
Quantitative estimation of muscle mass in older adults at risk of sarcopenia using ultrasound: a cross-sectional study
Introduction
Sarcopenia is an age-associated syndrome of decreased skeletal muscle function and loss of muscle mass (1,2). The prevalence of sarcopenia in people aged 65 years and over is 11.5% and 16.7% in men and women, respectively (3). Due to the continually declining birth rate and the increase in average life expectancy, population aging has gradually become a significant problem (4). Furthermore, sarcopenia-related disability has increased the burden of the disease to a considerable extent. Therefore, effective sarcopenia disease management is extremely important, and how to efficiently screen and target at-risk groups has become a key concern in the clinical management of sarcopenia.
At present, clinicians mainly diagnose and evaluate sarcopenia based on muscle strength, muscle mass, and physical performance. According to the European Working Group on Sarcopenia in Older People 2 (5), magnetic resonance imaging (MRI) and computed tomography (CT) are considered the gold standards for non-invasive muscle mass assessment. However, the high costs and lack of established cut-off values with these imaging techniques limit their clinical application. Dual-energy X-ray absorptiometry (DXA) is considered to be a preferred alternative method to MRI and CT. However, its use for community-dwelling older adults is not yet feasible. Moreover, DXA carries a radiation risk and is more expensive than bioelectrical impedance analysis (BIA), which discourages many older adults. Given the disadvantages of MRI, CT, and DXA, BIA has become the first-choice diagnostic method for sarcopenia in many medical institutions. BIA is usually based on DXA and other methods as a reference standard for modeling electrical impedance data and estimating muscle mass (6,7). However, BIA also has several shortcomings, and BIA equipment is not commonly available in primary medical institutions, which limits its application in community-based disease screening.
As a non-radiation and highly available medical imaging technology, ultrasound for musculoskeletal systems represents a breakthrough in the screening and diagnosis of sarcopenia and related diseases (8). Abe et al. (9,10) proposed a method that uses whole-body multisite ultrasound to estimate muscle mass. However, this method is time-consuming and does not meet the needs of disease screening. Establishing a scanning program that can take advantage of ultrasound would be a highly critical step in improving the clinical management of sarcopenia.
Our objective is to develop an ultrasound-derived algorithm for estimating muscle mass in older adults. We hypothesized that there would be a correlation between muscle mass and muscle thickness (MT) at different sites, and that MT at different sites would be a suitable algorithm component for estimating muscle mass. Based on this hypothesis, we designed this cross-sectional study to investigate whether ultrasound can be used to quantitatively estimate muscle mass in older adults, as an efficient method to assist in the diagnosis of sarcopenia.
We present the following article in accordance with the MDAR checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-21-685/rc).
Methods
Study participants
A total of 103 older adults who were at risk for sarcopenia according the Asian Working Group for Sarcopenia 2019 guidelines (11) were recruited to this cross-sectional study from the National Clinical Research Center for Geriatrics in West China Hospital between June 2020 and May 2021.
Individuals were primarily included if they met criterion (I) and any one of criteria (II) to (IV): (I) over 60 years of age; (II) a calf circumference of <34 cm (for males) or <33 cm (for females), or a Strength, Assistance with walking, Rise from a chair, Climb stairs and Falls (SARC-F) score of ≥4 or SARC-calf circumference (CalF) score of ≥11; (III) chronic disease status including diabetes, chronic obstructive pulmonary disease, and chronic kidney disease; and (IV) recent unintentional weight loss.
The exclusion criteria were as follows: (I) amputated arm or leg, (II) severe edema, (III) impaired consciousness, poor general health, or other reasons which would prevent the individual from completing the study.
BIA and ultrasound examinations were performed on all participants. BIA was performed by a geriatrician using the InBody 770 body composition analyzer (Seoul, South Korea). The obtained appendicular skeletal muscle (ASM, kg) results were divided by the square of the participant’s height (m) to obtain the skeletal muscle mass index (SMI, kg/m2). SMI was used in the analysis as described below. Ultrasound examinations were performed by an experienced and trained doctor in the Department of Medical Ultrasound. To ensure the comparability between different examinations, examinations of a particular participant were completed within the same day.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of West China Hospital of Sichuan University (No. 2020[258]), and informed consent was obtained from all participants.
