
Evaluation of connective tissue disease-related interstitial lung disease using ultrasound elastography: a preliminary study
Introduction
Connective tissue disease (CTD) is a group of systemic autoimmune diseases characterized by immune-mediated tissue damage, which may affect many different organs and systems (1). Interstitial lung disease (ILD) is a pulmonary complication of CTD with a high morbidity and mortality. For example, the incidence of systemic sclerosis (SSc)-related ILD varies from 40% to 80%, depending on different ascertainments (2). In both SSc and rheumatoid arthritis (RA), the second leading cause of death is ILD (3). Therefore, it is essential to find more effective methods to diagnose and evaluate CTD-ILD.
Currently, the gold standard of medical imaging to diagnose ILD is high-resolution computed tomography (HRCT). However, due to the high amount of radiation, the application of HRCT is restricted in the early diagnosis and follow-up evaluation of CTD-ILD. In recent years, many studies have confirmed that lung ultrasound (LUS), as a noninvasive, convenient, and nonionizing radiation examination method, plays an important role in the evaluation of CTD-ILD (4-8). The count of B-lines and the evaluation of pleural line thickness and morphology have been employed as valuable parameters for CTD-ILD detection and follow-up (9,10). Nevertheless, B-lines are subjective assessments which can be influenced by operators’ experience, and as nonspecific signs, they can be found in other interstitial changes of the lung (4,9). Furthermore, existing researches showed that ILD-induced lung fibrosis could result in stiffened lung tissue, which cannot be detected by either HRCT or LUS (11,12). To improve the detection and screening of ILD, it is important to seek quantitative and objective assessment methods for ILD and the potential stiffened lung tissue.
Ultrasound elastography (UE), a noninvasive technology, provides qualitative and quantitative tissue elasticity measurements to evaluate and diagnose disease. Strain elastography (SE), acoustic radiation force impulse (ARFI) technology, and shear wave elastography (SWE) are the main types of UE methods, and ARFI technology is divided into two parts: ARFI imaging, and quantitative ARFI methods (13). Both SE and ARFI imaging are semiquantitative methods to measure stiffness and obtain relative stiffness values. In contrast, the quantitative ARFI method and SWE use the acoustic radiation force emitted by the ultrasonic transducer to excite the tissue and generate shear waves. The shear wave velocity (SWV) is measured to quantitatively reflect the stiffness of the target tissue.
Various UE technologies have been applied to assess the stiffness of breast, thyroid, liver, muscle, and many other tissues or organs during evaluation and diagnosis (14-18). For lung diseases, there have only been a few studies in which UE was conducted to determine the stiffness of lung tumors and pleural effusion. Notably, a UE technology named lung ultrasound surface wave elastography (LUSWE) was proposed by Zhang et al. (19,20). This technology assesses lung surface elasticity by applying low-frequency harmonic vibrations, detecting lung surface waves with an ultrasound probe, and obtaining lung surface wave speed. It is mostly used to assess the lung elasticity of ILD patients. As mentioned above, lung tissue can eventually be damaged and stiffened with the progression of ILD-induced lung fibrosis (11,12). The peripheral and subpleural regions of the lung are major areas in which many ILDs typically distribute (12,21). These previous findings suggest that the elastic properties of lung surface tissue can be changed due to ILD. Therefore, using different UE tools to measure the surface elasticity of lungs affected by ILD could be possible and feasible.
To date, only Zhang et al. have researched LUSWE, and more work is needed to further clarify whether this technique is useful for patients with ILD. Among other UE technologies, SWE has the advantages of objective and quantitative measurements and a high level of repeatability. Moreover, there is still a lack of research on the application of SWE to evaluate the lungs of CTD-ILD patients and healthy people. Further research is also required to explore the reliability and repeatability of SWE measurements of lung surface stiffness and the value and significance of SWE assessment of CTD-ILD. We aimed to study the intra- and inter-reliability of SWE measurements on lung surface stiffness of healthy people, explore whether SWE can distinguish diseased patients from healthy controls (HCs), and determine whether SWE can be used to evaluate CTD-ILD by correlating it with other quantitative evaluation methods in this study. We present the following article in accordance with the GRRAS reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1205/rc).
Methods
Patients and controls
From March 2019 to November 2020, 65 patients diagnosed with CTD-ILD were consecutively recruited at the West China Hospital of Sichuan University according to the equation , where n1 is sample size, Z is statistic, α is confidence coefficient, Sen is sensitivity and δ is allowable error. A total of 60 healthy volunteers were enrolled at the West China Hospital of Sichuan University. This was approximate to the ratio of 1 patient to 1 volunteer. For the case group, patients with a history of respiratory diseases other than ILD, such as lung tumors and bulk pleural effusion, or with a history of other disorders (except for CTD) that may injure the respiratory system, such as heart disease, or with a history of medical treatment that may cause lung damage, such as radiotherapy and chemotherapy, were excluded from the study. For the control group, volunteers with a history of respiratory diseases, heart disease, rheumatic disease, or a history of medical treatment that may cause lung damage were excluded. Volunteers with respiratory symptoms and signs were excluded. Respiratory symptoms in volunteers were confirmed through a clinical examination performed by an experienced respiratory doctor. Meanwhile, pregnant women, people with skin scars at examination sites, and children (those under 18 years old) were not included in either group.
