
Coexistence of hypertrophic cardiomyopathy and left ventricular non-compaction cardiomyopathy—a description of two cases
Introduction
Embryogenesis of the heart and vascular system is extremely complicated and multistage. Numerous internal and external factors determine the normal course of this process. In the formation of the heart, not only are the structure of the heart and its correct location important, but so is the position of the heart cavities and the vessels of the circulatory system (1). Internal factors include processes that regulate the activity of numerous genes, protein pathways, growth factors, and the processes of apoptosis and cell death. Morphogenesis is regulated by transcription factors whose proteins are encoded by a number of genes (2-4).
When a gene mutates or inactivates at particular stages of heart development, congenital heart defects occur. These defects can be isolated or complex. Cardiomyopathies are also congenital heart defects. The pathophysiology of their origin is multifactorial and not fully understood (5). However, the very division of cardiomyopathy raises a lot of controversy, as evidenced by the fact that different specialists around the world use different classifications (6).
Case presentation
In clinical practice, more and more patients with features of different cardiomyopathies are being found. Increased detection is undoubtedly due to easier access to transthoracic echocardiography (TTE) and cardiovascular magnetic resonance (CMR) imaging. The cases of two boys are presented below; they were under the care of the Cardiology Outpatient Clinic at the Pediatric Hospital in Kielce (Poland).
The first patient was a 17-year-old boy who has been under the care of the Cardiology Outpatient Clinic since the age of 10 due to innocent heart murmurs. The patient did not report any respiratory or cardiovascular complaints. Biochemical and laboratory findings were unremarkable. There was no family history of sudden cardiac deaths. In the physical examination, no abnormalities were found apart from a murmur in the area of the aortic valve, classified as an innocent heart murmur. Basic medical tests were carried out, including TTE. In this test, features of myocardial hypertrophy around the cardiac apex were found, but they did not meet the criteria for hypertrophic cardiomyopathy (HCM). The patient was referred for CMR. Magnetic resonance imaging was performed with gadolinium contrast agent. The test was performed according to a standard protocol. Segment 1 was 27 mm in thickness and segment 2 was 25 mm, which confirmed the diagnosis of HCM based on Jenni’s criteria (7). This study also found that in segments 16, 15, 11, and 10, the ratio of non-compacted to compacted myocardium (NC/C ratio) was >2.3. After intravenous gadolinium contrast agent was administered, numerous punctual and linear areas of delayed contrast enhancement became apparent, which indicates the presence of connective tissue in hypertrophied myocardium. Fluid in the pericardial sac with a thickness of up to 4 mm was also found. Using Petersen’s criteria, left ventricular non-compaction cardiomyopathy (LVNC) can be diagnosed. The overall assessment of the magnetic resonance imaging supported the concurrence of HCM and LVNC.
Another patient is a boy who was admitted to the Cardiology Clinic at the age of 16. The reason for admission was irregularities detected during the physical examination qualifying him for sports activities. Electrocardiography (ECG) screening showed features of HCM. This boy, like the previous patient, had not reported any complaints. No sudden cardiac deaths were recorded in this patient’s family history, either. Blood and urine laboratory tests were normal. Further additional tests were performed, such as using a Holter monitor for 72 h, in which no arrhythmias were found. Blood pressure was also monitored for 24 h (ambulatory blood pressure monitoring—ABPM)—the readings were also normal. Diagnosis was expanded to include TTE, which revealed hypertrophy of the left ventricular basal segments with a thickness of up to 32 mm; this confirmed the diagnosis of HCM. In addition, an increased number of left ventricle (LV) muscular trabeculae were noted. A CMR was requested, following a standard protocol with intravenous gadolinium contrast agent administration. This test showed a thickening basal segments of the left ventricular wall and non-compacted myocardium in segments 10, 11, 15, and 16 and the NC/C ratio was >2.3. After contrast agent administration, areas of subtle contrast enhancement extending along the LV lateral and posterior walls were seen, supporting the presence of connective tissue. Thus, LVNC can also be diagnosed in this patient (8).
