggressive approach, respectively. Although the specificity was improved, the sensitivity of the combination of B-mode US and SWE using the aggressive approach was lower than that of B-mode US alone. As a result, 27.8% (5/18) of breast cancers (Emax from 65.3 to 75.8 kPa) were missed. This phenomenon is not acceptable in clinical practice. However, the sensitivity of the combination of B-mode US and SWE using the conservative approach was higher than that of US, without missing any cancerous lesions.
We found that malignant breast lesions had higher stiffness than benign breast lesions. Similar results have been reported in other studies (23,24). Among the malignant breast lesions, invasive ductal carcinomas (11/18) were the most common pathological type in our study. Of the 18 confirmed breast cancers, 16 breast lesions were correctly diagnosed by B-mode US, while the diagnosis of 1 cancer misdiagnosed using B-mode US alone was corrected using the combination of B-mode US and SWE (Emax 300 kPa) with both the conservative and aggressive approaches. One cancer was incorrectly classified as a benign lesion (BI-RADS category 3) with benign characteristics on the US and had low stiffness (Emax 75.8 kPa) shown by SWE with both the conservative and aggressive approaches.
Fibroadenomas were the most common and the softest benign breast tumors, with an Emax of 64.5±50.8 kPa in our study, which was similar to the report by Hari et al. (25). Among the benign lesions, some chronic inflammation and intraductal papillomas often mimic malignant lesions and pose diagnostic challenges (26-28). Two chronic inflammatory masses and 9 intraductal papillomas displayed malignancy features on US, which lead to their misclassification as malignant, but the addition of Emax to B-mode US resulted in two intraductal papillomas being properly classified as BI-RADS category 3 on the US with the aggressive approach; however, other inflammatory masses and intraductal papillomas with higher Emax values were still misdiagnosed with this approach. Borderline phyllodes tumors demonstrated higher Emax than other benign lesions, which were wrongly classified as malignant by the combined B-mode US and SWE. This might be because borderline phyllodes tumors are larger than other benign tumors. Furthermore, Chamming’s et al. (29) reported that tissue stiffness showed excellent correlation with tumor size in mice.
The combination of B-mode US with SWE improved the diagnostic performance of B-mode US alone. The AUC of B-mode US and B-mode US combined with SWE using the conservative and aggressive approaches were 0.721, 0.772, and 0.695, respectively. These results indicated that B-mode US combined with SWE using the conservative approach yielded the best diagnostic efficacy for breast lesions, and this finding was consistent with other studies (30).
There were several limitations to our study. First, as a single institutional study, our cohort included a relatively small number of malignant lesions. Furthermore, the low malignancy rate of 14.6% (18/123) may have led to a lower PPV. Second, Emax showed the best diagnostic performance, therefore only the diagnostic efficacy of B-mode US combined with the Emax of SWE was considered, and other SWE parameters were omitted. In addition, intraductal papillomas have an increased risk of breast cancer, therefore lesions suspected to be intraductal papillomas were classified as BI-RADS 4a and recommended for biopsy, so there was a higher rate of misdiagnosis. Moreover, the use of BI-RADS was too conservative, which led to a higher positive rate of BI-RADS 4a. In the future, we will correct our BI-RADS category norms. Finally, our study only included patients hospitalized for surgery. Future study should also be extended to outpatients.
Conclusions
The addition of SWE improves the diagnostic performance of breast US. Adding diagnostic criteria of SWE to the BI-RADS assessment of B-mode US, downgrading the lesion of Emax 30 kPa or less, and upgrading the lesion of Emax 160 kPa or more, would help discriminate low suspicion lesions from benign lesions to decrease false-positive findings and avoid missing cancer diagnoses.
Acknowledgments
We thank all the patients involved in this study for their participation.