Ultrasound examination
According to the study of Abe et al. (10), other related research (12-17), and our pre-experiment, 11 sites across the whole body were selected to measure MT, as explained below and as shown in Figure 1.
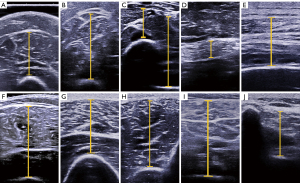
Site 1 (biceps and brachialis) and Site 2 (triceps): on the anterior and posterior surface 60% distal between the lateral epicondyle of the humerus and the acromial process of the scapula.
Site 3 (radial side: flexor pollicis longus, flexor digitorum superficialis, and brachioradialis) and Site 4 (ulnar side: flexor digitorum profundus, flexor digitorum superficialis, and flexor carpi radialis): on the anterior surface 30% proximal between the styloid process and the head of the radius.
Site 5 (rectus abdominis): approximately 3 cm to the right of the umbilicus.
Site 6 (back: latissimus dorsi and external intercostal): about 5 cm directly below the inferior angle of the scapula.
Site 7 (rectus femoris and vastus intermedius) and Site 8 (vastus lateralis and vastus intermedius): on the anterior and lateral surface midway between the lateral condyle of the femur and the greater trochanter.
Site 9 (tibialis anterior) and Site 10 (gastrocnemius, soleus, and flexor digitorum longus): on the anterior and posterior surface 30% proximal between the lateral malleolus of the fibula and the lateral condyle of the tibia.
Site 11 (waist: latissimus dorsi, serratus posterior inferior, longissimus thoracis, spinalis thoracis, and multifidus): at the first lumbar level. The 12th rib was located with ultrasound, and then the first lumbar spine was located, which corresponded to the whole muscle layer between the vertebral arch and the superficial fascia.
Bony landmarks were located by palpation or ultrasound, and then a soft ruler was used to locate the target point and a transverse line was drawn at these sites.
The sonographic equipment used was the Aixplorer Ultrasound system (SuperSonic Imagine, Aix-en-Provence, France), with an SL 10–2 (SuperSonic Imagine, Aix-en-Provence, France) multifrequency linear transducer. The superficial musculoskeletal setting used was the ‘general’ default mode. The depth was adjusted according to the scanned MT, and the focus was adjusted to the middle of the muscle layer. The frame rate was kept beyond 30 Hz.
The room temperature was maintained at 21 to 24 °C. After entering the examination room, each participant was asked to have a quiet rest, and the measuring sites were marked during this time. First, Sites 1, 3, 4, 5, 7, and 9 were scanned with the participant in a supine position. Then, Sites 2, 8, and 10 were scanned with the participant in a left lateral position. Finally, Sites 6 and 11 were scanned with the participant in a prone position. Throughout the procedure, the right upper limb of the participant was kept close to their trunk, and their palm was kept facing frontward.
Throughout the scanning process, the probe was coated with sufficient coupling agent and kept vertical to the skin without applying any pressure, allowing the gap between the skin layer and the probe to be filled with coupling agent. The muscle layer was observed continuously to check whether it was compressed. After obtaining the ultrasound images at the target sites, we measured the thickness of the selected muscles. The epimysium was not included in the measurement, and all measurements were recorded in mm.
BIA measurement
The participants were instructed not to eat, exercise, or drink water within the 2 hours before the BIA test, and they were asked to empty their bladder before measurement. During measurement, participants were instructed to remove metal accessories such as watches, stand barefoot on the pedal, fit the round sensor onto their heel, straighten and separate their arms, and hold the electrodes with both hands. During the test, participants were asked to keep their armpit and arm at an approximate 15-degree angle and to keep their inner thighs from touching each other. Participants were asked to remain quiet for the entire duration of the test, and each participant was tested only once.
