
Establishing a novel lens opacities classification system based on ultrasound biomicroscopy (UBM) for pediatric cataracts: reliability and availability
Introduction
Pediatric cataracts, which refer to the lens opacity of a patient at birth, can be treated by the extraction of the lens and the implantation of an artificial intraocular lens (1,2). Pediatric cataracts only occur in about 4 in every 1,000 babies (3); however, the disease has significant effects and may lead to deprivation amblyopia and even blindness (4,5). The lens opacity characteristics of pediatric cataracts are more diverse than those of age-related cataracts, which complicates pediatric cataract surgery (6). Due to pediatric cataracts' low morbidity and diversity, junior physicians lack an understanding of the disease and are slow to accumulate relevant clinical experience. However, surgeons need to be fully aware of the different types of pediatric cataracts and understand their surgery-related characteristics. Not only does such knowledge increase the success of surgery, but it also helps reduce postoperative complications. Characteristic abnormalities may also indicate the difficulties and risks of surgery, which may be helpful in preoperative preparation (7). Thus, an indirect experience that quickly provides professional knowledge and compensates for lack of direct experience is significant.
The Lens Opacities Classification System III (LOCS III) is a standard system used to compare lens opacity severity and distinguish among types of cataracts (8). The LOCS III is widely used in preoperative assessments and clinical studies of age-related cataracts. However, unlike age-related cataracts, pediatric cataracts are much more complicated, as they involve lens opacity and other developmental abnormalities. The LOCS III is unsuitable for pediatric cataracts. Thus, there is a need for a standardized classification system that compares lens opacities and distinguishes among types of pediatric cataracts. Such a system could also reflect the disease severity of pediatric cataracts more comprehensively and objectively. Additionally, a standardized method would reduce misunderstandings about the descriptions in medical records provided by different doctors and thus increase the efficiency of the treatment process.
The current main classification methods for pediatric cataracts (e.g., lamellar and sutural cataracts) are based on the location and morphology of lens opacity as observed by a slit lamp (9,10). This method can help surgeons to assess some types of pediatric cataracts and determine whether surgical intervention is required, but it does not help surgeons select the appropriate surgical procedure (10). Furthermore, when anterior capsule opaque, certain lens abnormities which behind of anterior capsule can be observed comprehensively operational time only after the anterior capsule be removed under a microscope. Thus, it is difficult to evaluate pediatric cataracts using a slit-lamp classification fully.
An ultrasound biomicroscopy (UBM) can provide images of structures in the anterior segment of the eyeball with high-frequency ultrasound and high resolution (11,12). Thus, unlike slit-lamp examinations, UBM can be used to detect lens opacity. In a previous study, we found that UBM imaging could be used before cataract surgery to evaluate lens morphology and density (13,14).
Presently, there is no systematic pediatric cataract classification protocol that provides a comprehensive assessment of the lens and assists in preoperative planning. Thus, a classification protocol based on UBM examinations for pediatric cataracts needs to be established. The current study sought to develop and validate an UBM-based diagnostic system for pediatric cataracts to standardize the classification of pediatric cataracts and reflect cataract features more comprehensively.
Methods
Patients
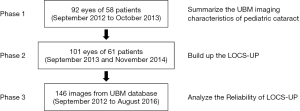
In this retrospective study, patients were involved in 3 phases (see Figure 1). In the first phase, UBM images of patients with pediatric cataracts (treated between September 2012 and October 2013) were used to establish the LOCS-UP based on our previous study (13). In the second phase, for validation purposes, pediatric cataract cases enrolled between September 2013 and November 2014 were assessed using the LOCS-UP. In the third phase, the images used to assess the diagnostic consistency between 2 physicians were obtained from pediatric cataract patients (treated between September 2012 and August 2016) in the UBM database.
To be eligible to participate in the study, patients had to meet the following Inclusion criteria were: (I) have pediatric cataracts (as detected by slit-lamp microscopy); (II) have undergone cataract surgery; (III) have undergone a UBM test before surgery (13). Cases with unclear UBM images were excluded from the study.
All patients were examined and underwent surgery at the Department of Ophthalmology in Guangzhou Women and Children’s Medical Center. The study was approved by the Institutional Review Board-Ethics Committee of Guangzhou Women and Children’s Medical Center. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants gave informed consent prior takingpart time.
