Changes in mitral annular morphology and function in young patients with type 1 diabetes mellitus—results from the three-dimensional speckle tracking echocardiographic MAGYAR-Path Study
Introduction
The mitral annulus (MA) separates the left atrium (LA) from the left ventricle (LV) and plays an important role in promoting LA and LV filling and emptying (1). The geometric shape of the MA approximates a hyperbolic paraboloid, which can be likened to a saddle (2). Alterations in MA size and function could be demonstrated in different clinical scenarios including different cardiomyopathies and ischaemic heart disease (3,4). Diabetes mellitus is a metabolic disease characterized by chronic hyperglycemia which causes micro- and macrovascular complications. There is a high prevalence of DM among subjects with calcific aortic stenosis and MA calcification, as well (5). However, it would be important if early changes in MA morphology and function could be detected in type 1 diabetes mellitus (T1DM) before MA calcification occurs. Three-dimensional speckle tracking echocardiography (3DSTE) is a new clinical tool validated for LA and LV chamber quantifications and functional featuring (6,7). Moreover, its capability in reproducible assessment of MA dimensions allowing simple calculation of LA ejection force has also been demonstrated (8). The present study designed to evaluate MA size and functional properties in young T1DM patients by 3DSTE and to compare their results to matched healthy controls.
Patients and methods
Patients population
The study comprised 18 non-obese patients with T1DM (mean age: 33.0±8.0 years, 8 males, duration of T1DM: 17.3±10.2 years, HbA1c: 8.2±1.8%, daily insulin dose: 37.6±7.1 IU, body mass index: 24.1±2.4 kg/m2) with subcutaneous insulin pump-treatment without cardiac symptoms. All T1DM patients with such treatment at the time of writing of the manuscript were involved into the present study from the patient population of the outpatient clinic of the diabetology division of the 1st Department of Medicine at the University of Szeged, Hungary. Their results were compared to 20 age- and gender-matched healthy controls (mean age: 37.8±10.9 years, 9 males, body mass index: 25.3±1.2 kg/m2). All subjects with known diseases or any condition which could affect the results were excluded from the control group. Healthy cases were primarily recruited on voluntary bases from the student and young fellows’ population at our university. All T1DM patients and controls had undergone complete two-dimensional (2D) Doppler echocardiography and 3DSTE. American Diabetes Association (9) and World Health Organization (10) criteria were used for definition of T1DM. Systolic or diastolic elevation of the blood pressure (>140/90 mmHg) or ongoing antihypertensive therapy were used for definition of hypertension. A total cholesterol level >5.0 mmol/L or current treatment with lipid-lowering medications were used for definition of hypercholesterolaemia. Weight and height of all subjects were recorded and body surface area (BSA) was calculated by the standard formula (weight0.425 in kilograms × height0.725 in centimeters ×0.007184). All subjects have been included in the MAGYAR-Path Study (Motion Analysis of the heart and Great vessels bY three-dimensionAl speckle-tRacking echocardiography in Pathological cases) (11-13). This has been organized at the Cardiology Center of the University of Szeged, Hungary to evaluate clinical significance of 3DSTE-derived parameters in pathological cases (‘magyar’ means ‘Hungarian’ in Hungarian language). The Ethics Committee of the University of Szeged, Hungary, approved the study and informed consent was obtained from all participants (14).
2D echocardiography
All subjects underwent conventional 2D Doppler echocardiography, performed using a commercially available ultrasonic device (Toshiba ArtidaTM; Toshiba Medical Systems, Tokyo, Japan) with a PST-30SBP (1-5 MHz) phased-array transducer. LV diameters and volumes and LA diameter were measured in parasternal long-axis view and the method of Teichholz et al. was used for calculation of ejection fraction (15). Visual grading was used for quantification of mitral regurgitation (MR). MA diameter (MAD2D) was obtained from an apical 4-chamber view at end-diastole (just before mitral valve closure).