Statistical analysis
Statistical analyses were performed using SPSS 24.0 software (IBM, Armonk, NY, USA). The Shapiro-Wilk test was used to confirm the normality of continuous variables. Continuous variables that conformed to a normal distribution were described as means ± standard deviations, and a t-test was used to assess differences between males and females. For other variables, the median (25th percentile to 75th percentile) was used, and a Mann-Whitney U test was applied for comparison of males and females in the case of nonnormally distributed continuous variables. The Pearson correlation test was used to test the correlation between MT measured using ultrasound and BIA results. Lastly, hierarchical multiple linear regression was used to establish a predictive model of MT under ultrasound for SMI, while entering age and sex into the regression and using a stepwise method to screen muscle layer thickness at 11 sites. Using this strategy, several sites that contributed to the SMI estimation were identified, and the site that made the biggest contribution was pinpointed. When sex was entered into the regression, values of 1 and 0 were assigned for males and females, respectively. After modeling, the parameters which contributed to the model were retained in the multiple linear regression. For multiple regression, there are many ways to calculate the required sample size, and results vary greatly depending on the method used to calculate the sample size (18). One particular approach recommends that the sample size be a minimum of five times the number of independent variables (19). A P value of <0.05 was used to indicate statistical significance for two-sided tests. A scatter plot of BIA results with age distribution among participants was drawn using R software (The R Foundation for Statistical Computing, Vienna, Austria) with the ggplot2 package.
Results
General characters of study participants
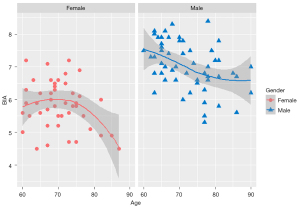
A total of 103 participants were included in this study, including 57 males and 46 females. No data were excluded from the analysis. The results for body mass index (BMI), age, and SMI are shown in Table 1 and Figure 2. No significant differences existed in age and BMI between male and female participants, but the SMI obtained by BIA for males was significantly higher than that obtained for females. Also, as depicted in Figure 2, the SMI exhibited a downward trend with age.
Table 1
| Characteristics | Male | Female | P value |
|---|---|---|---|
| Age (years) | 71.0 (65.5–78.0) | 70.0 (66.0–77.0) | 0.224 |
| BMI (kg/m2) | 23.3±3.0 | 23.3±3.8 | 0.994 |
| SMI (kg/m2) | 7.03±0.73 | 5.84±0.72 | <0.001 |
BMI, body mass index; SMI, skeletal muscle mass index.
Correlations between MT and SMI
Table 2 shows the MT at each site in males and females. In this study, the MT was significantly thicker in males than in females across all sites.
Table 2
| Sites | Muscle thickness (mm) | P value | |
|---|---|---|---|
| Male | Female | ||
| Site 1 | 28.9±4.0 | 23.8±4.0 | <0.001 |
| Site 2 | 27.3±7.9 | 23.1±8.3 | 0.014 |
| Site 3 | 17.6±3.0 | 13.9±2.5 | <0.001 |
| Site 4 | 35.6±2.9 | 30.6±2.7 | <0.001 |
| Site 5 | 7.9±1.9 | 6.3±1.5 | <0.001 |
| Site 6 | 13.4±3.5 | 11.9±3.3 | 0.034 |
| Site 7 | 29.7±7.8 | 26.2±7.1 | 0.020 |
| Site 8 | 18.7±4.8 | 14.9±3.7 | <0.001 |
| Site 9 | 26.3±2.6 | 23.1±3.0 | <0.001 |
| Site 10 | 54.1±8.0 | 50.3±6.9 | 0.015 |
| Site 11 | 22.6±4.5 | 20.2±4.0 | 0.007 |
Site 1: biceps and brachialis; Site 2: triceps; Site 3: flexor pollicis longus, flexor digitorum superficialis, and brachioradialis; Site 4: flexor digitorum profundus, flexor digitorum superficialis, and flexor carpi radialis; Site 5: rectus abdominis; Site 6: latissimus dorsi and external intercostal; Site 7: rectus femoris and vastus intermedius; Site 8: vastus lateralis and vastus intermedius; Site 9: tibialis anterior; Site 10: gastrocnemius, soleus, and flexor digitorum longus; Site 11: latissimus dorsi, serratus posterior inferior, longissimus thoracis, spinalis thoracis, and multifidus.