In both of these individuals, the CMR scan met Petersen’s criteria for LVNC and HCM criteria, with no clinical symptoms (Figure 1A-1E).
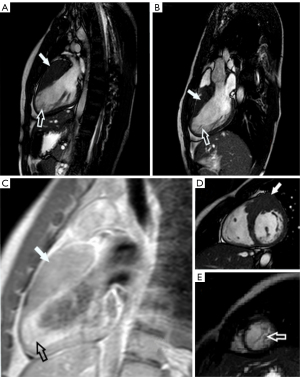
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images—both described patients are currently of legal age. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Cardiovascular morphogenesis begins in the early stages of embryo formation. It is regulated by transcription factors, whose proteins are encoded by different genes. Some of them are highly selective, e.g., Nkx 2.5, whose expression affects only the process of the formation of the cardiogenic plate and the surrounding endoderm, which corresponds to the formation of the neural crest in a later stage (1).
One of the most important growth factors (CR-1) is responsible for the development of a normal embryonic heart. It affects the activity of the anf, mhc-2a, and mhc-2b genes, which are responsible for the transformation of precursor cells towards mesodermal cardiomyocytes (9,10).
According to the latest scientific reports, the proper organogenesis of other organs in the cranial part of the embryo, e.g., parathyroid glands or the respiratory system, is also responsible for proper heart development (11).
One of the most important stages in the development of cardiac chambers is the formation of heart fields. From the first cardiac field, the LV is formed together with the atrioventricular canal (AVC) and the atria (2,3). The cells of the second heart field originate with the right ventricle (RV), the left ventricular outflow tract (LVOT), and the right ventricular outflow tract (RVOT). Some myocytes from the second heart field migrate towards LV and partially form the LV myocardium. Differentiation processes of heart field cells are regulated by fibroblast growth factors (FGF), bone morphogenetic proteins (BMP), transcription factors Tbx5, Gata4, Baf60c, and Wnt proteins, among other things (10,12). Along with the further development of the heart, the myocardium is formed. Its differentiation begins in the early stages of its creation. Endocardial endothelial cells secrete neuralgine, which—along with the constant activity of the mlc-2 gene—is responsible for the formation of the trabeculae carneae. This process is regulated by retinoic acid via the RXRalpha and RXRbeta receptors (1).
According to the very schematic description mentioned above, one gets the impression of an extremely complicated process of organogenesis. Disruption of any of the pathways of morphogenesis can lead to all sorts of disorders in the structure and functioning of not only the heart itself, but also of the circulatory system. The degree of organ dysfunction depends on both the type of damaged gene and the “level of damage” to the gene, which determines its expression. Cardiomyopathies are among the congenital heart defects most dependent on the level of gene expression.
The very division of cardiomyopathies is ambiguous, and various specialists around the world use different classifications (6). This is clearly seen in one of the more common cardiomyopathies—HCM and the less common LVNC, where there are different criteria for diagnosis and classification according to the European Society of Cardiology (ESC) and other criteria according to the American Heart Association (AHA) (6,9).
The CMR is the most important modality by which HCM and LVNC can be diagnosed.
In the latest ESC document from 2014, HCM was defined as a thickening of the left ventricular wall, and the diagnostic criterion was a value of ≥15 mm for the diastolic thickness of at least one left ventricular segment, measured by any imaging technique (TTE or CMR). Other secondary causes should also be excluded. In the course of this heart disease, the LVOT may narrow as a result of thickening of the base segments with simultaneous systolic displacement of the anterior mitral cusp towards the LVOT.
LVNC is a rare genetic form of cardiomyopathy that is characterized by abnormal left ventricular trabeculation and deep recesses between the trabeculae communicating with the LV. The lesions are located mainly in the apex cordis and in the lateral wall of the LV; they are less common in the ventricular septum and are rare in the right ventricle. TTE is a key test in diagnosing and monitoring disease progression (11,13).