Funding: This study was supported by grants from the National Natural Science Foundation of China (No. 81801701 to WZ; No. 81901749 to HZ; No. 81901703 to CY; No. 81830058 to CC).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-916/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-916/coif). All authors report that this work was supported by The National Natural Science Foundation of China (No. 81801701 to WZ; No. 81901749 to HZ; No. 81901703 to CY; No. 81830058 to CC). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of (as revised in 2013). This study was approved by the Ethical Review Committee of the Fudan University Shanghai Cancer Center (No. 2107238-18), and informed consent was provided by all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- You C, Li J, Zhi W, Chen Y, Yang W, Gu Y, Peng W. The volumetric-tumour histogram-based analysis of intravoxel incoherent motion and non-Gaussian diffusion MRI: association with prognostic factors in HER2-positive breast cancer. J Transl Med 2019;17:182. [Crossref] [PubMed]
- Berg WA, Mendelson EB, Cosgrove DO, Doré CJ, Gay J, Henry JP, Cohen-Bacrie C. Quantitative Maximum Shear-Wave Stiffness of Breast Masses as a Predictor of Histopathologic Severity. AJR Am J Roentgenol 2015;205:448-55. [Crossref] [PubMed]
- Evans A, Whelehan P, Thomson K, Brauer K, Jordan L, Purdie C, McLean D, Baker L, Vinnicombe S, Thompson A. Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. Br J Cancer 2012;107:224-9. [Crossref] [PubMed]
- Golatta M, Pfob A, Büsch C, Bruckner T, Alwafai Z, Balleyguier C, Clevert DA, Duda V, Goncalo M, Gruber I, Hahn M, Kapetas P, Ohlinger R, Rutten M, Tozaki M, Wojcinski S, Rauch G, Heil J, Barr RG. The Potential of Shear Wave Elastography to Reduce Unnecessary Biopsies in Breast Cancer Diagnosis: An International, Diagnostic, Multicenter Trial. Ultraschall Med 2021; Epub ahead of print. [Crossref] [PubMed]
- Choi JS, Han BK, Ko EY, Ko ES, Shin JH, Kim GR. Additional diagnostic value of shear-wave elastography and color Doppler US for evaluation of breast non-mass lesions detected at B-mode US. Eur Radiol 2016;26:3542-9. [Crossref] [PubMed]
- Mendelson EB, Böhm-Vélez M, Berg WA, Whitman GJ, Feldman MI, Madjar H, Rizzatto G, Baker JA, Zuley M, Stavros AT, Comstock C, Wear VVD. ACR BI-RADS ultrasound. In: D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA. editors. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System. Reston: American College of Radiology, 2013.
- Li DD, Xu HX, Guo LH, Bo XW, Li XL, Wu R, Xu JM, Zhang YF, Zhang K. Combination of two-dimensional shear wave elastography with ultrasound breast imaging reporting and data system in the diagnosis of breast lesions: a new method to increase the diagnostic performance. Eur Radiol 2016;26:3290-300. [Crossref] [PubMed]
- Altıntas Y, Bayrak M, Alabaz Ö, Celiktas M. A qualitative and quantitative assessment of simultaneous strain, shear wave, and point shear wave elastography to distinguish malignant and benign breast lesions. Acta Radiol 2021;62:1155-62. [Crossref] [PubMed]
- Liu C, Zhou J, Chang C, Zhi W. Feasibility of Shear Wave Elastography Imaging for Evaluating the Biological Behavior of Breast Cancer. Front Oncol 2022;11:820102. [Crossref] [PubMed]
- Cosgrove DO, Berg WA, Doré CJ, Skyba DM, Henry JP, Gay J, Cohen-Bacrie C. BE1 Study Group. Shear wave elastography for breast masses is highly reproducible. Eur Radiol 2012;22:1023-32. [Crossref] [PubMed]
- Berg WA, Cosgrove DO, Doré CJ, Schäfer FK, Svensson WE, Hooley RJ, Ohlinger R, Mendelson EB, Balu-Maestro C, Locatelli M, Tourasse C, Cavanaugh BC, Juhan V, Stavros AT, Tardivon A, Gay J, Henry JP, Cohen-Bacrie C. BE1 Investigators. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 2012;262:435-49. [Crossref] [PubMed]
- Zheng X, Li F, Xuan ZD, Wang Y, Zhang L. Combination of shear wave elastography and BI-RADS in identification of solid breast masses. BMC Med Imaging 2021;21:183. [Crossref] [PubMed]
- Klotz T, Boussion V, Kwiatkowski F, Dieu-de Fraissinette V, Bailly-Glatre A, Lemery S, Boyer L. Shear wave elastography contribution in ultrasound diagnosis management of breast lesions. Diagn Interv Imaging 2014;95:813-24. [Crossref] [PubMed]