The correlations between MT at each site and the SMI measured using BIA are shown in Figure 3. In the heat map, white represents a correlation coefficient of 0, and the darkest color represents a correlation coefficient of 0.8: the larger the correlation coefficient, the darker the color. There were sex differences in the correlations between different sites and the SMI. For instance, there was no correlation between the MT of Site 2 and SMI in males, but there was such a correlation in females. The MTs of Sites 6, 8, and 11 showed the opposite result: they were related to SMI in males but not in females.

There were also intrasexual differences in the correlations between different sites and the SMI. In males, the highest correlation was found for Site 7 (rectus femoris and intermedius femoris), with a correlation of 0.719. The lowest correlation in males existed for Site 2, with a correlation of 0.383. However, the correlation of Site 7 and SMI in females was only 0.585. Site 3 (flexor pollicis longus, flexor digitorum superficialis, and brachioradialis of the forearm) showed the highest correlation in females, with a correlation of 0.733.
Establishment of muscle mass estimation algorithms
Based on the correlation analysis results, hierarchical multiple linear regression was employed in an effort to establish a predictive model of MT under ultrasound for SMI. The MTs at Site 1 (biceps), Site 3 (flexor pollicis longus, flexor digitorum superficialis, and brachioradialis), Site 7 (rectus femoris and vastus intermedius), and Site 9 (tibialis anterior) were found to contribute to the model, with Site 7 making the largest contribution.
Based on the above results, a one-site prediction model suitable for rapid muscle mass estimation and a four-site predictive model with the highest diagnostic accuracy were established. The one-site and four-site prediction models are algorithms 1 and 2, respectively. The results are shown in Table 3. The R2 values were 0.697 and 0.806 for algorithms 1 and 2, respectively. The standard error of estimate (SEE) values were 0.519 and 0.404 kg/m2 for algorithms 1 and 2, respectively.
Table 3
| Designations | R2 | Algorithm (kg/m2) | SEE (kg/m2) |
|---|---|---|---|
| Algorithm 1 | 0.701 | Estimated-SMI=6.019+1.031×Sex−0.025×Age (years)+0.060×MT of Site7 (mm) | 0.519 |
| Algorithm 2 | 0.819 | Estimated-SMI =3.143+0.474×Sex−0.020×Age (years)+0.044×MT of Site1 (mm)+0.065×MT of Site3 (mm)+0.025×MT of Site7 (mm)+0.064×MT of Site9 (mm) | 0.404 |
SMI, skeletal muscle mass index; MT, muscle thickness; SEE, standard error of estimate; Sex: male =1; female =0; Site 1: biceps; Site 3: flexor pollicis longus, flexor digitorum superficialis, and brachioradialis; Site 7: rectus femoris and vastus intermedius; Site 9: tibialis anterior.
Discussion
Our results show that SMI was correlated with MT measured using ultrasound at some sites, and that there were sex differences in the correlations. This study has demonstrated that SMI can be quantitatively estimated by fitting algorithms that include sex, age, and MT, suggesting that ultrasound can be an efficient assistive method for diagnosing sarcopenia.
Sarcopenia is a syndrome of decreased muscle mass and muscle function in older adults (1,2). In the era of population ageing, sarcopenia and related ensuing falls and disability load a huge burden of disease onto society and families. Extremely large numbers of older adults are at risk of developing sarcopenia, and existing strains on medical resources demand higher requirements for disease screening.
At present, CT and MRI are the gold standards for non-invasive assessment of skeletal muscle mass according to the European Working Group on Sarcopenia in Older People. However, their cost and lack of defined cut-off values limit their clinical application. DXA, is the alternative preferred method, but its use is not yet feasible for community-dwelling older adults. Therefore, BIA has become the mainstream method for muscle mass estimation in adults at risk for sarcopenia. However, BIA still has some technical limitations, and BIA equipment is not available in many primary medical institutions. Therefore, it is extremely important to find a supplementary method for muscle mass estimation which can be implemented more widely. The application of ultrasound in sarcopenia has been extensively studied worldwide. Using ultrasound, Abe et al. found that combining the MT at eight sites throughout the body could predict the total body muscle mass with an error of 1.13 kg (20), and their subsequent research showed that the error in prediction using a single site on the forearm was about 1.95 kg (21).