All recruited participants underwent LUS and SWE examinations. Additionally, all enrolled CTD-ILD patients were scheduled for an HRCT examination and received a pulmonary function test (PFT) within 30 days before or after the LUS and SWE examinations. Recruited CTD-ILD patients who could not complete the HRCT examination within the limited time were not included in the case group. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the West China Hospital of Sichuan University, and informed consent was provided by all individual participants.
LUS and SWE examination
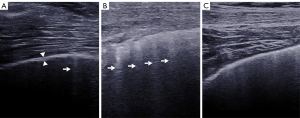
In this study, LUS and SWE examinations were performed using the Aixplorer US system (SuperSonic Imagine, Aix-en-Provence, France). An SL 10-2 linear probe (operating at 2–10 MHz), “General” mode, and the display scale (0–180 kPa) in the standard default setting were preselected. A total of 50 sites on two sides of the lung were all examined during LUS and SWE examination in the supine position and the seated position, as shown in Table 1 and Figure 1 (22). During the examination of each site, the probe tip was covered with ultrasound gel and gently and placed parallel to the lung intercostal spaces (LIS), perpendicular to the chest wall. No pressure was applied between the probe and chest wall skin. The B-lines were counted. A B-line was defined as a wedge-shaped hyperechoic artifact generated from the pleural line level extending to the edge of the screen (23) (Figure 2). The pleural line was defined as a horizontal hyperechoic line separating the chest wall and the lung, which can be observed to increase in ILD patients due to subpleural fibrotic scars (24). The measurement of the pleural line thickness was completed by sonographers when the B-mode image of the pleural line and the lung below were clearly shown on the screen (Figure 2). If there was a full white screen or a total of more than 10 B-lines in a single scanning site, the count of B-lines was recognized as 10 B-lines (25). Maximal contrast between all evaluated structures was obtained by manually adjusting image parameters (26).
Table 1
| Anatomical lines | 50 sites | Position | |
|---|---|---|---|
| Right | Left | ||
| Parasternal line | Second to fifth LIS | Second to fourth LIS | Supine |
| Midclavicular line | Second to fifth LIS | Second to fourth LIS | |
| Anterior axillary line | Second to fifth LIS | Second to fourth LIS | |
| Mid-axillary line | Second to fifth LIS | Second to fourth LIS | |
| Posterior axillary line | Seventh to eighth LIS | Seventh to eighth LIS | Sitting |
| Subscapular line | Seventh to eighth LIS | Seventh to eighth LIS | |
| Paravertebral line | Second to eighth LIS | Second to eighth LIS | |
LIS, lung intercostal spaces.
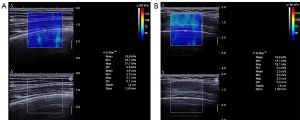
After switching to SWE mode, efforts were made to ensure that the central area of the real-time color-coded square region of interest (ROI) overlay on the grayscale image was placed at the pleural line level. In the meantime, the transducer remained stable. The participants were asked to take a deep breath and hold their breath for a few seconds at the end of the maximum inspiration to obtain a stable SWE image. The Q-box, a small circle, was placed on the pleural line (close to the middle line of the screen) to measure the lung surface stiffness. As ILD-induced abnormalities are frequently distributed in subpleural areas (11), the pleural line was defined as a horizontal hyperechoic line that separates the chest wall from the lung with increased thickness, and the irregularity is related to subpleural fibrotic scars (24). Therefore, pleural line stiffness was considered to correspond to lung surface stiffness. The diameter of the Q-box was set to its minimum size (1 mm), and, if the thickness of the pleural line was less than 1 mm, the Q-box should include the pleural line and part of the lung surface below. The image displayed the mean, maximum, minimum, and standard deviation values of the elastic moduli, including both Young’s modulus and SWV. These were automatically calculated and presented by the system according to the equation E = 3ρc2 (E = Young’s modulus, ρ = tissue density, c = SWV) (Figure 3). In this study, three measurements were taken at each site. The mean Young’s modulus value and the mean SWV value were representative values that we selected in each obtained measurement. Then, the averaged mean value of Young’s modulus (Emean, kPa) and the averaged mean value of SWV (Cmean, m/s) of the pleural line were recorded.
The SWE examination was performed and interpreted by sonographer A and sonographer B, each with at least 3 years’ experience examining musculoskeletal system and lungs, who were blinded to each other’s interpretation throughout the whole study. All participants were examined by sonographer A independently, while the third LIS of the right midclavicular line of 20 randomly selected healthy participants was examined again by sonographer B independently on the same day to assess inter-operator variability. For the assessment of intra-operator variability, repeated SWE measurements of the third LIS of the right midclavicular line of these 20 participants were performed independently by sonographer A one week later.