3DSTE-derived mitral annular measurements
3DSTE-derived pyramidal datasets were acquired in the apical view using a fully sampled PST-25SX matrix-array transducer (Toshiba Medical Systems, Tokyo, Japan) (7,8,11-13,16). The depth and angle were adjusted in order to reach optimal temporal and spatial resolution. Full volume images were stitched from six subvolumes in six consecutive cardiac cycles. Measurements were performed using 3D Wall Motion Tracking software version 2.7. The apical two-chamber (AP2CH) and four-chamber (AP4CH) views and three short-axis views at different LV levels from the base to the apex were automatically selected from the 3D echocardiographic pyramidal dataset at end-diastole by the software. The Q7 short axis view was positioned at the level of MA in order to calculate morphological MA parameters. AP2CH and AP4CH views helped to find optimal endpoints of the MA. Measurements were made both at end-diastole and end-systole. The following measures were obtained (1,3,4,17) (Figure 1):
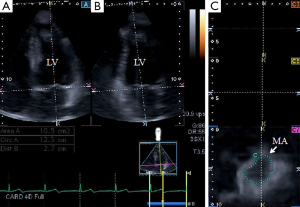
- MA diameter (MAD3D), defined as the perpendicular line drawn from the peak of MA curvature to the middle of the straight MA border both at systole and diastole;
- MA diameter index (MADI3D), defined as MAD3D/BSA;
- MA area (MAA3D), measured at end-diastole (just before mitral valve closure) and at end-systole (just before mitral valve opening);
- MA area index (MAAI3D), defined as MAA3D/BSA;
- MA perimeter (MAP3D);
- MA perimeter index (MAPI3D), defined as MAP3D/BSA;
- MA fractional shortening (MAFS3D), defined as: [(end-diastolic MAD3D—end-systolic MAD3D)/end-diastolic MAD3D ×100];
- MA fractional area change (MAFAC3D), defined as: [(end-diastolic MAA3D—end-systolic MAA3D)/end-diastolic MAA3D ×100].
Statistical analysis
Quantitative data were expressed as mean ± standard deviation. For all analyses, two-sided P<0.05 was defined as statistical significance. An independent-sample t-test was utilised for comparison between two groups. Categorical variables were compared using chi-square test and Fischer’s exact test. Pearson’s coefficient was calculated to examine correlations between MAD2D and MAD3D and MA and LV functional parameters. Commercially available MedCalc software was used for statistical calculations (MedCalc, Mariakerke, Belgium).
Results
Demographic and 2D echocardiographic data
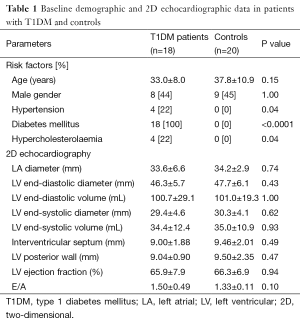
No significant differences could be demonstrated in demographic and standard echocardiographic parameters between the groups (Table 1). No T1DM patients or healthy controls showed significant (≥ grade 1) MR. No calcification could be demonstrated in any of T1DM patients. Diastolic MAD2D of T1DM patients and controls was 2.70±0.21 cm and 2.47±0.26 cm, respectively (P=0.05). Systolic MAD2D of T1DM patients and controls was 2.01±0.15 cm and 2.03±0.18 cm, respectively (P=0.81).
Full table
3DSTE-derived MA parameters
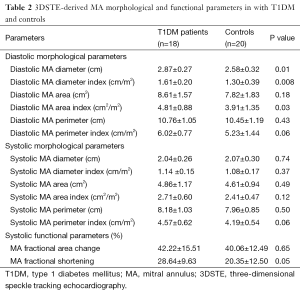
Significantly enlarged diastolic MA diameter, MA diameter index and MA area index could be demonstrated together with augmented MAFS3D in T1DM patients as compared to age- and gender matched healthy controls (Table 2).
Full table
Correlations between MAD2Dvs. MAD3D
Measurements of MAD2D and MAD3D were well correlated both in T1DM patients and controls (r=0.80, P<0.01 and r=0.81, P<0.01, respectively).
MA function and LV function
MAFAC3D did not correlate with LVEF2D in any subjects or patients. MAFS3D correlated with LVEF2D in control subjects (r=0.50, P=0.03).
Discussion
Morphologic and functional aspects of the MA in young patients with T1DM were assessed by 3DSTE in the present work. To the best of authors’ knowledge this is the first study in which early alterations in MA size and function could be detected in young T1DM patients without MR/valvular calcifications by 3DSTE. Enlarged diastolic MA dimensions and augmented MA function could be demonstrated as compared to matched healthy controls.