UBM examination and surgery
Before the examination, all patients were placed in the supine position sober or under sedation with oral chloral hydrate. The same physician performed the UBM examination. The doctor, sitting on the Patient’s right side, inspected the Patient’s bilateral eyeballs with an ultrasonic probe, while a nurse held the Patient’s head with their hands. The UBM unit (Quantel Medical, MT, 50 MHz probes) was assembled; however, the small water bag was replaced by a standard plastic shell. Lens opacity features were observed and recorded perioperatively, and UBM imaging data were compared to lens opacity characteristics in intraoperative videos. All surgeries under general anesthesia were performed by the same chief physician (Dr. Xiang), who has 28 years of experience in the field of ophthalmology.
The standard of naming and grading under the LOCS-UP
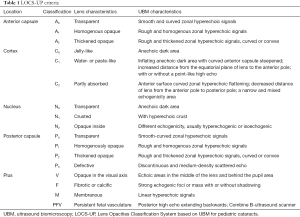
Previously, we observed that regional opacity in pediatric cataracts is characteristic in UBM images. Accumulated evidence indicates that most cataracts can be divided into 4 groups based on the following locations: the anterior capsule (A); the cortex (C); the nucleus (N); and the posterior capsule (P). Lens opacity in various positions also presents different performances of the echo shape, strength, and trait. Our results suggested that UBM data could describe lens opacity in detail and be used in an imaging-based diagnosis. We summarized opaque lens features by assessing the UBM images of patients treated at the hospital between September 2012 and October 2013. Next, the UBM data were compared to the data used in the intraoperative diagnosis. When the UBM diagnosis was inconsistent with the intraoperative diagnosis, the UBM description was modified until the consistency was achieved. All lens abnormalities were distinguished based on unique features of echoes in the UBM images.
We classified anterior capsule abnormalities by assessing whether (I) the echo on the lens surface was smooth or rough, (II) the echo was thickened, and (III) the echo was enhanced. We defined cortex abnormalities by evaluating echo strength, lens morphology, and thickness. The nucleus abnormalities were classified based on echolocation and intensity. The nucleus characteristics of the hyperechoic crust were also considered. Posterior capsule opacity was defined based on the echoes reflected by the lens surface. The classification of cataracts was determined by 2 professional doctors (attending physicians). The 2 doctors were blind to each other. When their cataract classifications differed, a final determination was reached by consensus.
The key points were as follows: the echo was smooth or rough, homogenous or thickened, or continuous or discontinuous. During the study, special opacities were found. Fibrotic or calcific changes were observed which shows a strong echo in UBM images. This kind of lens opacity could be found in any lens layer and could be hard to be removed during operation. Also, it is important to find the opacities that locate in the visual axis. We used the letter “V” to name the opacity located in the visual axis and the letter “F” to name the opacity with strong echogenic mass in any layer as a “plus” diagnosis. In addition, some cataracts were not included in the A/C/N/P criteria because of the special morphology and clinical meanings. Membranous cataract presents as linear strong echo in UBM image. We used “M” to name membranous cataract as a “plus” diagnosis. A diagnosis of persistent fetal vasculature (PFV) often required the use of a B-ultrasound scanner. We used “PFV” to name persistent fetal vasculatre as a “plus” diagnosis.
The establishment of the LOCS-UP
Based on the above, 6 categories with different subtypes of lens opacity classification were defined. Any pediatric cataract patient can be classified using this method. The classification criteria are summarized in Table 1. Finally, we obtained a new classification method to link the UBM-based imaging diagnosis to the gold standard. We named this method the “LOCS-UP”.
Full table
The reliability of the UBM-based diagnosis name
Two observers (ophthalmologists) were asked to classify the UBM images according to the LOCS-UP independently. Before the actual test, the observers met to better classify some UBM images together to understand the LOCS-UP better. In case of inconsistency between the 2 clinicians, a final diagnosis was reached by consensus.
To verify the method applicability, specificity was determined as TN/(TN + FP), sensitivity as TP/(TP + FN), and accuracy as (TP + TN)/number of all eyes, were FP, FN, TP, and TN referred to a false positive, false negative, true positive, and true negative cases, respectively.
