
Evaluation of ulcerative colitis activity using transabdominal ultrasound shear wave elastography
Introduction
Ulcerative colitis (UC) is a disease that causes diffuse and nonspecific inflammation of unknown etiology in the colonic mucosa. It is often characterized by relapses and remissions during the clinical course and is associated with a risk of carcinogenesis. Colonoscopy is almost always used to diagnose UC and evaluate the effectiveness of treatment (1). In recent years, mucosal healing based on endoscopic findings has been proposed as the goal of treatment (2), and periodical colonoscopy is performed. Endoscopy may also be repeated within a short period to evaluate the effectiveness of treatment (1). However, colonoscopy is painful for some patients and may cause complications or the worsening of colitis due to irritation caused by endoscopic procedures, including bowel preparation (3). Examination without bowel preparation is possible, but the risk of pain and complications of the procedure itself remains. The acceptability of endoscopy as a monitoring tool for inflammatory bowel disease (IBD) has been shown to be low, and alternative methods need to be developed (4).
Transabdominal ultrasound (US) has lower risk and is a less painful test than colonoscopy. The European Crohn’s and Colitis Organisation (ECCO) and the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) guidelines include US as one of the diagnostic tools for IBD, and gastrointestinal US can be performed without bowel preparation (5). Several papers have reported the utility of US for UC, and it has also been found to be effective in assessing intestinal wall thickness and blood flow on color Doppler (6-8). Regarding the hardness of the intestinal wall, it has been reported that the hardness of the intestine differs between normal subjects and UC patients (9,10). Nair et al. reported that the Young’s modulus of the intestine of UC patients was found to be lower than that of normal subjects via hardness measurements using optical coherence tomography in an ex vivo study (9,10). Elastography is a noninvasive technique for measuring the elasticity of tissues. US elastography can be divided into two types: strain elastography, which visualizes the distribution of distortions, and shear wave elastography (SWE), which quantifies tissue elasticity by measuring the shear wave speed (11). We have previously reported that the pattern of distortion distribution on strain elastography correlates with endoscopic findings in patients with UC (12). Some recent papers have shown a correlation between the activity of UC and SWE (13,14). SWE visualizes the propagation of shear waves and noninvasively quantifies tissue elasticity based on propagation speed. Recently, it has also become possible to simultaneously measure tissue viscosity by shear wave dispersion (SWD). Quantitative testing has the advantage of maintaining objectivity. SWD has been reported to reflect inflammation and adipogenesis in the liver and pancreaticobiliary region (15-17).
In this study, we investigated how SWE and SWD obtained via US are related to the activity of UC. The primary endpoint was the correlation of the severity of inflammation in UC with SWE and SWD. The secondary endpoints were the correlation of the severity of inflammation with wall thickness in patients with UC and verifying the validity and reliability of SWE and SWD obtained via US.
Methods
Out of 39 UC patients aged 20 years or older who were hospitalized in our hospital between April 2019 and March 2020 for whether exacerbation of UC, periodic examination of UC, or diseases other than UC, consent was obtained from 26 patients for inclusion in the study. Of these, 22 had exacerbation of UC, 1 had a periodic examination of UC, and 3 were hospitalized for other diseases. All patients underwent US within 2 days before or after colonoscopy. Although bowel preparation was not necessarily required prior to the US procedure, polyethylene glycol-electrolyte lavage for the patients who underwent bowel preparations. US and SWE/SWD measurements were performed by a single gastroenterologist (KY), who has performed more than 3,000 US examinations and belongs to the Japan Society of Ultrasonics in Medicine. The gastroenterologist was blinded to the endoscopic results and clinical symptoms but not the diagnosis of UC. An Aplio i900 ultrasound system (Canon Medical Systems Corp.) with a convex probe (i8CX1) was used to measure SWE and SWD. All SWE/SWD measurements were performed on the sigmoid colon due to the high degree of delineation on US (7). B-mode images were used to delineate the bowel in the short axis, and the measurement region of interest (ROI) was set to a size large enough to contain the entire delineated bowel wall on the ventral side, including all layers from mucosa to serosa, avoiding blood vessels. SWE/SWD measurements were performed using propagation displays and color mapping, respectively (Figure 1). The measurements were performed at least 5 times based on previous reports (17-19). In the propagation view, if the ROI included a region with parallel contours of propagation with a constant interval, it was regarded as evaluable. Measurements were repeated a maximum of 10 times, and if a parallel region was acquired fewer than five times, the case was regarded as unevaluable. The intestinal wall thickness was measured manually as the perpendicular distance between the mucosa and the serosa on the screen on which SWE/SWD was measured (20).