In the current study, we referred to the inclusion criteria of the Asian Working Group for Sarcopenia 2019 for patients at risk for sarcopenia. Our aim was to include the broadest possible scope of older individuals with potential risk of sarcopenia after screening. Consequently, a considerable number of the older adults recruited did not have sarcopenia or even a low SMI, and the average SMIs for the male and female participants were 7.03 and 5.84 kg/m2, respectively. Given that the objective of our research was to establish a muscle mass estimation algorithm which is applicable to older adults, we believe it was necessary to include relatively healthy participants in this study.
In our study, male SMI and MT at all studied sites were significantly higher than those of females, and the SMI showed a downward trend with age. We also found sex differences in the correlations between SMI and MT at different sites. Overall, the correlation between MT and SMI was stronger in male participants. In males, the MT of 10 out of the 11 sites scanned was correlated with the SMI. In contrast, for females, there was no significant correlation between the MT and SMI at 3 out of the 11 sites. Even for the MT at the same site, the correlations with SMI differed between men and women. We speculate that these differences may be due to the following reasons. Firstly, from the perspective of the microenvironment, skeletal muscle is affected by a variety of hormones, including testosterone, glucocorticoids, growth hormone (GH), and insulin-like growth factor-1 (IGF-1), and there are six differences in the levels of these hormones and corresponding receptor levels. Studies have demonstrated that excess glucocorticoids may cause muscle weakness and atrophy with age through increased levels of the glucocorticoid-amplifying enzyme 11 beta-hydroxysteroid dehydrogenase type 1 (11βHSD1) in muscle (22), the expression of which was found to be increased in older women, with no age-associated differences observed in men (23). In addition to this, the GH/IGF-1 axis has been proven to be correlated with body composition, function, and metabolism (24,25), and the correlation between muscle power and IGF-1 has been reported to only exist in older women and not in men (26). From a macroperspective, muscle loss with age has been proven to be site-specific (9,27,28), which means that the muscles at some sites may atrophy at a faster rate, resulting in differences in the correlations between SMI and MT at different sites. Moreover, sex differences in morphology and muscle loss with age have been observed and verified in previous studies (27,29), which leads to differences in the correlation between regional MT and the total muscle mass of men and women. Our results suggest that sex is a factor that must be carefully considered when using ultrasound to estimate muscle mass.
Based on the correlation and multiple regression modeling results, we constructed a one-site ultrasound algorithm designed for quick estimation of muscle mass and a four-site ultrasound algorithm with the highest accuracy. The four-site ultrasound algorithm achieved an SEE value of 0.404 kg/m2, and the one-site ultrasound algorithm achieved an SEE value of 0.519 kg/m2. Apart from its accuracy, the main advantage of ultrasound lies in its availability and efficiency. Ultrasound equipment is available in almost all primary medical institutions, and portable ultrasound machines can further broaden the usage scenarios. For one-site ultrasound scanning, the process is simple and time-saving, taking less than a minute. Moreover, the reliability and validity of MT measurement have been verified in a considerable number of studies (30-33). Although BIA also has many portable solutions, it still has some technical limitations, including the fact that results are affected by hydration status and water distribution in the body (34). Also, for older adults who work in agriculture in rural areas or manual labor, thickened callosities in palms or feet may significantly affect measurement results (35). In well-equipped medical institutions, these influencing factors can be detected in time and controlled by medical personnel. However, in the community-screening setting, it is difficult for operators to conduct a strict assessment of these potential influencing factors for every older adult, which limits the use of BIA as a screening tool in the community.