We extracted the clinical data of patients diagnosed with pediatric cataracts at the hospital between September 2012 and August 2016. All UBM images were imported into the database and randomly selected and classified by researchers according to the LOCS-UP standard. In this study, a total of 146 images were selected to assess the reliability of the UBM-based diagnoses.
Statistical analysis
Central anterior chamber depth (CACD) and lens thickness (LT) were compared by a 1-way analysis of variance (ANOVA) with Bonferroni's post-hoc test using SPSS 25.0 software. The kappa coefficients were determined to assess the consistency between the 2 observers. Data are expressed as mean ± standard deviation (SD). A P<0.05 was considered statistically significant.
Results
LOCS-UP criteria
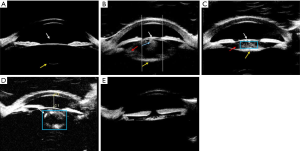
Fifty-eight patients (92 eyes) with pediatric cataracts participated in phase 1. By repeatedly comparing UBM and intraoperative images, the imaging diagnoses were made as consistent as the intraoperative diagnoses. The final classification criteria of the LOCS-UP are summarized in Table 1. In the LOCS-UP, letters with numbers represent different types of pediatric cataracts. The UBM diagnosis was obtained by combining these letters in order (see Figure 2).
Patient characteristics
Sixty-one patients (101 eyes) with pediatric cataracts participated in phase 2. Patients’ age groups and gender distribution are set out in Table S1. Of the patients, 52.46% were aged between 2 and 6 months, 8.20% were 6 to 12 months old, 13.11% were 12 to 24 months old, 18.03% were 24 to 36 months old, and 8.20% were over 36 months old. The overall male-to-female ratio was 1.5:1 (37:24).
We also used slit lamps to classify the examined cataracts, and common types are shown in Figure 3. Of the 101 eyes, 9.90% (10/101), 5.94% (6/101), 0.99% (1/101), 3.96% (4/101), 41.58% (42/101), 13.86% (14/101), and 23.76% (24/101) showed membranous, polar, sutural, embryonic nuclear, nuclear, lamellar, and complete cataracts, respectively.
Comparison of UBM and intraoperative diagnoses
Diagnostic differences in anterior capsule cataracts
The consistency between the UBM and intraoperative diagnoses was considered. In the diagnosis of anterior capsule abnormalities, homogenous opaque and thickened opaque lenses were considered abnormal. Most UBM diagnoses (94 eyes, 97.91%) were consistent with the intraoperative diagnoses. 1 case of homogenous opacity was misdiagnosed. 1 case of thickened opacity was defined as homogenous opacity (see Table S2). In this study, 22.77% (23/101) of the assessed eyes had anterior capsule abnormalities.
Diagnostic differences in cortex cataracts
We compared CACD among jelly-like, water- or paste-like, and partly absorbed groups (see Table S3). CACD was the most shallow and deepest in the water- or paste-like and partly absorbed groups, respectively. LT was the biggest and smallest in the water- or paste-like and partly absorbed groups, respectively. We considered lenses with a water- or paste-like opacity or a partly absorbed cortex to be abnormal for cortex abnormalities. Most eyes (93 eyes, 96.88%) had the same UBM and intraoperative diagnoses. However, 3 cases of water- or paste-like opacity were misdiagnosed (see Table S4). The cortex was abnormal in 37.5% of the eyes.
Diagnostic differences in nuclear cataracts
A nucleus of the lens that was crusted or opaque was considered abnormal. Most eyes (93 eyes, 96.88%) had a correct UBM diagnosis. However, 3 eyes (3.13%) were misdiagnosed (see Table S5). 1 opaque nucleus case was a missed diagnosis, and 2 crusted nucleus cases were misdiagnosed as opaque nucleus cases. 44.79% (43/96) of eyes had nucleus abnormalities.
Diagnostic differences in posterior capsule cataracts
Concerning the UBM-based diagnoses of posterior capsule abnormalities (homogenously opaque, thickened opaque, or defective), 86 eyes were correctly diagnosed. The diagnoses of 2 homogenously opaque cases and 1 thickened opaque posterior capsule case was missed (see Table S6). 19.80% (20/101) of eyes showed posterior capsule abnormalities in this study.
