Ultrasound for molecular imaging and therapy in cancer
Abstract
Over the past decade, molecularly-targeted contrast enhanced ultrasound (ultrasound molecular imaging) has attracted significant attention in preclinical research of cancer diagnostic and therapy. Potential applications for ultrasound molecular imaging run the gamut from early detection and characterization of malignancies to monitoring treatment responses and guiding therapies. There may also be a role for ultrasound contrast agents for improved delivery of chemotherapeutic drugs and gene therapies across biological barriers. Currently, a first Phase 0 clinical trial in patients with prostate cancer assesses toxicity and feasibility of ultrasound molecular imaging using contrast agents targeted at the angiogenic marker vascular endothelial growth factor receptor type 2 (VEGFR2). This mini-review highlights recent advances and potential applications of ultrasound molecular imaging and ultrasound-guided therapy in cancer.
Key words
Ultrasound; molecular imaging; contrast-enhanced; targeted; cancer imaging; microbubbles; nanoparticles; VEGFR2; integrin
Introduction
Contrast-enhanced ultrasound using non-targeted contrast microbubbles is clinically approved in more than 50 countries, primarily in Europe and Asia, and is also used in Canada. It already has an established role in the clinic for several indications including the assessment of myocardial function and perfusion as well as the detection and characterization of focal liver lesions, including screening surveillance for HCC in high risk patients, to mention a few (1,2). Contrast-enhanced ultrasound imaging for the characterization of liver lesions is currently in Phase III clinical testing in the USA and FDA approval for contrast-enhanced liver imaging in the USA is expected soon. Its gaining acceptance is due in part to some of the inherent advantages of ultrasound, namely its lack of ionizing radiation, noninvasiveness, and high spatial and temporal sensitivity. Ultrasound devices are also widely available and relatively inexpensive compared to other imaging modalities. Furthermore, the contrast agents specifically used in contrast-enhanced ultrasound have proven to be safe and can be used in patients with renal insufficiency since there is no known nephrotoxic potential of current ultrasound contrast agents (1,3).
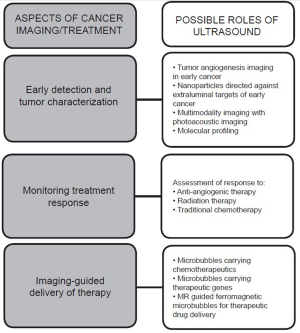
Ultrasound molecular imaging, which involves the use of molecularly targeted contrast agents, combines the aforementioned advantages of contrast-enhanced ultrasound with the ability to characterize neoplastic processes on a molecular level. The hope is to be able to image genetic and cellular alterations prior to any visible morphological or anatomic changes. To date, the majority of preclinical studies have employed the use of contrast microbubbles, which are gas-liquid emulsions of several micrometeres in size that are confined to the intravascular space. Newer, smaller nanoparticles that are able to extravasate through tumor vasculature are also being actively investigated. The potential applications of ultrasound molecular imaging are vast - from early cancer detection and tumor characterization to monitoring treatment response and guiding cancer therapies (Figure 1). Ultrasound molecular imaging contrast agents also have the potential for use within the theranostic realm as vehicles for the delivery of chemotherapeutic drugs and gene therapies. Several groups are also investigating multimodality imaging, which combines ultrasound with photoacoustic or magnetic resonance imaging modalities to further characterize malignancies. To date, the vast majority of investigations into molecular ultrasound for cancer imaging are in the preclinical stage; however, a recently launched first clinical trial explores the safety and efficacy of targeted contrast microbubbles for ultrasound molecular imaging in patients. In this mini-review we discuss recent advances and potential applications of ultrasound molecular imaging and ultrasound-guided therapy in cancer.