The applicable criteria for the diagnosis of LVNC in echocardiography, as established by Jenni et al. (7), include thickening of the myocardium and its division into 2 layers—a thin, compact epicardial layer and a thickened endocardial layer with numerous protruding trabeculae carneae and deep recesses; a ratio of non-compacted to compacted layers of >2:1 in the end-systolic phase from the parasternal short-axis view; the presence of blood flow in colored Doppler ultrasound (US) in the inter-trabecular recesses; and trabeculation protruding into the lumen of the LV within the apex cordis and/or the middle segments of the inferior and lateral walls.
To make a diagnosis it is required to meet all of the above criteria, assessed from the parasternal short-axis view at the level of the basal, mid-cavity, and apical segments. Additional parameters to be assessed may be disturbances in segmental contractility (hypokinesis) of non-compacted segments and adjacent segments or a maximum systolic thickening of the compacted layer of <8.1 mm, which is particularly useful in the diagnosis of LVNC in patients with aortic stenosis. A less common criterion for the diagnosis of LVNC has an X/Y ratio greater than or equal to 0.5, where X is the width of the compacted layer and Y is the width of the compacted + non-compacted layers, assessed in trabeculated apical segments from a subcostal or apical four-chamber view in ECG. The least popular criteria are those established by Stöllberger, according to which LVNC can be diagnosed in TTE when more than three trabeculae carneae protrude from the left ventricular wall, distal to the papillary muscles, observed from a single view, and blood flow is found at the inter-trabecular recesses on colored Doppler US (5,6).
In order to visualize the myocardial morphology, CMR is the best examination. Based on Petersen’s criteria—a NC/C ratio of >2.3, in the final diastolic phase—and/or the criteria of Jacquier—when the mass of the trabecular part of the myocardium accounts for >20% of the total myocardial mass—LVNC can be diagnosed. With the help of a gadolinium contrast agent, the degree of myocardial fibrosis can be assessed, which reflects the clinical stage of the disease.
HCM was called “sarcomeric disease” when it was discovered that the first three genes responsible for the disease encode components of the myocardial contractile apparatus. The most common are mutations of the MYH7 gene, coding for the heavy chain of b-myosin, and the MYBPC3 gene, coding for the myosin-binding protein C, cardiac type (cMyB-C)—mutations of each of these genes account for about 25–33% of all cases of disease (14,15).
In addition, other gene mutations have been reported: lamin A/C (LMNA); alpha-dystrobrevin (DTNA), or SCN5A protein (5,16).
In both cardiomyopathies, mutations are found in so-called sarcomere and non-sarcomere genes. It seems right to continue genetic testing to answer the question of whether a single gene mutation can cause different forms of cardiomyopathy and whether the presence of a strictly defined sequence of mutated genes is the sine qua non for the occurrence of a given cardiomyopathy.
Miszalski-Jamka et al. (15) presented in their work a thorough analysis of the co-occurrence of various types of cardiomyopathy and a list of gene mutations that are responsible for them. The most frequently reported mutations in patients with LVNC and HCM were concerned the TTN and MYH7 genes as well as the MYPN and LDB3 genes. Also in the work of van Waning et al. (17) devoted to genotype-phenotype correlation in the course of LVNC, the most common were single mutations in sarcomere genes, e.g., MYH7, MYBPC3 and ACTC1.
Both cardiomyopathies are autosomal, dominant, inherited diseases caused by mutations of multiple genes and are characterized by a large variation in gene expression, and thus a different clinical picture. They may include exercise-induced dyspnea (the most common symptom), angina (chest pain), heart palpitations, dizziness, fainting (especially in HCM with LVOT obstruction), or sudden cardiac death. Patients with LVNC mainly have symptoms of heart failure and atrioventricular conduction disturbances, which was described in the article Schultze-Berndt et al. (18).