The diagnostic value of the LOCS-UP in pediatric cataracts
As Table 2 shows, based on the intraoperative diagnoses, the sensitivity of the LOCS-UP reached 100% for anterior capsule abnormalities, 91.67% for cortex defects, 97.67% for nucleus abnormalities, and 50% for posterior capsule abnormalities. The specificity of the LOCS-UP for lens abnormalities was 98.96% for the anterior capsule, 96.88% for the cortex, 98.96% for the nucleus, and 89.59% for the posterior capsule. The UBM-based diagnoses had accuracies of 98.96%, 96.88%, 98.96%, and 89.59% for the anterior capsule, cortex, nucleus, and posterior capsule abnormities, respectively.

Full table
The reliability of the LOCS-UP
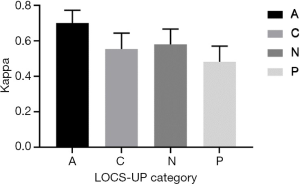
By extracting UBM images from the database for analysis (in phase 3), 100% (146/146) of the images were classified using the LOCS-UP. The kappa coefficient (0.70) for anterior capsule classification fell in the substantial range, followed by those for the nucleus and cortex (0.56 and 0.58, respectively), which were moderate. The kappa value for the posterior capsule classification was 0.48 (see Figure 4).
Discussion
The LOCS III is a useful tool for grading age-related cataracts
Slit-lamp microscopy is one of the most common examination methods used to detect lens opacity in cataracts (10,15,16). The LOCS (a classification system of age-related cataracts based on the slit-lamp method) was created by Leo T. Chylack to provide a reliable and reproducible grading scheme that was simpler for clinicians to use accounting for important cataract characteristics (17). The LOCS has become more objective and standardized and provides ophthalmologists with a way to communicate clinical findings and compare new developments in diagnostic technologies for cataracts. For age-related cataracts, this method allows ophthalmologists to follow a cataract over a period to assess its progression and to share data with other physicians in a clear and standardized way. The LOCS III may also aid in communications between physicians and patients, as it can provide patients with understandable information about the diagnosis, progression, and importance of treatment concerning their cataracts, which also allows for improved patient care. Further, the LOCS III can also be used in epidemiological studies (16,18).
A grading system of lens opacities for pediatric cataracts is necessary
Age-related cataracts are caused by the aging of fully developed lenses (19), and opacity mainly occurs in the cortex and nucleus and sometimes in the posterior capsule. Genetic factors or developmental abnormalities cause pediatric cataracts, and opacity is located from the anterior capsule to the posterior capsule (20). There are not many cases of anterior capsule opacity; however, this type of cataracts should be covered by any pediatric cataract classification system. Lens abnormalities in pediatric cataracts are not only limited to opacity. The LOCS III is not an appropriate tool for pediatric cataracts; thus, we sought to establish a classification standard for pediatric cataracts.
The current classification of pediatric cataracts is based on the position and the appearance of the lens opacity as observed by a slit lamp. This classification of pediatric cataracts can help surgeons estimate whether cataracts' type meets operation indications (10). Notably, due to the low incidence of pediatric cataracts (3), even specialized cataract doctors perform very few surgeries yearly. According to a survey of 125 American Pediatric Ophthalmology and Strabismus Association (AAPOS) doctors managing pediatric cataracts, 21% and 27.4% perform <5 and >20 cataract surgeries yearly, respectively (21). Thus, the LOCS-UP would benefit clinicians, especially young physicians, and provide them with a better understanding of pediatric cataracts.
The establishment of the LOCS-UP
We developed the LOCS-UP, which was used to assess 61 pediatric patients with cataracts. Based on the intraoperative findings, the diagnostic effectiveness of the LOCS-UP was satisfactory. We found that UBM allowed good visualization of pediatric cataracts. Pediatric cataracts can be graded based on local opacity characteristics (echo position, intensity, and shape) in UBM images. The method for pediatric cataracts based on A/C/N/P corresponds to an intraoperative diagnosis. To improve the accuracy of the UBM diagnostic criteria, the UBM description of lens opacity was repeatedly ameliorated by comparing examination and perioperative images.