HRCT assessment
The HRCT examinations were conducted with computed tomography (CT) scanners from Siemens (Siemens Healthineers, Erlangen, Germany), Philips (Philips, Best, The Netherlands), and GE (GE Healthcare, Milwaukee, WI, USA) according to the standard protocol. The whole lung (from the apex to the base) was scanned at full inspiration with the participants in the supine position. The slice thickness and spacing of scans were both 1 mm. The HRCT images were interpreted and scored by an experienced radiologist who was blinded to the PFTs, LUS, and SWE findings according to the Warrick scoring method (score range: 0 to 30) (27) (Table 2).
Table 2
| HRCT abnormalities | Severity | Extent of disease—lung segments involved | ||
|---|---|---|---|---|
| 1–3 | 4–9 | >9 | ||
| Ground glass opacities | 1 | 1 | 2 | 3 |
| Irregular pleural margin | 2 | 1 | 2 | 3 |
| Septal or subpleural lines | 3 | 1 | 2 | 3 |
| Subpleural cyst | 4 | 1 | 2 | 3 |
| Honeycomb | 5 | 1 | 2 | 3 |
HRCT, high-resolution computed tomography.
The PFT
A PFT was performed using the Masterscreen pulmonary function measurement system (Jaeger, Wuerzburg, Germany). Forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DLCO) were chosen as indicators for further analysis in this study, and were expressed as percentages of predicted values.
Statistical analysis
Statistical analysis was carried out using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). In this study, continuous variables were presented as the mean ± standard deviation for data with normal distributions and as the median (upper quartile, lower quartile) for data with abnormal distributions. Categorical variables were expressed as numbers and percentages. The demographic data statistics of the control group and the CTD-ILD group were determined using the χ2 test and t-test for continuous variables and categorical variables, respectively. Additionally, the receiver operating characteristic (ROC) curve, area under the curve (AUC), and cutoff values with associated sensitivity and specificity were analyzed. The comparison of the pleural line elasticity between the control group and the CTD-ILD group was undertaken using the Mann–Whitney U rank sum test. Pearson’s correlation analysis (r) was used to determine the relation between the pleural line elasticity, including both Emean and Cmean, and to interpret the results of LUS, HRCT, and PFTs. The single-measure intraclass correlation coefficient (ICC) using a two-way random-effects model and absolute type was used in intra- and interobserver repeatability assessments. Consistency analysis was conducted using Pearson’s correlation. A two-sided P value of <0.05 was considered statistically significant.
Results
We enrolled 65 CTD-ILD patients and 60 HCs according to the criteria mentioned previously. All HCs were confirmed to have no respiratory symptoms or signs by clinical examination. In the case group, most patients were diagnosed with either SSc (n=23) or idiopathic inflammatory myopathy (IIM) (n=22). There was no statistically significant difference between the CTD-ILD case group and the control group in terms of gender, age, body mass index (BMI), or smoking status. The detailed demographic and clinical characteristics of all participants are shown in Table 3.
Table 3
| Characteristics | Case group (n=65) | Control group (n=60) | P value |
|---|---|---|---|
| CTD classification | / | / | |
| SSc | 23 | ||
| IIM | 22 | ||
| ASS | 6 | ||
| MCTD | 3 | ||
| SS | 3 | ||
| RA | 2 | ||
| RA + SSc | 2 | ||
| UCTD | 2 | ||
| AAV | 1 | ||
| SLE | 1 | ||
| Age, years, mean ± SD | 49.14±10.97 | 46.53±12.61 | 0.219 |
| Gender, male/female | 20/45 | 19/41 | 0.914 |
| BMI, kg/m2, mean ± SD | 22.91±3.55 | 22.54±2.78 | 0.517 |
| Smoke (yes/no) | 10/55 | 7/53 | 0.545 |
CTD, connective tissue disease; SSc, systemic sclerosis; IIM, idiopathic inflammatory myopathy; ASS, anti-synthetase syndrome; MCTD, mixed CTD; SS, Sjogren’s syndrome; RA, rheumatic arthritis; UCTD, undifferentiated CTD; AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis; SLE, systemic lupus erythematosus; BMI, body mass index.
Reliability of SWE measurements
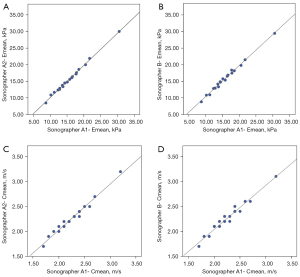
Table 4 illustrates the intra- and inter-observer repeatability of the SWE quantification in the third LIS pleural line of the right midclavicular line of 20 HCs. The interobserver reliability was evaluated with two sonographers’ measurements in this study. For both Emean and Cmean, the ICC value was >0.97 for intra- and inter-observer reliability (P<0.01). The R2 value was also >0.96 (P<0.01), which showed the correlation of intra- and inter-observer ICCs analogously (Figure 4).