There are known cases of patients diagnosed with HCM and/or LVNC who did not manifest clinical symptoms during follow-up (16). There is also a group of patients with low symptoms or non-specific symptoms, like the woman with LVNC HCM and syncope described by Hotta et al. (19). Nevertheless, asymptomatic patients with LVNC HCM should be under the constant supervision of a cardiologist (20).
Two boys with concurrent HCM and LVNC have been described above, but in the literature, you can find descriptions of patients with LVNC and other cardiomyopathies coinciding, e.g., with restrictive cardiomyopathy or dilated cardiomyopathy (16,21,22). LVNC is a genetically and phenotypically heterogeneous disease, and although it is increasingly recognized in clinical practice, there are no universally accepted diagnostic criteria (15).
Patients with severe disease course have been described in previous reports of each cardiomyopathy. In later studies, however, it was observed that some patients have a mild and sometimes atypical form of the disease.
Varying penetration of individual mutated genes, in effect, leads to varying clinical presentation, from a lack of clinical symptoms to severe heart failure or sudden cardiac death. Therefore, in close relatives of patients diagnosed with LVNC, who generally have a 50% chance of having the disease before the test, it would be reasonable to use different, slightly less strict diagnostic criteria than the currently valid ones (15,22).
Clinicians taking care of such genetically burdened families are currently using complex diagnostic algorithms to interpret minor irregularities. Unfortunately, LVNC is often diagnosed in more than just by people with a family history. One special group is young people who actively practice sport and professional athletes. There are studies in which as many as 8% of the athletes examined who met the criteria for LVNC diagnosis did not report any clinical symptoms related to LVNC during the 24-month follow-up period. In some at-risk asymptomatic patients, impaired left ventricular systolic or diastolic function, thrombus, or abnormal papillary muscle structure can be found in TTE (23). Studies have not shown a correlation between the number of non-compacted segments and left ventricular systolic dysfunction (9).
Acknowledgments
Funding: This work was supported under the program of the Minister of Science and Higher Education under name “Regional Initiative of Excellence in 2019–2022 project number: 024/RID/2018/19, financing amount: 11.999.000,00 PLN”.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-730/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images—both described patients are currently of legal age. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kobylińska J, Dworzański W, Cendrowska-Pinkosz M, Dworzańska A, Hermanowicz-Dryka T, Kiszka J, Starosławska E, Burdan F. Morphological and molecular bases of cardiac development. Postepy Hig Med Dosw (Online) 2013;67:950-7. [Crossref] [PubMed]
- Epstein JA, Franklin H. Epstein Lecture. Cardiac development and implications for heart disease. N Engl J Med 2010;363:1638-47. [Crossref] [PubMed]
- Niszczota C, Koleśnik A. The development and clinical morphology of normal fetal and children's heart. Sequential segmental analysis of congenitally malformed heart. Pediatr Pol 2012;87:78-90.