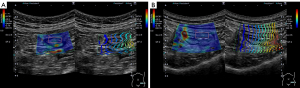
The Lichtiger index and the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) were used to determine the clinical severity (21) and endoscopic activity (22), respectively. For the UCEIS, the value of the sigmoid colon as assessed by a skilled endoscopist at the time of the examination was used. The shear wave speed and dispersion were defined as the SWE value (m/s) and SWD value [(m/s)/kHz], respectively, which were the median values of five measurements. The median of five measurements of intestinal wall thickness was also recorded as the wall thickness (mm). For the SWE and SWD values, the interquartile range (IQR)/median for each measurement was calculated, and the reliability of the obtained values was evaluated.
Based on a previous report (23), which stated that a UCEIS score of 0 points would be endoscopically assessed as mucosal healing, patients with a UCEIS score of 0 points were classified as belonging to the mucosal healing group, and those with a UCEIS score of 1 or more points were classified as belonging to the active phase group. The SWE and SWD values were compared between the two groups, and if there was a significant difference, the area under the receiver operating characteristic curve (AUCROC) was calculated to assess the ability of that value to predict mucosal healing and determine the appropriate cutoff value.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of our hospital (approval number 2014-0399) and informed consent was taken from all the patients (Clinical trial registration number: UMIN 000016497).
Statistical analysis
SPSS (ver. 27, SPSS Inc.) was used for the statistical analysis. Spearman rank correlation coefficients (rs) were used to evaluate correlations as weak (|rs|<0.2), mild (0.2<|rs|<0.4), moderate (0.4<|rs|<0.7), or strong (|rs|≥0.7).
A nonparametric test (Mann-Whitney U-test) was used for the comparisons of continuous variables.
The AUCROC was defined as indicating low (AUCROC =0.5–0.7), moderate (AUCROC =0.7–0.9) or high (AUCROC =0.9–1.0) accuracy. Cutoff values were determined by maximizing the Youden index (sensitivity + specificity -1). The sensitivity and specificity were calculated for the cutoff value.
Results
SWE/SWD was measurable in all 26 cases. The median age was 48 (IQR, 37.0–63) years, and the male to female ratio was 20:6. The median body mass index (BMI) was 18.2 (IQR, 17.8–19.8) kg/m2. The median duration of the disease was 125 (IQR, 51.8–216) months. Regarding the colitis type, 19 cases were classified as the pancolitis type and 7 cases were classified as the left-sided colitis type based on the extent of inflammation at the most active phase in each patient. Bowel preparation was performed in 4 patients and was not performed in 22 patients. There was no fluid pooling in the lumen in the 4 patients who underwent US after bowel preparation. The median measured depth from the probe to the top of the ROI for SWE/SWD was 1.85 (IQR, 1.50–2.08) cm (Table 1).
Table 1
| Characteristics | N=26 |
|---|---|
| Age (years), median (IQR) | 48 [37–63] |
| Sex, male: female | 20:6 |
| BMI, median (IQR) | 18.2 (17.8–19.8) |
| Duration of disease (months), median (IQR) | 125 (51.8–216) |
| Bowel preparation, yes:no | 4:22 |
| Location of colitis, pancolitis:left-sided colitis | 19:7 |
| Albumin (mg/dL), median (IQR) | 3.4 (2.55–3.95) |
| Hemoglobin (g/L), median (IQR) | 11.7 (9.03–14.2) |
| C-reactive protein (mg/dL), median (IQR) | 1.07 (0.22–2.58) |
| ESR (mm), median (IQR) | 45 [20–60] |
| Depth (cm), median (IQR) | 1.85 (1.50–2.08) |
IQR, interquartile range; BMI, body mass index; ESR, erythrocyte sedimentation rate.