Table 4
| SWE measurements | Sonographer A1 | Sonographer A2 | Sonographer B | ICC (95% confidence interval) | P value | |
|---|---|---|---|---|---|---|
| Intraobserver | Interobserver | |||||
| Cmean (m/s)† | 2.26±0.34 | 2.27±0.32 | 2.27±0.30 | 0.986 (0.966–0.994) | 0.976 (0.941–0.969) | <0.01* |
| Emean (kPa)† | 15.74±4.79 | 15.67±4.64 | 15.73±4.47 | 0.998 (0.994–0.999) | 0.994 (0.985–0.998) | <0.01* |
†, mean ± standard deviation; *, P<0.01. SWV, shear wave velocity; SWE, shear wave elastography; Emean, averaged mean Young’s modulus; Cmean, averaged mean shear wave velocity; Sonographer A1, first measurement of Sonographer A; Sonographer A2, second measurement of sonographer A; ICC, intraclass correlation coefficient.
Comparison of LUS and SWE measurements between the case group and the control group
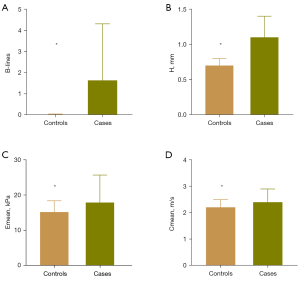
The LUS and SWE examinations were scheduled for a total of 3,250 sites in 65 cases, of which 100 sites could not be scanned or measured because they were covered by different organs, such as the liver, spleen, or heart. Moreover, there were 2 sites that were not measured by SWE due to the deep location of the pleural line. In addition, there were 4 sites where the pleural line thickness could not be measured due to the extreme irregularity of the pleural line. For the control group, a total of 3,000 sites in 60 healthy volunteers were examined by LUS and SWE. The number of B-lines, pleural line thickness, and Emean and Cmean of the pleural line in the case group were generally higher than those of the HCs, and the difference was statistically significant (P<0.001) (Tables 5-7; Figure 5). Comparison of each parameter between the case group and the HC group at each site showed that more B-lines and thicker pleural lines were found in the CTD-ILD group at all 50 sites (Table S1; Table S2), while statistically higher Emean and Cmean of the case group were only found in the following 22 sites (P<0.05): the fifth LIS of the right parasternal line and right anterior axillary line, the second to fifth LIS of the right midclavicular line, the fourth LIS of the bilateral midaxillary line, the eighth LIS of the right posterior axillary line, the second to fourth LIS of the right paravertebral line, the second to fourth LIS of the left midclavicular line, the third and fourth LIS of the left anterior axillary line and the second to sixth LIS of the left paravertebral line (Table S3; Table S4). Additionally, at the second LIS of the bilateral midclavicular line and of the left midaxillary line, the Cmean of CTD-ILD cases was higher than that of HCs (P<0.05), and statistically higher values of the pleural line Young’s modulus of CTD-ILD cases were found at the fifth LIS of the right paravertebral line (P<0.05).
Table 5
| Parameters | The case group (n=3,148) | The control group (n=3,000) | P value |
|---|---|---|---|
| Emean (kPa) | 17.90 (12.20, 25.68) | 15.20 (12.30, 18.40) | <0.001* |
| Cmean (m/s) | 2.40 (2.00, 2.90) | 2.20 (2.00, 2.50) | <0.001* |
Data are presented as the median (upper quartile, lower quartile). *, P<0.001. Emean, averaged mean Young’s modulus; Cmean, averaged mean shear wave velocity.
Table 6
| Parameters | The case group (n=3,150) | The control group (n=3,000) | P value |
|---|---|---|---|
| Number of B-lines | 0.00 (0.00, 2.00) | 0.00 (0.00, 0.00) | <0.001* |
Data are presented as the median (upper quartile, lower quartile). *, P<0.001. LUS, lung ultrasound; H, pleural line thickness.
Table 7
| Parameters | The case group (n=3,146) | The control group (n=3,000) | P value |
|---|---|---|---|
| H (mm) | 1.10 (0.80, 1.40) | 0.70 (0.70, 0.80) | <0.001* |
Data are presented as the median (upper quartile, lower quartile). *, P<0.001. LUS, lung ultrasound; H, pleural line thickness.
Analysis of ROC curve for SWE measurements for CTD-ILD
The AUC of Emean and Cmean for assessing CTD-ILD were 0.646 and 0.647, respectively. This indicated that the diagnostic value of SWE measurements for CTD-ILD was acceptable (Figure 6). The cutoff values for Emean and Cmean were 15.81 kPa and 2.31 m/s, respectively, which could help distinguish CTD-ILD from healthy lungs (Table 8).