This new classification system does not depend entirely on subjective decisions. CACD and LT were employed for the diagnosis of cortical opacity. A UBM examination showing a shallow anterior chamber and a thick lens likely indicate water- or paste-like cortex opacity in a child patient. Thus, UBM diagnoses that were highly consistent with confirmed diagnostic findings were achieved, and the LOCS-UP was developed based on UBM.
The local opacity of the lens was divided into the following 4 series: A (anterior capsule), C (cortex), N (nucleus), and P (posterior capsule). A0-A2, C0-C2, N0-N2, and P0-P3 were used to name the different grands of the opacity of the anterior capsule, cortex, nucleus, and posterior capsule. However, membranous cataracts and PFV were listed separately due to their particularities.
We speculated that the LOCS-UP could also play a role in evaluating intraoperative risks, as different lens abnormalities have different effects on the surgery. Thus, the imaging diagnosis provided by the LOCS-UP is clinically applicable.
Comparing the LOCS III and the LOCS-UP
LOCS III divides the local opacity of age-related cataracts into the following 3 parts: the nucleus (N), the cortex (C), and the posterior capsule (P), but does not consider the opacity of the anterior capsule. Anterior capsule opacity is rare in age-related cataracts but is a common anomaly in pediatric cataracts. According to the LOCS III, the grading of cortical opacity (C1–C5) is determined by the range of opacity. However, the grade of pediatric cataract cortex opacity is complex, and the opacity range alone cannot represent its characteristics. Cortical opacity was named C0, C1, and C2 in the LOCS-UP to represent a jelly-like, paste-like, and partially absorbed cortex. The optical detection method cannot distinguish the posterior structure of the lens as the UBM does when the anterior capsule is completely cloudy. Some authors also tried to preoperative evaluation of the posterior lens capsule using B-scan and As-OCT imaging in traumatic cataract (22). In addition, the LOCS III grades the color or opalescence of the lens nucleus, which has great significance in preoperative evaluations. However, the opacity of the nucleus of the lens in pediatric cataracts is mostly white, and other abnormalities, such as calcification and fibrosis, also hamper the removal of the lens, as does a hard nuclear (23,24). Thus, color grading alone cannot reflect the characteristics of nuclear opacity of pediatric cataracts, nor can it guide preoperative evaluations. Further, unlike in the LOCS III, “P” in the local opacity lens series stands for the posterior capsule itself, whole in the LOCS-UP, “P” stands for the posterior subcapsular. Posterior capsule defects are also not included in the LOCS III classification criteria.
The reliability and application value of the LOCS-UP
All pediatric cataracts assessed in this research received standardized names with details under the LOCS-UP. In most cases in phase 2, the lens opacities of pediatric cataracts were accurately detected by the UBM. The LOCS-UP had the highest sensitivity and specificity in diagnosing anterior capsule defects and the lowest sensitivity in diagnosing posterior capsule abnormalities. Posterior capsule defects might have been challenging to diagnose because the 50-MHz frequency selected for this study was insufficient to penetrate the lens perfectly. In future experiments, a lower frequency ultrasound (that is more penetrating) should be considered for the examinations (25). For example, 25-MHz UBM has been applied to measure the degree of lens opacity in age-related cataracts, but no classification was performed (26). The diagnosis of cortex and nucleus abnormalities is much more complex than that of capsule defects (27). Thus, the comprehensive consideration of lens features (opacity location, echo strength, and lens morphology) and numerical value (CACD and LT) could help improve the diagnosis rate. In brief, the LOCS-UP is a comprehensive and accurate pediatric cataract grading method.
The reproducibility of the LOCS-UP
In this study, the kappa coefficient for anterior capsule classification fell within the substantial range, indicating that the LOCS-UP could be easily reproducible in the anterior capsule. The anterior capsule is the most superficial structure of the lens in which ultrasonic wave do not easily be interfered. A clear image improves the accuracy of the diagnosis. The consistency of posterior capsule classifications between the 2 observers was relatively low. The blurred images might have led to divergent classification results.
Additionally, the kappa coefficients for the nucleus and cortex classifications were moderate. Nucleus and cortex opacities may confuse observers, as they are complex and diverse. Thus, image quality and classification complexity may affect the reproducibility of the LOCS-UP. The consistency of results may be improved if the observers were to discuss the classification methods to increase their proficiency in the LOCS-UP.