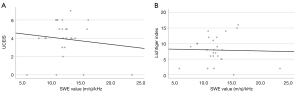
The median Lichtiger index score, UCEIS score, SWE value, SWD value, and wall thickness were 8 (IQR, 5.3–10.8), 4 (IQR, 3.3–5), 1.69 (IQR, 1.49–2.16) m/s, 11.9 (IQR, 10.9–13.3) (m/s)/kHz, and 5.0 (IQR, 4.1–5.9) mm, respectively. The IQR/median for SWE and SWD values were 0.21 and 0.34, respectively. The Lichtiger index and UCEIS scores were negatively correlated with the SWE values (rs=−0.404, P=0.041 and rs=−0.506, P=0.008, respectively) (Figure 2).

The Lichtiger index and UCEIS scores were not correlated with the SWD values (rs=0.004, P=0.986 and rs=0.002, P=0.993, respectively) (Figure 3). The Lichtiger index and UCEIS scores were moderately positively correlated (rs=0.608, P=0.001). There was no correlation between wall thickness and SWE, SWD, Lichtiger index or UCEIS scores (rs=−0.209, P=0.306; rs=−0.010, P=0.960; rs=0.252, P=0.214; and rs=0.342, P=0.087, respectively).
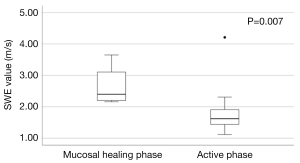
Based on the UCEIS score, there were 4 patients in the mucosal healing group and 22 patients in the active phase group. The SWE values differed significantly between the two groups, with 2.40 (IQR, 2.18–3.38) m/s and 1.62 (IQR, 1.44–1.95) m/s in the mucosal healing and active groups, respectively (P=0.007) (Figure 4). The median SWD values were 13.1 (IQR, 7.05–21.6) (m/s)/kHz and 11.9 (IQR, 11.1–13.2) (m/s)/kHz in the mucosal healing and active groups, respectively, with no significant difference between the groups (P=0.918). There was no correlation between SWE and duration of disease in the active phase group (rs=0.080, P=0.723).
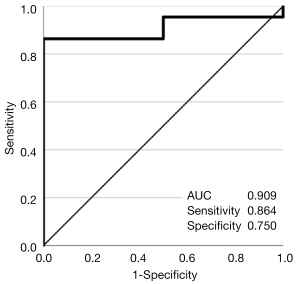
The AUCROC [95% confidence interval (CI)] for the assessment of mucosal healing by the SWE value was 0.909 (0.794–1.000) (Figure 5). With a cutoff value of 2.20, the sensitivity and specificity were 0.864 and 0.750, respectively.
Discussion
In this study, we investigated the correlation between SWE and SWD values and the Lichtiger index and UCEIS scores in patients with UC. SWE values were shown to negatively correlate with both the clinical and endoscopic severity of UC.
Previous reports have shown a correlation between wall thickness and inflammation on US (6-8,13), but in the present study, there was no statistically significant correlation between wall thickness and UC activity, although a trend was suggested. On the other hand, there was a statistical correlation between SWE and UC activity. These results indicate that measurement of the SWE value in transabdominal ultrasonography could be a noninvasive method of evaluating the severity of UC.
There are several reports investigating the elasticity of the intestine in UC patients, but conflicting results have been reported. In two previous reports comparing SWE in healthy volunteers and UC patients, SWE and UC activity were found to be positively correlated (13), and velocity was found to be higher in UC patients than in healthy subjects (14). On the other hand, two other studies using optical coherence elastography to measure intestinal stiffness in healthy and UC murine models reported that the intestinal tracts of UC subjects were softer than those of healthy controls (9,10). Therefore, the stiffness of the intestinal wall in UC patients remains controversial. Multiple factors may contribute to stiffness, such as inflammation and fibrosis. Fibrosis is likely to lead to stiff tissue, but the effect of inflammation on stiffness is unclear. Differences in the disease period may also affect the severity of fibrosis or inflammation. In addition, the histology of the intestinal wall could be heterogeneous in UC patients, especially in those in the active phase. Given these factors, differences in the subjects enrolled and measurement methods used can yield different results, as shown above. In the present study, to reduce the effect of histological heterogeneity and selection bias, we measured SWE and SWD in the sigmoid colon in all patients and set the ROI to include the entire delineated bowel, including all layers from the mucosa to the serosa. In this setting, our study revealed a negative correlation between SWE and clinical severity and endoscopic activity.
Delineation of the sigmoid colon was possible in all cases, as previously reported (7). Both SWE and SWD could be measured in all cases, and they could be measured at the same time. The reason for the high success rate of SWE and SWD measurements was thought to be that the sigmoid colon targeted in this study was easy to visualize in B-mode, and the segmental bowel measurement depth was shallow. It has been suggested that the measurement depth may affect evaluations of SWE and SWD (24).