It cannot be concluded that sites that were not included in our algorithm are of no value in sarcopenia. Our research focused on the quantitative estimation of muscle mass, looking to determine sites with predictive value for muscle mass; however, we are aware of some studies on the value of muscles at other sites. Ido et al. (36), for instance, found that ultrasound-derived abdominal MT could provide a better assessment of sarcopenia in patients with obesity; however, such patients were not included in our research. Also, Fukumoto et al. (37) found that calf MT can predict low SMI for sarcopenia and is more accurate than the quadriceps femoris. However, our results showed that the quadriceps may be more valuable than calf muscles in the quantitative estimation of muscle mass. We speculate that the calf muscles may atrophy obviously with age, but this atrophy may be nonlinear and not in parallel to the overall SMI decline, making the calf muscles a good indicator for predicting low SMI but not a suitable indicator for quantitative estimation of SMI. Furthermore, the implementation details of ultrasound scanning in the two studies were different: the participants in our study were in a supine or lateral position, while the participants in the former study were in a sitting position. This factor may also be one of the sources of the difference in our observations. In fact, every muscle has a unique medical value due to muscles having different physiological functions. Further research is needed to verify whether the muscles at other sites in our study have a profound effect on the occurrence and development of sarcopenia.
Regarding the ultrasound scanning program used in this study, there are still some limitations, such as the inability to quantitatively determine the state of fat or fibrous infiltration in muscles, which may result in the overestimation of muscle mass. Although some studies have used echo intensity to estimate fat infiltration (38), the echo intensity of the muscle layer is affected by the thickness of the subcutaneous fat layer (39). Even with the same ultrasonographic settings, the echo intensity of the target muscle could be different. Moreover, some studies have also found that there may be differences in the rate of muscle atrophy at different sites and between patients of different races (40,41). This suggests that when ultrasound is used as a screening tool in different countries and regions, the parameters of the predicting algorithm should be corrected or adjusted separately based on the data of the local population.
Conclusions
MT measured by ultrasound at some sites is correlated with SMI measured by BIA, and the correlation is different in males and females. When sex and age were included in the algorithm, the MTs of Site 1 (biceps), Site 3 (flexor pollicis longus, flexor digitorum superficialis, and brachioradialis), Site 7 (rectus femoris and intermedius femoris), and Site 9 (tibialis anterior) were valuable in estimating the SMI, with Site 7 being the most valuable parameter among them. Ultrasound-derived algorithms can achieve a satisfying fitting effect and can provide new solutions for muscle mass estimation in older adults.
Acknowledgments
Funding: This study was funded by the 1·3·5 Project for Disciplines of Excellence Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2020HXFH001); the Chinese National Science and Technology Pillar Program (No. 2020YFC2005600); and the National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (No. Y2021LC002).
Footnote
Reporting Checklist: The authors completed the MDAR checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-21-685/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-685/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the West China Hospital of Sichuan University (No. 2020[258]), and informed consent obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni MEuropean Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95-101. [Crossref] [PubMed]