Several methodologies including different echocardiographic techniques (1,18,19), computer tomography (CT) (20) and cardiac magnetic resonance imaging (cMRI) (21) could be used for non-invasive quantification of MA size and function. Volumetric real-time 3D echocardiography (RT3DE) and real-time 3D transesophageal echocardiography (RT3DTEE) has been demonstrated to be useful for non-(semi-)invasive estimation of MA morphology and functional properties (1,3,4,17,19). 3DSTE seems to be a promising tool based on “block-matching” algorithm by strain analysis for evaluation of atrial and ventricular volumes, strains, rotational and dyssynchrony parameters (7,8,12,13,16). In a recent study capability of 3DSTE in reproducible assessment of MAD and MAA has also been confirmed (8). 3DSTE-derived MA parameters, especially MAD however, showed somewhat lower values as demonstrated before for that of healthy subjects by RT3DE (17). However, in other 2D echocardiographic studies, MAA values were in the similar range as in the present 3DSTE study (22,23). Our results could be explained by 3DSTE-related technical limitations including low image quality and temporal and spatial resolutions. Moreover, definition of normal MA is quite variable, probably caused by the complex 3D saddle-shaped geometry of the MA, which could give an opportunity for inadequate positioning of the cross-sectional plane on it during measurements (17). These facts could lead for underestimation of MA parameters. It should also be taken into consideration that mostly young patients without any risk factors or other conditions were involved into the study. Theoretically there were no reasons for MA dilatation or functional alterations in these cases. Results could highlight on the importance on additional validation of the method which requires serial comparative studies with other imaging methodologies.
In recent studies, despite MA dilatation in patients with hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM), MA function was found to be augmented in HCM patients and impaired in DCM patients as assessed by RT3DE-derived MAFAC and MAFS (3). Similar alterations could be detected in noncompaction cardiomyopathy (NCCM): MA dilatation was associated with decreased MA functional properties by RT3DE (4). It is also known, that calcification within the MA results from a degenerative process in the cardiovascular fibrous skeleton, which is reported to be accelerated by several risk factors including DM (24,25). With the present study, MA dilatation and compensating enhancement in MA function could be demonstrated in young T1DM patients before MA calcification would have been developed. These findings could draw our attention on the fact, that valvular remodeling starts even in younger ages with compensating functional alterations in T1DM. However, further studies are warranted to confirm our findings focusing on clinical implications.
Limitation section
The following important limitations should be taken into consideration over mentioned above:
- 3DSTE has been demonstrated to be suitable for the evaluation of volumetric, strain, rotational and dyssynchrony parameters of different heart chambers. However, the present study did not aim to analyse these parameters;
- Higher grade of MR should have affected results. However, none of T1DM patients and controls had ≥ grade 1 MR;
- A limited number of T1DM patients and controls were examined and compared in this study. This fact should be taken into consideration when interpreting results;
- Patients in non-sinus rhythm would have been excluded due to the nature of 3DSTE. However, all subjects were in sinus rhythm;
- Further validation studies are warranted for the usefulness of 3DSTE in the evaluation of valvular morphology and functional properties.
Conclusions
Early alterations in MA size and function could be demonstrated in young patients with T1DM by 3DSTE.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nemes A, Geleijnse ML, Soliman OI, Vletter WB, McGhie JS, Forster T, Ten Cate FJ. Evaluation of the mitral valve by transthoracic real-time three-dimensional echocardiography. Orv Hetil 2010;151:854-63. [PubMed]
- Silbiger JJ. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am Heart J 2012;164:163-76. [PubMed]
- Anwar AM, Soliman OI, Nemes A, Germans T, Krenning BJ, Geleijnse ML, Van Rossum AC, ten Cate FJ. Assessment of mitral annulus size and function by real-time 3-dimensional echocardiography in cardiomyopathy: comparison with magnetic resonance imaging. J Am Soc Echocardiogr 2007;20:941-8. [PubMed]
- Nemes A, Anwar AM, Caliskan K, Soliman OI, van Dalen BM, Geleijnse ML, ten Cate FJ. Non-compaction cardiomyopathy is associated with mitral annulus enlargement and functional impairment: a real-time three-dimensional echocardiographic study. J Heart Valve Dis 2008;17:31-5. [PubMed]
- Cronin CC, O'Sullivan DJ, Mitchell TH. Medial arterial calcification, calcific aortic stenosis and mitral annular calcification in a diabetic patient with severe autonomic neuropathy. Diabet Med 1996;13:768-70. [PubMed]
- Kleijn SA, Brouwer WP, Aly MF, Rüssel IK, de Roest GJ, Beek AM, van Rossum AC, Kamp O. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur Heart J Cardiovasc Imaging 2012;13:834-9. [PubMed]
- Nemes A, Domsik P, Kalapos A, Lengyel C, Orosz A, Forster T. Comparison of three-dimensional speckle tracking echocardiography and two-dimensional echocardiography for evaluation of left atrial size and function in healthy volunteers (results from the MAGYAR-Healthy study). Echocardiography 2014;31:865-71. [PubMed]
- Piros GA, Domsik P, Kalapos A, Lengyel C, Orosz A, Forster T, Nemes A. Left atrial ejection force correlates with left atrial strain and volume-based functional properties as assessed by three-dimensional speckle-tracking echocardiography (From the MAGYAR-Healthy Study). Rev Port Cardiol. In press.