The IQR/median is reported to indicate the quality of the data when assessing liver fibrosis by elastography, and a value less than 0.30 in the liver region is considered reliable (25). The IQR/median for the SWE and SWD values measured in this study were 0.21 and 0.34, respectively. The IQR/median for SWE was less than 0.30 in this study. Although the IQR/median for SWD was higher than 0.30, it was close to 0.30. Thus, the SWE and SWD values obtained in this study were considered evaluable. We reported that the data obtained from the measurement of SWE values were highly reliable when the contour lines were measured parallel to each other using a propagation display (19), and we assume that the measurement using the propagation display led to the high accuracy of the examinations performed in this study.
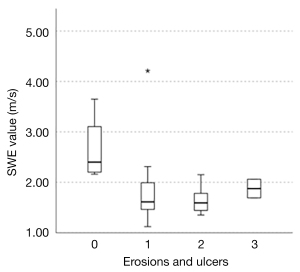
In a previous study evaluating the endoscopic findings and strain elastography in patients with UC reported by our institution (12), edema and erosions were depicted as soft areas, and the decrease in SWE values may also reflect these changes in the colon wall caused by inflammation. In addition, pathological biopsy results in patients with UC have shown more crypt abscesses and necrosis as the disease severity increases, as shown in Matts’ classification (26). Because abscesses and necrosis are reported to be soft (27-30), the progression of abscesses and necrosis with increasing severity may be one of the reasons for the negative correlation between endoscopic activity and SWE values. Next, we focused on the presence of erosions and ulcers. The UCEIS is a total score of three items: vascular pattern, bleeding, and erosions and ulcers. The erosions and ulcers score were 3 for deep ulcers, 2 for superficial ulcers, 1 for erosion and 0 for none (22). In this study, this score was 3 in 2 cases, 2 in 9 cases, 1 in 11 cases, and 0 in 4 cases; the median SWE for each score was 1.86, 1.59, 1.61, and 2.40, respectively (Figure 6). The SWE value tended to be higher for score 3 than for score 2. However, when comparing score 3 to score 0, the SWE value was significantly lower for score 3. This suggests that the SWE value is higher in the absence of erosions and ulcers than in the presence of deep ulcers. It is noteworthy that there is a correlation with the UCEIS, indicating that SWE measurements can noninvasively predict the severity of inflammation in patients with UC. SWE could be a noninvasive alternative to endoscopy for the assessment of disease severity, especially in patients with severe cases in whom the risk of disease aggravation or complications due to endoscopy is high or in patients who do not wish to undergo endoscopy. SWE may be an objective and acceptable method of determining the severity of inflammation in patients with UC due to the small interrater variability (31) and quantitative results.
The SWE value was highly accurate for the assessment of mucosal healing (UCEIS 0 points), with a high AUCROC value (0.909). Good sensitivity and specificity (0.864 and 0.750, respectively) were obtained by setting the cutoff SWE value to 2.20. It was also suggested that SWE could be used to determine the effectiveness of treatment for the same patient, thereby reducing the number of endoscopic procedures.
However, no correlation was found between SWD values and UC activity. In the liver region, SWD is said to reflect inflammation, necrosis and adipogenesis (15,16). We also reported that SWD reflects adipogenesis in the pancreas (17). Inflammation in UC is not accompanied by adipogenesis, which may be one of the reasons why the severity of inflammation in UC did not correlate with the SWD values. Another reason for the lack of utility of SWD may be that the four different layers, that is, the mucosa, submucosa, muscularis propria, and serosa, were measured together in this study.
There are several limitations of this study. First, this was a single-center study. Therefore, there was a bias in the severity of UC among the included patients. The number of patients in the remission phase was relatively small, which may have affected some of the results. Second, we were not able to longitudinally assess the course of treatment and changes in SWE values. Third, comparisons with pathological findings were not possible. Finally, evaluations in segments other than the sigmoid colon and in other IBDs, such as Crohn's disease, were not performed. A prospective study should be conducted in the future.