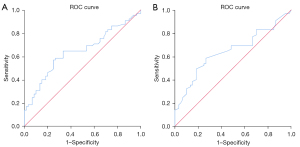
Table 8
| Parameters | AUC (95% CI) | Standard errora | Pb | Cutoff value | Sensitivity | Specificity | 95% CI |
|---|---|---|---|---|---|---|---|
| Emean (kPa) | 0.646 (0.549–0.743) | 0.050 | 0.005* | 15.81 | 0.646 | 0.667 | 0.549–0.743 |
| Cmean (m/s) | 0.647 (0.549–0.744) | 0.050 | 0.005* | 2.31 | 0.585 | 0.733 | 0.549–0.744 |
a, under the nonparametric assumption; b, null hypothesis: true area =0.5; *, P<0.05. SWE, shear wave elastography; CTD-ILD, connective tissue disease-related interstitial lung disease; Emean, averaged mean Young’s modulus; Cmean, averaged mean shear wave velocity; AUC, area under the curve; CI, confidence interval.
Quantitative SWE measurements of the case group in relation to the LUS interpretation
In the case group, two SWE values related to pleural line elasticity and the number of B-lines had no significant correlations (P>0.05), while a weakly negative correlation was found between Emean and Cmean of the pleural line and the pleural line thickness (r=−0.284 and r=−0.316, respectively, P<0.05). The results are outlined in Table 9. For meaningful sites (a total of 22 sites) which had a difference in both pleural line elastic moduli between cases and HCs, the values of the pleural line Young’s modulus and SWV were extracted and analyzed. Those values still had no relation to the number of B-lines (P>0.05). Similarly, Emean (r=−0.245; P<0.05) and Cmean (r=−0.269; P<0.05) of the pleural line were negatively associated with pleural line thickness (Table 10).
Table 9
| Parameters | The number of B-line | H (mm) | |||
|---|---|---|---|---|---|
| r | P value | r | P value | ||
| Emean (kPa) | 0.053 | >0.05 | −0.284* | <0.05 | |
| Cmean (m/s) | 0.044 | >0.05 | −0.316* | <0.05 | |
*, P<0.05. H, pleural line thickness; Emean, averaged mean Young’s modulus; Cmean, averaged mean shear wave velocity.
Table 10
| Parameters | The number of B-line | H (mm) | |||
|---|---|---|---|---|---|
| r | P value | r | P value | ||
| Emean (kPa) | 0.025 | >0.05 | −0.245* | <0.05 | |
| Cmean (m/s) | 0.033 | >0.05 | −0.269* | <0.05 | |
*, P<0.05. H, pleural line thickness; Emean, averaged mean Young’s modulus; Cmean, averaged mean shear wave velocity.
Quantitative SWE measurements of the case group in relation to the HRCT and PFT results
The average Warrick score of all 65 CTD-ILD patients was 14.43±6.60. Regarding PFTs, 25 patients did not undergo the examination in time, and 3 patients could not complete the whole examination process to obtain full results. Therefore, only 37 patients successfully underwent PFTs. The average values of FVC and DLCO were 87.5%±17.2% and 72.6%±17.7%, respectively. Table 11 shows that there was no significant correlation between Emean and Cmean of the pleural line and Warrick score in the case group (r=−0.010 and r=−0.015, respectively, P>0.05). Similarly, no significant correlation was found between the pleural line elastic values and PFT results (P>0.05; Table 11). Moreover, in the 22 meaningful sites, the pleural line elastic values had no significant relation to the HRCT assessment of the PFT results (P>0.05; Table 12).
Table 11
| Parameters | HRCT (n=65), r (P) | PFT (n=37), r (P) | |||
|---|---|---|---|---|---|
| Warrick score | FVC, % | DLCO, % | |||
| Emean (kPa) | −0.010 (>0.05) | 0.040 (>0.05) | 0.305 (>0.05) | ||
| Cmean (m/s) | −0.015 (>0.05) | 0.025 (>0.05) | 0.306 (>0.05) | ||
HRCT, high-resolution computed tomography; PFT, pulmonary function test; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; H, pleural line thickness; Emean, averaged mean Young’s modulus; Cmean, averaged mean shear wave velocity.
Table 12
| Parameters | HRCT (n=65), r (P) | PFT (n=37), r (P) | |||
|---|---|---|---|---|---|
| Warrick score | FVC, % | DLCO, % | |||
| Emean (kPa) | −0.053 (>0.05) | −0.018 (>0.05) | 0.279 (>0.05) | ||
| Cmean (m/s) | −0.040 (>0.05) | −0.005 (>0.05) | 0.319 (>0.05) | ||
HRCT, high-resolution computed tomography; PFT, pulmonary function test; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; H, pleural line thickness; Emean, averaged mean Young’s modulus; Cmean, averaged mean shear wave velocity.
Discussion
As reported, changes in biomechanical properties of the lung can be found in some lung diseases, such as ILD (12). Many types of ILDs distributed in the subpleural regions can lead to stiffened lung surface tissue (12). Observation of the change in lung surface stiffness may help diagnose ILD. Currently, HRCT is the gold standard of medical imaging for ILD diagnosis, and LUS plays an important role in the evaluation of CTD-ILD. However, the high radiation dose restrains the application of HRCT. Furthermore, the B-line is a nonspecific sign of interstitial lung processes, which decreases the diagnostic specificity for CTD-ILD of LUS. Moreover, both methods are not capable of examining the stiffened lung surface tissue that occurs with lung fibrosis. As a noninvasive technology that can quantitatively measure tissue stiffness, SWE may provide a promising and novel method to assess CTD-ILD.