Microbubble and nanoparticle contrast agents
The majority of preclinical research with ultrasound molecular imaging has involved the use of microbubbles as contrast agents. Microbubbles are gas-liquid emulsions consisting of a gaseous core surrounded by a soft or hard shell. The gas core can be air or heavier gases such as perfluorocarbon (PFC), while the outer shell can consist of a variety of substances including albumin, polymers or a lipid bilayer (4-7). In order to functionalize microbubble contrast agents to attach to a particular molecular target, a linking agent such as avidin or streptavidin can be incorporated into the shell. This allows for straight-forward noncovalent binding to virtually any biotinylated ligand, such as antibodies, peptides or small molecules and represents a practical platform for rapid testing of new targeted contrast agents in animal models of human diseases. Because the streptavidin-biotin complex can be immunogenic, however, these agents are not suitable for clinical use. Several different strategies have been adopted to circumvent this issue and are reviewed elsewhere (8). Pochon et al. used a human VEGFR2 (KDR) targeted lipopeptide that was directly incorporated into the microbubble shell to create a clinical grade molecularly targeted ultrasound contrast agent (BR55) (9). Pysz et al. showed that this contrast agent could be used for quantification and monitoring of tumor angiogenesis during anti-angiogenic treatment of human colon cancer xenografts in mice (10). An alternative clinical grade VEGFR2-targeted contrast agent was synthesized by covalently binding a recombinant single-chain vascular endothelial growth factor construct (scVEGF) onto the surface of microbubbles (11).
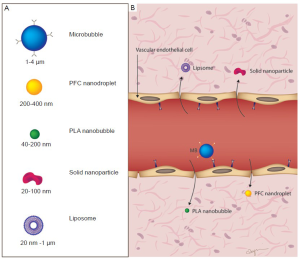
Like other contrast agents, microbubbles are injected intravenously, and due to their sizes of several microns (usually ranging between 1 and 4 μm; slightly smaller than red blood cells) they are confined to the intravascular space (Figure 2). Targeted microbubbles have binding ligands on their surfaces that come in contact with specific molecular targets on vascular endothelial cells and accumulate at tissue sites that overexpress those molecular targets. Several groups have shown that attachment of dual-targeting of microbubbles, i.e. equipping microbubbles with two ligands, can be more effective than single-targeted microbubbles (12,13). For example, microbubbles that were designed for imaging tumor angiogenesis in ovarian cancer xenografts in mice have been shown to better accumulate in tumors using microbubbles dual-targeted at the two angiogenesis markers VEGFR2 and αvβ3 integrin than single-targeted microbubbles to either VEGFR2 or αvβ3 integrin alone (13).
Microbubbles are detectable by ultrasound because they oscillate non-linearly when exposed to typical clinically used ultrasound pulses with frequencies ranging between 2 and 10 MHz that coincide with the resonance frequencies of most contrast microbubbles (14-16). In contrast, surrounding tissue structures do not or only minimally show non-linear behavior and give rise to echoes that simply mirror the incident ultrasound pulse. Exploiting this differential behavior of microbubbles and surrounding tissue, several dedicated ultrasound pulse sequences have been developed (e.g., pulse inversion) to differentiate imaging signal from ultrasound contrast agents and background tissue signal which substantially increases signal to background imaging signal and which allows detection of small amounts of accumulating microbubbles (17). Phantom studies by Klibanov et al. showed that ultrasound may detect a single microbubble, suggesting picogram sensitivity of ultrasound molecular imaging (18). Another interesting feature of microbubbles is that microbubbles rupture when the peak negative pressure (the “ultrasound power”) of the ultrasound pulse is increased from the “diagnostic range” (usually below 100 kPa) to the “therapeutic range” (several hundred kPa to several MPa). This increase of the ultrasonic pressure causes the microbubbles to vigorously oscillate and eventually collapse, releasing the energy into the surrounding tissue and causing transient cell membrane permeabilization (a process which is called sonoporation) (19). Secondary ultrasound bioeffects, such as microstreaming and other convective phenomena, are also thought to contribute to this phenomenon (20,21) which can be leveraged for using ultrasound within the theranostic realm, and which will be discussed in a subsequent section.