Conclusions
SWE may have clinical utility for the assessment of UC; it could be used as a noninvasive method of assessing the severity of the disease. Although SWD was not correlated with disease activity, the clinical benefit of SWD should be further evaluated.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/qims-21-403). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of our hospital (approval number 2014-0399) and informed consent was taken from all the patients (Clinical trial registration number: UMIN 000016497).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet 2017;389:1756-70. [Crossref] [PubMed]
- Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol 2015;110:1324-38. [Crossref] [PubMed]
- Kothari ST, Huang RJ, Shaukat A, Agrawal D, Buxbaum JL, Abbas Fehmi SM, et al. ASGE review of adverse events in colonoscopy. Gastrointest Endosc 2019;90:863-876.e33. [Crossref] [PubMed]
- Buisson A, Gonzalez F, Poullenot F, Nancey S, Sollellis E, Fumery M, et al. Comparative Acceptability and Perceived Clinical Utility of Monitoring Tools: A Nationwide Survey of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis 2017;23:1425-33. [Crossref] [PubMed]
- Sturm A, Maaser C, Calabrese E, Annese V, Fiorino G, Kucharzik T, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis 2019;13:273-84. [Crossref] [PubMed]
- Maaser C, Petersen F, Helwig U, Fischer I, Roessler A, Rath S, Lang D, Kucharzik T. German IBD Study Group and TRUST&UC study group. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut 2020;69:1629-36. [Crossref] [PubMed]
- Kinoshita K, Katsurada T, Nishida M, Omotehara S, Onishi R, Mabe K, Onodera A, Sato M, Eto K, Suya M, Maemoto A, Hasegawa T, Yamamoto J, Mitsumori D, Yoshii S, Ono K, Sakamoto N. Usefulness of transabdominal ultrasonography for assessing ulcerative colitis: a prospective, multicenter study. J Gastroenterol 2019;54:521-9. [Crossref] [PubMed]
- Smith RL, Taylor KM, Friedman AB, Gibson RN, Gibson PR. Systematic Review: Clinical Utility of Gastrointestinal Ultrasound in the Diagnosis, Assessment and Management of Patients With Ulcerative Colitis. J Crohns Colitis 2020;14:465-79. [Crossref] [PubMed]
- Nair A, Liu CH, Das S, Ho T, Du Y, Soomro S, Mohan C, Larin KV. Detecting murine Inflammatory Bowel Disease using Optical Coherence Elastography. Annu Int Conf IEEE Eng Med Biol Soc 2018;2018:830-3. [Crossref] [PubMed]
- Nair A, Liu CH, Singh M, Das S, Le T, Du Y, Soomro S, Aglyamov S, Mohan C, Larin KV. Assessing colitis ex vivo using optical coherence elastography in a murine model. Quant Imaging Med Surg 2019;9:1429-40. [Crossref] [PubMed]
- Shiina T. JSUM ultrasound elastography practice guidelines: basics and terminology. J Med Ultrason (2001) 2013;40:309-23. [Crossref] [PubMed]
- Ishikawa D, Ando T, Watanabe O, Ishiguro K, Maeda O, Miyake N, Nakamura M, Miyahara R, Ohmiya N, Hirooka Y, El-Omar EM, Goto H. Images of colonic real-time tissue sonoelastography correlate with those of colonoscopy and may predict response to therapy in patients with ulcerative colitis. BMC Gastroenterol 2011;11:29. [Crossref] [PubMed]
- Goertz RS, Lueke C, Schellhaas B, Pfeifer L, Wildner D, Neurath MF, Strobel D. Acoustic radiation force impulse (ARFI) shear wave elastography of the bowel wall in healthy volunteers and in ulcerative colitis. Acta Radiol Open 2019;8:2058460119840969 [Crossref] [PubMed]
- Marin AM, Calapod OP, Moldoveanu AC, Tribus LC, Fierbințeanu-Braticevici C. Non-invasive Ultrasonographic Score for Assessment of the Severity of Inflammatory Bowel Disease. Ultrasound Med Biol 2021;47:932-40. [Crossref] [PubMed]
- Sugimoto K, Moriyasu F, Oshiro H, Takeuchi H, Yoshimasu Y, Kasai Y, Furuichi Y, Itoi T. Viscoelasticity Measurement in Rat Livers Using Shear-Wave US Elastography. Ultrasound Med Biol 2018;44:2018-24. [Crossref] [PubMed]