- Rozwadowska K, Raczak G, Sikorska K, Fijałkowski M, Kozłowski D, Daniłowicz-Szymanowicz L. Influence of hereditary haemochromatosis on left ventricular wall thickness: does iron overload exacerbate cardiac hypertrophy? Folia Morphol (Warsz) 2019;78:746-53. [Crossref] [PubMed]
- Faria R, Santos W, Camacho A, Marques N, Ferrinha R, Marques V, de Jesus I. One patient, one mutation and two cardiomyopathies - hypertrophic cardiomyopathy and left ventricular noncompaction. Rev Port Cardiol 2012;31:317-9. [Crossref] [PubMed]
- Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Ku¨hl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008;29:270-6. [Crossref] [PubMed]
- Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 2001;86:666-71. [Crossref] [PubMed]
- Haland TF, Saberniak J, Leren IS, Edvardsen T, Haugaa KH. Echocardiographic comparison between left ventricular non-compaction and hypertrophic cardiomyopathy. Int J Cardiol 2017;228:900-5. [Crossref] [PubMed]
- Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, McKenna WJ, Sharma S, Elliott PM. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J 2008;29:89-95. [Crossref] [PubMed]
- Lincoln J, Yutzey KE. Molecular and developmental mechanisms of congenital heart valve disease. Birth Defects Res A Clin Mol Teratol 2011;91:526-34. [Crossref] [PubMed]
- Lin YN, Wang YQ, Yu Y, Cao Q, Wang F, Chen SY. Left ventricular noncompaction cardiomyopathy: a case report and literature review. Int J Clin Exp Med 2014;7:5130-3. [PubMed]
- Ong LL, Kim N, Mima T, Cohen-Gould L, Mikawa T. Trabecular myocytes of the embryonic heart require N-cadherin for migratory unit identity. Dev Biol 1998;193:1-9. [Crossref] [PubMed]
- Watkins H, Ashrafian H, Redwood C. Inherited cardiomyopathies. N Engl J Med 2011;364:1643-56. [Crossref] [PubMed]
- Vatta M, Mohapatra B, Jimenez S, Sanchez X, Faulkner G, Perles Z, et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol 2003;42:2014-27. [Crossref] [PubMed]
- Miszalski-Jamka K, Jefferies JL, Mazur W, Głowacki J, Hu J, Lazar M, et al. Novel Genetic Triggers and Genotype-Phenotype Correlations in Patients With Left Ventricular Noncompaction. Circ Cardiovasc Genet 2017;10:e001763. [Crossref] [PubMed]
- Lofiego C, Biagini E, Pasquale F, Ferlito M, Rocchi G, Perugini E, Bacchi-Reggiani L, Boriani G, Leone O, Caliskan K, ten Cate FJ, Picchio FM, Branzi A, Rapezzi C. Wide spectrum of presentation and variable outcomes of isolated left ventricular non-compaction. Heart 2007;93:65-71. [Crossref] [PubMed]
- van Waning JI, Moesker J, Heijsman D, Boersma E, Majoor-Krakauer D. Systematic Review of Genotype-Phenotype Correlations in Noncompaction Cardiomyopathy. J Am Heart Assoc 2019;8:e012993. [Crossref] [PubMed]
- Schultze-Berndt A, Kühnisch J, Herbst C, Seidel F, Al-Wakeel-Marquard N, Dartsch J, Theisen S, Knirsch W, Jenni R, Greutmann M, Oechslin E, Berger F, Klaassen S. Reduced Systolic Function and Not Genetic Variants Determine Outcome in Pediatric and Adult Left Ventricular Noncompaction Cardiomyopathy. Front Pediatr 2021;9:722926. [Crossref] [PubMed]
- Hotta VT, Monge NMS, Fernandes F, Arteaga-Fernandez E. Rare association of left ventricular non-compaction and hypertrophic cardiomyopathy. Eur Heart J Case Rep 2020;4:1-2. [Crossref] [PubMed]
- Komissarova SM, Rineiska NM, Chakova NN, Niyazova SS. Overlapping Phenotype: Left Ventricular non-Compaction and Hypertrophic Cardiomyopathy. Kardiologiia 2020;60:137-45. [Crossref] [PubMed]
- Biagini E, Ragni L, Ferlito M, Pasquale F, Lofiego C, Leone O, Rocchi G, Perugini E, Zagnoni S, Branzi A, Picchio FM, Rapezzi C. Different types of cardiomyopathy associated with isolated ventricular noncompaction. Am J Cardiol 2006;98:821-4. [Crossref] [PubMed]
- Rapezzi C, Leone O, Ferlito M, Biagini E, Coccolo F, Arpesella G. Isolated ventricular non-compaction with restrictive cardiomyopathy. Eur Heart J 2006;27:1927. [Crossref] [PubMed]
- Abela M, D'Silva A. Left Ventricular Trabeculations in Athletes: Epiphenomenon or Phenotype of Disease? Curr Treat Options Cardiovasc Med 2018;20:100. [Crossref] [PubMed]