- American Diabetes Association. Available online: www.diabetes.org
- World Health Organization. Available online: www.who.int/diabetes
- Domsik P, Kalapos A, Lengyel C, Orosz A, Forster T, Nemes A. Correlations between mitral annular and left atrial function as assessed by three-dimensional speckle-tracking echocardiography in healthy volunteers. Results from the MAGYAR-Healthy Study. Orv Hetil 2014;155:1517-23. [PubMed]
- Domsik P, Kalapos A, Chadaide S, Sepp R, Hausinger P, Forster T, Nemes A. Three-dimensional speckle tracking echocardiography allows detailed evaluation of left atrial function in hypertrophic cardiomyopathy--insights from the MAGYAR-Path Study. Echocardiography 2014;31:1245-52. [PubMed]
- Kalapos A, Domsik P, Forster T, Nemes A. Left ventricular strain reduction is not confined to the noncompacted segments in noncompaction cardiomyopathy-insights from the three-dimensional speckle tracking echocardiographic MAGYAR-Path study. Echocardiography 2014;31:638-43. [PubMed]
- WMA. WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. Available online: http://www.wma.net/en/30publications/10policies/b3/index.html
- Teichholz LE, Cohen MV, Sonnenblick EH, Gorlin R. Study of left ventricular geometry and function by B-scan ultrasonography in patients with and without asynergy. N Engl J Med 1974;291:1220-6. [PubMed]
- Nemes A, Kalapos A, Domsik P, Forster T. Three-dimensional speckle-tracking echocardiography -- a further step in non-invasive three-dimensional cardiac imaging. Orv Hetil 2012;153:1570-7. [PubMed]
- Anwar AM, Soliman OI, ten Cate FJ, Nemes A, McGhie JS, Krenning BJ, van Geuns RJ, Galema TW, Geleijnse ML. True mitral annulus diameter is underestimated by two-dimensional echocardiography as evidenced by real-time three-dimensional echocardiography and magnetic resonance imaging. Int J Cardiovasc Imaging 2007;23:541-7. [PubMed]
- Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611-44. [PubMed]
- Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, Reeves ST, Shanewise JS, Siu SC, Stewart W, Picard MH. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26:921-64. [PubMed]
- Gordic S, Nguyen-Kim TD, Manka R, Sündermann S, Frauenfelder T, Maisano F, Falk V, Alkadhi H. Sizing the mitral annulus in healthy subjects and patients with mitral regurgitation: 2D versus 3D measurements from cardiac CT. Int J Cardiovasc Imaging 2014;30:389-98. [PubMed]
- Fernandes AM, Rathi V, Biederman RW, Doyle M, Yamrozik JA, Willians RB, Hedge V, Graunt S, Aras R Jr. Cardiovascular magnetic resonance imaging-derived mitral valve geometry in determining mitral regurgitation severity. Arq Bras Cardiol 2013;100:571-8. [PubMed]
- Ormiston JA, Shah PM, Tei C, Wong M. Size and motion of the mitral valve annulus in man. I. A two-dimensional echocardiographic method and findings in normal subjects. Circulation 1981;64:113-20. [PubMed]
- Yiu SF, Enriquez-Sarano M, Tribouilloy C, Seward JB, Tajik AJ. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation 2000;102:1400-6. [PubMed]
- Qasim AN, Rafeek H, Rasania SP, Churchill TW, Yang W, Ferrari VA, Jha S, Master SM, Mulvey CK, Terembula K, Dailing C, Budoff MJ, Kawut SM, Reilly MP. Cardiovascular risk factors and mitral annular calcification in type 2 diabetes. Atherosclerosis 2013;226:419-24. [PubMed]
- Movahed MR, Saito Y, Ahmadi-Kashani M, Ebrahimi R. Mitral annulus calcification is associated with valvular and cardiac structural abnormalities. Cardiovasc Ultrasound 2007;5:14. [PubMed]