Although SWE has been used to quantify the stiffness of the skin, liver, and breast masses, and the consistency analysis results have been optimistic (28-30), no previous studies have investigated the reliability and repeatability of SWE measurements on lung surface stiffness. In the present study, intra- and inter-group consistency for examining the lung surface stiffness of the mid-anterior lung site of 20 volunteers was performed and the results were excellent. However, only one site in a HC was chosen to perform the repeated SWE examination. This site was the most superficial and the technically simplest site to obtain and was examined only in the supine position, which cannot indicate how the depth of different lung sites and the body habitus may influence the consistency results. Moreover, CTD-ILD patients were not included in the consistency analysis. Different results may be obtained from CTD-ILD patients because of their more complicated situations, such as tachypnea or difficulties obtaining lung imaging, which may influence the results. Therefore, the results in the present study only indicated that the repeatability of SWE measurements on healthy lung surface stiffness in the supine position was excellent (Table 4; Figure 4). These limitations need to be further studied. We also found that not all selected sites of each patient could be detected during the examination because they were covered by adjacent organs, which is similar to the situation mentioned by Vassalou et al. (26). The number of B-lines and the pleural line thickness between the case group and the HC group were significantly different both in the overall 50-site scheme and at each site, and the measured values in the case group were greater, which is consistent with many other studies (31,32). In addition, the values of the pleural line Young’s modulus and SWV of the case group were higher than those of the control group at certain sites (Tables 8,9). Other studies that have used LUSWE to evaluate lung surface stiffness in patients with ILD (mostly SSc-ILD) and in healthy people have indicated that the lung surface wave velocity in ILD patients is higher than that in HCs (11,12,33,34). The results of those studies are similar to those of the present study, which all implied that the ILD-affected lung is stiffer than the normal lung. Therefore, the pleural line Young’s modulus and SWV may provide new indicators to distinguish healthy lungs from lungs with ILD. Sites where stiffness quantification values were significantly different between cases and controls may be potential examination sites to differentiate between healthy and diseased lungs when using SWE, while the pathophysiologic and other influencing factors should be considered. Nevertheless, to the best of our knowledge, there have been no other studies on SWE measurements of the lungs affected by CTD-ILD. However, in view of the limited number of recruited participants, more research is still needed for further exploration. The ROC curve suggested that SWE values can be used to distinguish CTD-ILD and healthy lungs with passable diagnostic efficacy. Furthermore, the calculated cutoff values of both Emean and Cmean may be used as cutoff values for distinguishing CTD-ILD from healthy lungs. Nevertheless, we also found that the sensitivity and specificity associated with these cutoff values were not very high. In view of the relatively low number of enrolled cases and HCs, the analysis of the ROC curve can be reference only, and further study is needed.
In the present study, the values measured using SWE, namely, Young’s modulus and SWV of the pleural line, showed weakly negative correlations with pleural line thickness (Tables 9,10). This weak correlation suggests that these may be considered to have no specific clinical significance. Furthermore, when examining the relationship between SWE measurements and other methods that are useful in the diagnosis and evaluation of CTD-ILD, the study found that SWE elastic moduli had no relation to other parameters to evaluate LUS (the number of B-lines) or interpreting the results of HRCT and PFTs in both the 50-site protocol and 22 selected sites. According to the above results, we consider that SWE assessment of the severity of CTD-ILD may be of limited value. We suppose that the SWE measurement of lung surface stiffness may be influenced by many factors, such as age, BMI, adjacent organs, and different examination areas, which may result in nonsignificant correlations between SWE results and other evaluation methods. These relationships need to be further studied. In consideration of the uncertain influencing factors and the lack of similar studies, the value of SWE for CTD-ILD evaluation needs to be further explored and confirmed.
There are some other limitations to the current study. First, an analysis of factors that influenced SWE measurements of lung surface stiffness was not included in the study. When the pleural line thickness of the participant was less than 1 mm, the Q-box (minimum diameter of 1 mm) in the SWE examination contained the pleural line and the lung surface below it. This measuring method may affect the SWE quantification of the pleural line and needs to be further studied and improved. Moreover, 30 days between LUS/SWE and HRCT examinations may cause dramatically different results between different examinations after induction steroid therapy and other treatments. Although the mean interval between LUS/SWE and HRCT was 4.17 days in the present study, which may not cause obvious dyssynchrony of the LUS/SWE and HRCT results, the interval may need to be reduced in future research. Finally, because this study is a preliminary study, CTD-ILD patients were not grouped in accordance to different CTD types to compare the LUS and SWE data. In the future, these limitations need to be further addressed.
Conclusions
In addition to LUS, which can be used to evaluate CTD-ILD and distinguish CTD-ILD-affected lungs from healthy lungs, SWE can also be performed to measure the lung surface stiffness of CTD-ILD patients and HCs to differentiate them at certain sites. Cutoff values were calculated to help the differentiation. Moreover, measurement of healthy anterior lung surface stiffness in the supine position was found to be a reliable approach. This result suggested that SWE may provide a promising imaging method to assess lung surface stiffness. However, the value of SWE assessment for the severity of CTD-ILD is limited in the present study, and further study of the ability and reliability of SWE for lung surface stiffness assessment is needed.