- Kitamura A, Seino S, Abe T, Nofuji Y, Yokoyama Y, Amano H, Nishi M, Taniguchi Y, Narita M, Fujiwara Y, Shinkai S. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle 2021;12:30-8. [Crossref] [PubMed]
- Zeng Y. Towards Deeper Research and Better Policy for Healthy Aging --Using the Unique Data of Chinese Longitudinal Healthy Longevity Survey. China Economic J 2012;5:131-49. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. [Crossref] [PubMed]
- Kyle UG, Genton L, Hans D, Pichard C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin Nutr 2003;22:537-43. [Crossref] [PubMed]
- Sergi G, De Rui M, Veronese N, Bolzetta F, Berton L, Carraro S, Bano G, Coin A, Manzato E, Perissinotto E. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin Nutr 2015;34:667-73. [Crossref] [PubMed]
- Han DS, Wu WT, Hsu PC, Chang HC, Huang KC, Chang KV. Sarcopenia Is Associated With Increased Risks of Rotator Cuff Tendon Diseases Among Community-Dwelling Elders: A Cross-Sectional Quantitative Ultrasound Study. Front Med (Lausanne) 2021;8:630009. [Crossref] [PubMed]
- Abe T, Sakamaki M, Yasuda T, Bemben MG, Kondo M, Kawakami Y, Fukunaga T. Age-related, site-specific muscle loss in 1507 Japanese men and women aged 20 to 95 years. J Sports Sci Med 2011;10:145-50. [PubMed]
- Abe T, Kondo M, Kawakami Y, Fukunaga T. Prediction equations for body composition of Japanese adults by B-mode ultrasound. Am J Hum Biol 1994;6:161-70. [Crossref] [PubMed]
- Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300-307.e2. [Crossref] [PubMed]
- Hides JA, Cooper DH, Stokes MJ. Diagnostic ultrasound imaging for measurement of the lumbar multifidus muscle in normal young adults. Physiother Theory Pract 1992;8:19-26. [Crossref]
- Gibbons D, Ahern DP, Curley AE, Kepler CK, Butler JS. Impact of Sarcopenia on Degenerative Lumbar Spondylosis. Clin Spine Surg 2021;34:43-50. [Crossref] [PubMed]
- Sanada K, Kearns CF, Midorikawa T, Abe T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol 2006;96:24-31. [Crossref] [PubMed]
- Minetto MA, Caresio C, Menapace T, Hajdarevic A, Marchini A, Molinari F, Maffiuletti NA. Ultrasound-Based Detection of Low Muscle Mass for Diagnosis of Sarcopenia in Older Adults. PM R 2016;8:453-62. [Crossref] [PubMed]
- Turton P, Hay R, Taylor J, McPhee J, Welters I. Human limb skeletal muscle wasting and architectural remodeling during five to ten days intubation and ventilation in critical care - an observational study using ultrasound. BMC Anesthesiol 2016;16:119. [Crossref] [PubMed]
- Boutin RD, Katz JR, Chaudhari AJ, Yabes JG, Hirschbein JS, Nakache YP, Seibert JA, Lamba R, Fananapazir G, Canter RJ, Lenchik L. Association of adipose tissue and skeletal muscle metrics with overall survival and postoperative complications in soft tissue sarcoma patients: an opportunistic study using computed tomography. Quant Imaging Med Surg 2020;10:1580-9. [Crossref] [PubMed]
- Green SB. How Many Subjects Does It Take To Do A Regression Analysis. Multivariate Behav Res 1991;26:499-510. [Crossref] [PubMed]
- Fidell LS. Using Multivariate Statistics. 6th edition. 2012.
- Abe T, Thiebaud RS, Loenneke JP, Young KC. Prediction and validation of DXA-derived appendicular lean soft tissue mass by ultrasound in older adults. Age (Dordr) 2015;37:114. [Crossref] [PubMed]
- Abe T, Loenneke JP, Thiebaud RS. The use of ultrasound for the estimation of muscle mass: one site fits most? J Cachexia Sarcopenia Muscle 2018;9:213-4. [Crossref] [PubMed]
- Kilgour AH, Gallagher IJ, MacLullich AM, Andrew R, Gray CD, Hyde P, Wackerhage H, Husi H, Ross JA, Starr JM, Chapman KE, Fearon KC, Walker BR, Greig CA. Increased skeletal muscle 11βHSD1 mRNA is associated with lower muscle strength in ageing. PLoS One 2013;8:e84057. [Crossref] [PubMed]
- Hassan-Smith ZK, Morgan SA, Sherlock M, Hughes B, Taylor AE, Lavery GG, Tomlinson JW, Stewart PM. Gender-Specific Differences in Skeletal Muscle 11β-HSD1 Expression Across Healthy Aging. J Clin Endocrinol Metab 2015;100:2673-81. [Crossref] [PubMed]