- Choi HJ, Ko KH, Jung HK. Shear Wave Elastography for Surgically Verified Breast Papillary Lesions: Is It Effective for Differentiation Between Benign and Malignant Lesions? J Ultrasound Med 2017;36:2007-14. [Crossref] [PubMed]
- Lee EJ, Jung HK, Ko KH, Lee JT, Yoon JH. Diagnostic performances of shear wave elastography: which parameter to use in differential diagnosis of solid breast masses? Eur Radiol 2013;23:1803-11. [Crossref] [PubMed]
- Kim H, Lee J, Kang BJ, Kim SH. What shear wave elastography parameter best differentiates breast cancer and predicts its histologic aggressiveness? Ultrasonography 2021;40:265-73. [Crossref] [PubMed]
- Park SY, Kang BJ. Combination of shear-wave elastography with ultrasonography for detection of breast cancer and reduction of unnecessary biopsies: a systematic review and meta-analysis. Ultrasonography 2021;40:318-32. [Crossref] [PubMed]
- Choi HY, Seo M, Sohn YM, Hwang JH, Song EJ, Min SY, Kang HJ, Han DY. Shear wave elastography for the diagnosis of small (≤2 cm) breast lesions: added value and factors associated with false results. Br J Radiol 2019;92:20180341. [Crossref] [PubMed]
- Song EJ, Sohn YM, Seo M. Diagnostic performances of shear-wave elastography and B-mode ultrasound to differentiate benign and malignant breast lesions: the emphasis on the cutoff value of qualitative and quantitative parameters. Clin Imaging 2018;50:302-7. [Crossref] [PubMed]
- Huang Y, Li F, Han J, Peng C, Li Q, Cao L, Liu Y, Zhou J. Shear Wave Elastography of Breast Lesions: Quantitative Analysis of Elastic Heterogeneity Improves Diagnostic Performance. Ultrasound Med Biol 2019;45:1909-17. [Crossref] [PubMed]
- Shang J, Ruan LT, Wang YY, Zhang XJ, Dang Y, Liu B, Wang WL, Song Y, Chang SJ. Utilizing size-based thresholds of stiffness gradient to reclassify BI-RADS category 3-4b lesions increases diagnostic performance. Clin Radiol 2019;74:306-13. [Crossref] [PubMed]
- Lin X, Chang C, Wu C, Chen Q, Peng Y, Luo B, Tang L, Li J, Zheng J, Zhou R, Cui G, Li A, Wang X, Qian L, Zhang J, Wen C, Gay J, Zhang H, Li A, Chen Y. Confirmed value of shear wave elastography for ultrasound characterization of breast masses using a conservative approach in Chinese women: a large-size prospective multicenter trial. Cancer Manag Res 2018;10:4447-58. [Crossref] [PubMed]
- Suvannarerg V, Chitchumnong P, Apiwat W, Lertdamrongdej L, Tretipwanit N, Pisarnturakit P, Sitthinamsuwan P, Thiravit S, Muangsomboon K, Korpraphong P. Diagnostic performance of qualitative and quantitative shear wave elastography in differentiating malignant from benign breast masses, and association with the histological prognostic factors. Quant Imaging Med Surg 2019;9:386-98. [Crossref] [PubMed]
- Sefidbakht S, Haseli S, Khalili N, Bazojoo V, Keshavarz P, Zeinali-Rafsanjani B. Can shear wave elastography be utilized as an additional tool for the assessment of non-mass breast lesions? Ultrasound 2022;30:44-51. [Crossref] [PubMed]
- Hari S, Paul SB, Vidyasagar R, Dhamija E, Adarsh AD, Thulkar S, Mathur S, Sreenivas V, Sharma S, Srivastava A, Seenu V, Prashad R. Breast mass characterization using shear wave elastography and ultrasound. Diagn Interv Imaging 2018;99:699-707. [Crossref] [PubMed]
- Liu SQ, Liu YP, Zhou BG, Deng XH, Li XL, Xiang LH, Ren WW, Xu HX. Two-dimensional shear wave elastography for differential diagnosis between mastitis and breast malignancy. Clin Hemorheol Microcirc 2018;70:347-54. [Crossref] [PubMed]
- Kokubu Y, Yamada K, Tanabe M, Izumori A, Kato C, Horii R, Ohno S, Matsueda K. Evaluating the usefulness of breast strain elastography for intraductal lesions. J Med Ultrason (2001) 2021;48:63-70. [PubMed]
- Sousaris N, Barr RG. Sonographic Elastography of Mastitis. J Ultrasound Med 2016;35:1791-7. [Crossref] [PubMed]
- Chamming’s F. Latorre-Ossa H, Le Frère-Belda MA, Fitoussi V, Quibel T, Assayag F, Marangoni E, Autret G, Balvay D, Pidial L, Gennisson JL, Tanter M, Cuenod CA, Clément O, Fournier LS. Shear wave elastography of tumour growth in a human breast cancer model with pathological correlation. Eur Radiol 2013;23:2079-86.
- Youk JH, Gweon HM, Son EJ, Han KH, Kim JA. Diagnostic value of commercially available shear-wave elastography for breast cancers: integration into BI-RADS classification with subcategories of category 4. Eur Radiol 2013;23:2695-704. [Crossref] [PubMed]