The application prospects and limitations of the LOCS-UP
In summary, LOCS-UP could supply available message for clinicians to cataract characteristics as well as be easy operated. It provides good evaluations of physical characteristics and the location of any opacity. Such information is useful in predicting the surgical difficulty of the lens. Additionally, from an epidemiological perspective, by characterizing pediatric cataract type and severity, the LOCS-UP could increase understanding of populations' health and treatment needs.
Our research had several limitations. It was performed at a single center with a relatively small number of cases. Thus, the future application of the LOCS-UP at a multicenter would increase the value of the pediatric cataract grading standard and extend its application range.
Conclusions
We developed the LOCS-UP to grade lens opacities accurately based on UBM for pediatric cataracts. Its diagnostic value (sensitivity, specificity, and accuracy) concerning preoperative pediatric cataracts was verified. The newly developed system provides detailed and accurate information based on local opacity. Under this system, each pediatric cataract patient can be assigned an individualized and digital classification. Notably, the cataract classification variability between the 2 doctors was low. This standardized diagnostic tool could reduce the difficulties of clinical training for ophthalmologists, and could also improve treatment results. Based on our preliminary assessments of these clinical studies, the LOCS-UP represents a very promising method for classifying pediatric cataracts.
Acknowledgments
Funding: This work was supported by the Guangdong Province Science and Technology Major Plan Project (2014B020212019) and the Guangdong Province Special Fund of Science and Technology Development (2016A020212002). The present study was supported by the Guangzhou Featured Project (2019TS54).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/qims-20-1028). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of this work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. This study was approved by the Institutional Review Board-Ethics Committee of Guangzhou Women and Children Medical Center, Guangzhou Medical University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants gave informed consent prior takingpart time.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ledoux DM, Trivedi RH, Wilson ME Jr, Payne JF. Pediatric cataract extraction with intraocular lens implantation: visual acuity outcome when measured at age four years and older. J AAPOS 2007;11:218-24. [Crossref] [PubMed]
- Plager DA, Kipfer H, Sprunger DT, Sondhi N, Neely DE. Refractive change in pediatric pseudophakia: 6-year follow-up. J Cataract Refract Surg 2002;28:810-5. [Crossref] [PubMed]
- Wu X, Long E, Lin H, Liu Y. Global prevalence and epidemiological characteristics of congenital cataract: a systematic review and meta-analysis. Lancet 2016;388:S55. [Crossref]
- Hwang S, Lim DH, Lee S, Choi DD, Chung ES, Chung TY. Temporary Piggyback Intraocular Lens Implantation Versus Single Intraocular Lens Implantation in Congenital Cataracts: Long-Term Clinical Outcomes. Invest Ophthalmol Vis Sci 2018;59:1822-7. [Crossref] [PubMed]
- Nagamoto T, Oshika T, Fujikado T, Ishibashi T, Sato M, Kondo M, Kurosaka D, Azuma N. Clinical characteristics of congenital and developmental cataract undergoing surgical treatment. Jpn J Ophthalmol 2015;59:148-56. [Crossref] [PubMed]
- Wang W, Yan W, Muller A, He M. A Global View on Output and Outcomes of Cataract Surgery With National Indices of Socioeconomic Development. Invest Ophthalmol Vis Sci 2017;58:3669-76. [PubMed]
- Traboulsi EI, Freedman SF, Wilson ME Jr, Lambert SR. Cataract morphology and risk for glaucoma after cataract surgery in infants with unilateral congenital cataract. J Cataract Refract Surg 2017;43:1611-2. [Crossref] [PubMed]