- Borst SE, Lowenthal DT. Role of IGF-I in muscular atrophy of aging. Endocrine 1997;7:61-3. [Crossref] [PubMed]
- Giovannini S, Marzetti E, Borst SE, Leeuwenburgh C. Modulation of GH/IGF-1 axis: potential strategies to counteract sarcopenia in older adults. Mech Ageing Dev 2008;129:593-601. [Crossref] [PubMed]
- Kostka T, Arsac LM, Patricot MC, Berthouze SE, Lacour JR, Bonnefoy M. Leg extensor power and dehydroepiandrosterone sulfate, insulin-like growth factor-I and testosterone in healthy active elderly people. Eur J Appl Physiol 2000;82:83-90. [Crossref] [PubMed]
- Kim KM, Lim S, Oh TJ, Moon JH, Choi SH, Lim JY, Kim KW, Park KS, Jang HC. Longitudinal Changes in Muscle Mass and Strength, and Bone Mass in Older Adults: Gender-Specific Associations Between Muscle and Bone Losses. J Gerontol A Biol Sci Med Sci 2018;73:1062-9. [Crossref] [PubMed]
- Abe T, Patterson KM, Stover CD, Geddam DA, Tribby AC, Lajza DG, Young KC. Site-specific thigh muscle loss as an independent phenomenon for age-related muscle loss in middle-aged and older men and women. Age (Dordr) 2014;36:9634. [Crossref] [PubMed]
- Jensen RK. Human morphology: its role in the mechanics of movement. J Biomech 1993;26:81-94. [Crossref] [PubMed]
- Ishida Y, Carroll JF, Pollock ML, Graves JE, Leggett SH. Reliability of B-mode ultrasound for the measurement of body fat and muscle thickness. Am J Hum Biol 1992;4:511-20. [Crossref] [PubMed]
- Tillquist M, Kutsogiannis DJ, Wischmeyer PE, Kummerlen C, Leung R, Stollery D, Karvellas CJ, Preiser JC, Bird N, Kozar R, Heyland DK. Bedside ultrasound is a practical and reliable measurement tool for assessing quadriceps muscle layer thickness. JPEN J Parenter Enteral Nutr 2014;38:886-90. [Crossref] [PubMed]
- Øverås CK, Myhrvold BL, Røsok G, Magnesen E. Musculoskeletal diagnostic ultrasound imaging for thickness measurement of four principal muscles of the cervical spine -a reliability and agreement study. Chiropr Man Therap 2017;25:2. [Crossref] [PubMed]
- Chang PH, Chen YJ, Chang KV, Wu WT, Özçakar L. Ultrasound measurements of superficial and deep masticatory muscles in various postures: reliability and influencers. Sci Rep 2020;10:14357. [Crossref] [PubMed]
- Kushner RF, Gudivaka R, Schoeller DA. Clinical characteristics influencing bioelectrical impedance analysis measurements. Am J Clin Nutr 1996;64:423S-7S. [Crossref] [PubMed]
- Roekenes J, Strømmen M, Kulseng B, Martins C. The Impact of Feet Callosities, Arm Posture, and Usage of Electrolyte Wipes on Body Composition by Bioelectrical Impedance Analysis in Morbidly Obese Adults. Obes Facts 2015;8:364-72. [Crossref] [PubMed]
- Ido A, Nakayama Y, Ishii K, Iemitsu M, Sato K, Fujimoto M, Kurihara T, Hamaoka T, Satoh-Asahara N, Sanada K. Ultrasound-Derived Abdominal Muscle Thickness Better Detects Metabolic Syndrome Risk in Obese Patients than Skeletal Muscle Index Measured by Dual-Energy X-Ray Absorptiometry. PLoS One 2015;10:e0143858. [Crossref] [PubMed]
- Fukumoto Y, Ikezoe T, Taniguchi M, Yamada Y, Sawano S, Minani S, Asai T, Kimura M, Ichihashi N. Cut-off Values for Lower Limb Muscle Thickness to Detect Low Muscle Mass for Sarcopenia in Older Adults. Clin Interv Aging 2021;16:1215-22. [Crossref] [PubMed]
- Pillen S, Tak RO, Zwarts MJ, Lammens MM, Verrijp KN, Arts IM, van der Laak JA, Hoogerbrugge PM, van Engelen BG, Verrips A. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol 2009;35:443-6. [Crossref] [PubMed]
- Neto Müller J, Lanferdini FJ, Passos Karam JY, de Brito Fontana H. Examination of the confounding effect of subcutaneous fat on muscle echo intensity utilizing exogenous fat. Appl Physiol Nutr Metab 2021;46:473-8. [Crossref] [PubMed]
- Abe T, Kawakami Y, Kondo M, Fukunaga T. Comparison of ultrasound-measured age-related, site-specific muscle loss between healthy Japanese and German men. Clin Physiol Funct Imaging 2011;31:320-5. [Crossref] [PubMed]
- Reimers CD, Harder T, Saxe H. Age-related muscle atrophy does not affect all muscles and can partly be compensated by physical activity: an ultrasound study. J Neurol Sci 1998;159:60-6. [Crossref] [PubMed]