Acknowledgments
Funding: This work was supported by the 1·3·5 Project for Disciplines of Excellence–Clinical Research Incubation Project (No. 2020HXFH001) and the National Clinical Research Center for Geriatrics (No. Z2021LC002).
Footnote
Reporting Checklist: The authors have completed the GRRAS reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-1205/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-1205/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the West China Hospital of Sichuan University, and informed consent was provided by all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mira-Avendano I, Abril A, Burger CD, Dellaripa PF, Fischer A, Gotway MB, Lee AS, Lee JS, Matteson EL, Yi ES, Ryu JH. Interstitial Lung Disease and Other Pulmonary Manifestations in Connective Tissue Diseases. Mayo Clin Proc 2019;94:309-25. [Crossref] [PubMed]
- Gutsche M, Rosen GD, Swigris JJ. Connective Tissue Disease-associated Interstitial Lung Disease: A review. Curr Respir Care Rep 2012;1:224-32. [Crossref] [PubMed]
- Salaffi F, Carotti M, Baldelli S, Bichi Secchi E, Manganelli P, Subiaco S, Salvolini L. Subclinical interstitial lung involvement in rheumatic diseases. Correlation of high resolution computerized tomography and functional and cytologic findings. Radiol Med 1999;97:33-41. [PubMed]
- Wang Y, Gargani L, Barskova T, Furst DE, Cerinic MM. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: a literature review. Arthritis Res Ther 2017;19:206. [Crossref] [PubMed]
- Ferro F, Delle Sedie A. The use of ultrasound for assessing interstitial lung involvement in connective tissue diseases. Clin Exp Rheumatol 2018;36:165-70. [PubMed]
- Xie HQ, Zhang WW, Sun S, Chen XM, Yuan SF, Gong ZH, Liu L. A simplified lung ultrasound for the diagnosis of interstitial lung disease in connective tissue disease: a meta-analysis. Arthritis Res Ther 2019;21:93. [Crossref] [PubMed]
- Volpicelli G. Lung Ultrasound B-Lines in Interstitial Lung Disease: Moving From Diagnosis to Prognostic Stratification. Chest 2020;158:1323-4. [Crossref] [PubMed]
- Vicente-Rabaneda EF, Bong DA, Castañeda S, Möller I. Use of ultrasound to diagnose and monitor interstitial lung disease in rheumatic diseases. Clin Rheumatol 2021;40:3547-64. [Crossref] [PubMed]
- Fairchild R, Chung M, Yang D, Sharpless L, Li S, Chung L. Development and Assessment of Novel Lung Ultrasound Interpretation Criteria for the Detection of Interstitial Lung Disease in Systemic Sclerosis. Arthritis Care Res (Hoboken) 2021;73:1338-42. [Crossref] [PubMed]
- Pinal-Fernandez I, Pallisa-Nuñez E, Selva-O'Callaghan A, Castella-Fierro E, Simeon-Aznar CP, Fonollosa-Pla V, Vilardell-Tarres M. Pleural irregularity, a new ultrasound sign for the study of interstitial lung disease in systemic sclerosis and antisynthetase syndrome. Clin Exp Rheumatol 2015;33:S136-41. [PubMed]
- Clay R, Bartholmai BJ, Zhou B, Karwoski R, Peikert T, Osborn T, Rajagopalan S, Kalra S, Zhang X. Assessment of Interstitial Lung Disease Using Lung Ultrasound Surface Wave Elastography: A Novel Technique With Clinicoradiologic Correlates. J Thorac Imaging 2019;34:313-9. [Crossref] [PubMed]
- Zhang X, Osborn T, Zhou B, Meixner D, Kinnick RR, Bartholmai B, Greenleaf JF, Kalra S. Lung Ultrasound Surface Wave Elastography: A Pilot Clinical Study. IEEE Trans Ultrason Ferroelectr Freq Control 2017;64:1298-304. [Crossref] [PubMed]
- Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol 2015;41:1126-47. [Crossref] [PubMed]
- Yin L, Cheng L, Wang F, Zhu X, Hua Y, He W. Application of intraoperative B-mode ultrasound and shear wave elastography for glioma grading. Quant Imaging Med Surg 2021;11:2733-43. [Crossref] [PubMed]
- Jia W, Luo T, Dong Y, Zhang X, Zhan W, Zhou J. Breast Elasticity Imaging Techniques: Comparison of Strain Elastography and Shear-Wave Elastography in the Same Population. Ultrasound Med Biol 2021;47:104-13. [Crossref] [PubMed]
- Tang X, Wang L, Guo R, Huang S, Tang Y, Qiu L. Application of ultrasound elastography in the evaluation of muscle strength in a healthy population. Quant Imaging Med Surg 2020;10:1961-72. [Crossref] [PubMed]