- Bencić G, Zorić-Geber M, Sarić D, Corak M, Mandić Z. Clinical importance of the lens opacities classification system III (LOCS III) in phacoemulsification. Coll Antropol 2005;29:91-4. [PubMed]
- Long E, Lin Z, Chen J, Liu Z, Cao Q, Lin H, Chen W, Liu Y. Monitoring and Morphologic Classification of Pediatric Cataract Using Slit-Lamp-Adapted Photography. Transl Vis Sci Technol 2017;6:2. [Crossref] [PubMed]
- Forster JE, Abadi RV, Muldoon M, Lloyd IC. Grading infantile cataracts. Ophthalmic Physiol Opt 2006;26:372-9. [Crossref] [PubMed]
- Gao K, Li F, Li Y, Li X, Huang W, Chen S, Liu Y, Aung T, Zhang X. Anterior Choroidal Thickness Increased in Primary Open-Angle Glaucoma and Primary Angle-Closure Disease Eyes Evidenced by Ultrasound Biomicroscopy and SS-OCT. Invest Ophthalmol Vis Sci 2018;59:1270-7. [Crossref] [PubMed]
- Shi Y, Wang H, Han Y, Cao K, Vu V, Hu M, Xin C, Zhang Q, Wang N. Correlation Between Trabeculodysgenesis Assessed by Ultrasound Biomicroscopy and Surgical Outcomes in Primary Congenital Glaucoma. Am J Ophthalmol 2018;196:57-64. [Crossref] [PubMed]
- Xiang D, Chen L, Hu L, Song S, Xie W, Long J. Image features of lens opacity in pediatric cataracts using ultrasound biomicroscopy. J aapos 2016;20:519-22.e4. [Crossref] [PubMed]
- Long J, Xiang D, Guo Z, Chen L, Chen F, Wang J, Xie W, He S. Clinical Characteristics and Surgical Procedures for Children with Congenital Membranous Cataract. J Ophthalmol 2017;2017:2370969 [Crossref] [PubMed]
- Vasavada AR, Praveen MR, Nath V, Dave K. Diagnosis and management of congenital cataract with preexisting posterior capsule defect. J Cataract Refract Surg 2004;30:403-8. [Crossref] [PubMed]
- Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993;111:831-6. [Crossref] [PubMed]
- Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993;111:831-6. [Crossref] [PubMed]
- Tan AC, Loon SC, Choi H, Thean L. Lens Opacities Classification System III: cataract grading variability between junior and senior staff at a Singapore hospital. J Cataract Refract Surg 2008;34:1948-52. [Crossref] [PubMed]
- Liu XF, Hao JL, Xie T, Malik TH, Lu CB, Liu C, Shu C, Lu CW, Zhou DD. Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell 2017;16:934-42. [Crossref] [PubMed]
- Mohammadpour M, Shaabani A, Sahraian A, Momenaei B, Tayebi F, Bayat R, Mirshahi R. Updates on managements of pediatric cataract. J Curr Ophthalmol 2018;31:118-26. [Crossref] [PubMed]
- Koo EB, VanderVeen DK, Lambert SR. Global Practice Patterns in the Management of Infantile Cataracts. Eye Contact Lens 2018;44:S292-6. [Crossref] [PubMed]
- Tabatabaei A, Hasanlou N, Kheirkhah A, Mansouri M, Faghihi H, Jafari H, Arefzadeh A, Moghimi S. Accuracy of 3 imaging modalities for evaluation of the posterior lens capsule in traumatic cataract. J Cataract Refract Surg 2014;40:1092-6. [Crossref] [PubMed]
- Miyata K, Nagamoto T, Maruoka S, Tanabe T, Nakahara M, Amano S. Efficacy and safety of the soft-shell technique in cases with a hard lens nucleus. J Cataract Refract Surg 2002;28:1546-50. [Crossref] [PubMed]
- Sauer A, Bourcier T, Gaucher D, Candolfi E, Speeg-Schatz C. Intraocular cytokines imbalance in congenital cataract and its impact on posterior capsule opacification. Graefes Arch Clin Exp Ophthalmol 2016;254:1013-8. [Crossref] [PubMed]
- Tabatabaei A, Kiarudi MY, Ghassemi F, Moghimi S, Mansouri M, Mirshahi A, Kheirkhah A. Evaluation of posterior lens capsule by 20-MHz ultrasound probe in traumatic cataract. Am J Ophthalmol 2012;153:51-4. [Crossref] [PubMed]
- Shi MY, Han X, Zhang JS, Yan QC. Comparison of 25 MHz and 50 MHz ultrasound biomicroscopy for imaging of the lens and its related diseases. Int J Ophthalmol 2018;11:1152-7. [PubMed]
- Barakat E, Ginat DT. Magnetic resonance imaging (MRI) features of cataracts in pediatric and young adult patients. Quant Imaging Med Surg 2020;10:428-431. [Crossref] [PubMed]