- Yoo J, Lee JM, Joo I, Lee DH, Yoon JH, Kang HJ, Ahn SJ. Prospective Validation of Repeatability of Shear Wave Dispersion Imaging for Evaluation of Non-alcoholic Fatty Liver Disease. Ultrasound Med Biol 2019;45:2688-96. [Crossref] [PubMed]
- Suzuki H, Kawashima H, Ohno E, Ishikawa T, Hashimoto S, Nakamura M, Miyahara R, Ishigami M, Hirooka Y, Fujishiro M. What is the role of measuring shear wave dispersion using shear wave elastography in pancreatic parenchyma? J Med Ultrason (2001) 2020;47:575-81. [Crossref] [PubMed]
- Kuwahara T, Hirooka Y, Kawashima H, Ohno E, Sugimoto H, Hayashi D, Morishima T, Kawai M, Suhara H, Takeyama T, Yamamura T, Funasaka K, Nakamura M, Miyahara R, Watanabe O, Ishigami M, Shimoyama Y, Nakamura S, Hashimoto S, Goto H. Quantitative evaluation of pancreatic tumor fibrosis using shear wave elastography. Pancreatology 2016;16:1063-8. [Crossref] [PubMed]
- Hashizume K, Hirooka Y, Kawashima H, Ohno E, Ishikawa T, Kawai M, et al. The Propagation Display Method Improves the Reproducibility of Pancreatic Shear Wave Elastography. Ultrasound Med Biol 2019;45:2242-7. [Crossref] [PubMed]
- Nylund K, Maconi G, Hollerweger A, Ripolles T, Pallotta N, Higginson A, Serra C, Dietrich CF, Sporea I, Saftoiu A, Dirks K, Hausken T, Calabrese E, Romanini L, Maaser C, Nuernberg D, Gilja OH. EFSUMB Recommendations and Guidelines for Gastrointestinal Ultrasound. Ultraschall Med 2017;38:273-84. [Crossref] [PubMed]
- Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, Michelassi F, Hanauer S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994;330:1841-5. [Crossref] [PubMed]
- Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lichtenstein GR, Marteau PR, Reinisch W, Sands BE, Yacyshyn BR, Schnell P, Bernhardt CA, Mary JY, Sandborn WJ. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology 2013;145:987-95. [Crossref] [PubMed]
- Vuitton L, Peyrin-Biroulet L, Colombel JF, Pariente B, Pineton de Chambrun G, Walsh AJ, Panes J, Travis SP, Mary JY, Marteau P. Defining endoscopic response and remission in ulcerative colitis clinical trials: an international consensus. Aliment Pharmacol Ther 2017;45:801-13. [Crossref] [PubMed]
- Alfuraih AM, O'Connor P, Hensor E, Tan AL, Emery P, Wakefield RJ. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: Variables affecting reliability of SWE. J Clin Ultrasound 2018;46:108-15. [Crossref] [PubMed]
- Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, Garra B, Myers RP, Wilson SR, Rubens D, Levine D. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2015;276:845-61. [Crossref] [PubMed]
- MATTS SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med 1961;30:393-407. [PubMed]
- Sousaris N, Barr RG. Sonographic Elastography of Mastitis. J Ultrasound Med 2016;35:1791-7. [Crossref] [PubMed]
- Bertolotto M, Muça M, Currò F, Bucci S, Rocher L, Cova MA. Multiparametric US for scrotal diseases. Abdom Radiol (NY) 2018;43:899-917. [Crossref] [PubMed]
- Bhatia KS, Yuen EH, Cho CC, Tong CS, Lee YY, Ahuja AT. A pilot study evaluating real-time shear wave ultrasound elastography of miscellaneous non-nodal neck masses in a routine head and neck ultrasound clinic. Ultrasound Med Biol 2012;38:933-42. [Crossref] [PubMed]
- Lim CK, Chung CL, Lin YT, Chang CH, Lai YC, Wang HC, Yu CJ. Transthoracic Ultrasound Elastography in Pulmonary Lesions and Diseases. Ultrasound Med Biol 2017;43:145-52. [Crossref] [PubMed]
- Fang C, Konstantatou E, Romanos O, Yusuf GT, Quinlan DJ, Sidhu PS. Reproducibility of 2-Dimensional Shear Wave Elastography Assessment of the Liver: A Direct Comparison With Point Shear Wave Elastography in Healthy Volunteers. J Ultrasound Med 2017;36:1563-9. [Crossref] [PubMed]