- Qiu Y, Xing Z, Liu J, Peng Y, Zhu J, Su A. Diagnostic reliability of elastography in thyroid nodules reported as indeterminate at prior fine-needle aspiration cytology (FNAC): a systematic review and Bayesian meta-analysis. Eur Radiol 2020;30:6624-34. [Crossref] [PubMed]
- Nierhoff J, Chávez Ortiz AA, Herrmann E, Zeuzem S, Friedrich-Rust M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur Radiol 2013;23:3040-53. [Crossref] [PubMed]
- Zhang X, Osborn T, Kalra S. A noninvasive ultrasound elastography technique for measuring surface waves on the lung. Ultrasonics 2016;71:183-8. [Crossref] [PubMed]
- Zhang X, Qiang B, Hubmayr RD, Urban MW, Kinnick R, Greenleaf JF. Noninvasive ultrasound image guided surface wave method for measuring the wave speed and estimating the elasticity of lungs: A feasibility study. Ultrasonics 2011;51:289-95. [Crossref] [PubMed]
- Desai SR, Veeraraghavan S, Hansell DM, Nikolakopolou A, Goh NS, Nicholson AG, Colby TV, Denton CP, Black CM, du Bois RM, Wells AU. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004;232:560-7. [Crossref] [PubMed]
- Tardella M, Gutierrez M, Salaffi F, Carotti M, Ariani A, Bertolazzi C, Filippucci E, Grassi W. Ultrasound in the assessment of pulmonary fibrosis in connective tissue disorders: correlation with high-resolution computed tomography. J Rheumatol 2012;39:1641-7. [Crossref] [PubMed]
- Gutierrez M, Salaffi F, Carotti M, Tardella M, Pineda C, Bertolazzi C, Bichisecchi E, Filippucci E, Grassi W. Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders--preliminary results. Arthritis Res Ther 2011;13:R134. [Crossref] [PubMed]
- Manolescu D, Davidescu L, Traila D, Oancea C, Tudorache V. The reliability of lung ultrasound in assessment of idiopathic pulmonary fibrosis. Clin Interv Aging 2018;13:437-49. [Crossref] [PubMed]
- Gargani L, Doveri M, D'Errico L, Frassi F, Bazzichi ML, Delle Sedie A, Scali MC, Monti S, Mondillo S, Bombardieri S, Caramella D, Picano E. Ultrasound lung comets in systemic sclerosis: a chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology (Oxford) 2009;48:1382-7. [Crossref] [PubMed]
- Vassalou EE, Raissaki M, Magkanas E, Antoniou KM, Karantanas AH. Lung Ultrasonography in Patients With Idiopathic Pulmonary Fibrosis: Evaluation of a Simplified Protocol With High-Resolution Computed Tomographic Correlation. J Ultrasound Med 2018;37:689-96. [Crossref] [PubMed]
- Warrick JH, Bhalla M, Schabel SI, Silver RM. High resolution computed tomography in early scleroderma lung disease. J Rheumatol 1991;18:1520-8. [PubMed]
- Ferraioli G, Tinelli C, Zicchetti M, Above E, Poma G, Di Gregorio M, Filice C. Reproducibility of real-time shear wave elastography in the evaluation of liver elasticity. Eur J Radiol 2012;81:3102-6. [Crossref] [PubMed]
- Hong S, Woo OH, Shin HS, Hwang SY, Cho KR, Seo BK. Reproducibility and diagnostic performance of shear wave elastography in evaluating breast solid mass. Clin Imaging 2017;44:42-5. [Crossref] [PubMed]
- Xiang X, Yan F, Yang Y, Tang Y, Wang L, Zeng J, Qiu L. Quantitative Assessment of Healthy Skin Elasticity: Reliability and Feasibility of Shear Wave Elastography. Ultrasound Med Biol 2017;43:445-52. [Crossref] [PubMed]
- Moazedi-Fuerst FC, Zechner PM, Tripolt NJ, Kielhauser SM, Brickmann K, Scheidl S, Lutfi A, Graninger WG. Pulmonary echography in systemic sclerosis. Clin Rheumatol 2012;31:1621-5. [Crossref] [PubMed]
- Buda N, Piskunowicz M, Porzezińska M, Kosiak W, Zdrojewski Z. Lung Ultrasonography in the Evaluation of Interstitial Lung Disease in Systemic Connective Tissue Diseases: Criteria and Severity of Pulmonary Fibrosis - Analysis of 52 Patients. Ultraschall Med 2016;37:379-85. [PubMed]
- Zhou B, Bartholmai BJ, Kalra S, Osborn TG, Zhang X. Lung US Surface Wave Elastography in Interstitial Lung Disease Staging. Radiology 2019;291:479-84. [Crossref] [PubMed]
- Zhang X, Zhou B, Kalra S, Bartholmai B, Greenleaf J, Osborn T. An Ultrasound Surface Wave Technique for Assessing Skin and Lung Diseases. Ultrasound Med Biol 2018;44:321-31. [Crossref] [PubMed]