While microbubbles are largely confined to the intravascular space due to their size of several microns (pure intravascular contrast agents), there have been several investigations into smaller, submicron sized contrast agents that are able to enter the extravascular space of tumors and, thus, could theoretically be targeted against a larger number of disease specific markers beyond the targets expressed luminally on the vascular endothelial cells. It has been hypothesized that extravasation of these nanoparticles could be aided by the enhanced permeability and retention (EPR) effect. EPR is the theory wherein tumor vasculature is thought to be leaky and defective, which, when combined with poor lymphatic drainage, results in increased accumulation of macromolecular agents within the tumor (22). Nanoparticle contrast agents thus may be able to extravasate more easily through the tumor neovasculature. Investigative contrast agents such as liposomes (20 nm -10 μm), nanodroplets (200-400 nm) and solid nanoparticles (20-100 nm) are some of the agents currently being investigated (Figure 2) (4). The ability of nano-sized contrast agents to extravasate is being exploited in various ways, to be discussed in the following section.
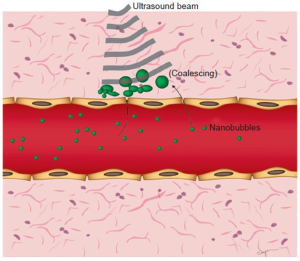
While the challenge remains of retaining ultrasound detectability while reducing the size of those agents, several groups have shown this may be possible in preclinical studies. Patel and colleagues have investigated whether submicron double-walled microspheres were acoustically detectable in vitro and if they could be used for imaging of sentinel lymph nodes (23,24). Submicron and micron sized contrast agents were injected into eleven canines’ distal extremities and popliteal and cervical lymph nodes were imaged using ultrasound with a high degree of accuracy (25). Yin et al. synthesized small lipid-based nanobubbles (average size 436.8±5.7 nm) that were able to retain similar echogenicity compared to microbubbles in vitro (26). In vivo imaging of orthotopically implanted tumors in nude rats was also performed; a robust signal was detectable at the tumor site, suggesting passive targeting. The nanobubbles were also labeled with fluorescent dye and their presence within the extravascular space was confirmed with confocal laser scanning microscopy (26). Rapoport et al. demonstrated that perfluoropentane (PFP) nanobubbles were able to extravasate into tumoral tissue in human breast cancer xenografts in nude mice and provide a long-lasting contrast agent (27). The group also showed that heating their nanobubbles to physiologic temperatures in vitro caused coalescence into microbubble sized collections; it was hypothesized that coalescence of the nanobubbles into larger collections in the extravascular space makes them more detectable by ultrasound (Figure 3). Furthermore, when nanoparticle contrast agents are vaporized within the extravascular space, micron-sized gas collections form, which are detectable by ultrasound (28). However, while many tumor models in animals have shown substantial EPR effects and, thus, timely and prolonged accumulation of nanoscale contrast agents could be observed in vivo in preclinical studies, it remains to be shown whether the EPR effect is high enough in different cancer types in patients to allow detection of these nanoscale contrast agents using currently available clinic imaging equipment.
Early cancer detection and monitoring treatment response
Early cancer detection remains one of the most elusive goals of oncological imaging. It is a near universal axiom that the early detection of cancer is the most important determinant of outcome; for nearly every malignancy the survival rates are inversely related to the cancer stage at the time of diagnosis. While many blood based and imaging biomarkers for various malignancies have been discovered, very few have acceptable sensitivities and specificities to be clinically useful for early cancer detection (29). The hope for molecular imaging is to be able to detect and image genetic or biochemical changes in early cancer prior to any gross anatomic alterations.
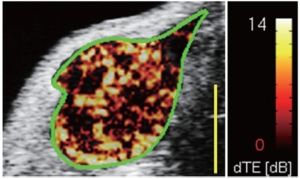
The imaging of tumor angiogenesis, the development of new vessels in growing tumors, has been the most studied and promising target of ultrasound molecular imaging using microbubbles in cancer. Angiogenesis is one of the hallmarks of early cancer development (30) since most tumors rely on increased vascularity once they grow beyond 1-2 mm in size (31). The new vessels are needed to meet the increased demand of growing tumors for oxygen and nutrition and molecular imaging of markers associated with angiogenesis could be leveraged to visualize cancer at an early still treatable stage (4,32). There are several molecular markers associated with tumor angiogenesis in early cancer that can be visualized by molecular imaging including VEGFR2, integrins and endoglin to mention a few. Several groups have shown that microbubbles targeted against VEGFR2, αvβ3 integrin and endoglin could be used to successfully image angiogenesis in various mouse models of cancer (9,10,33-36) (Figure 4). Recently, Bachawal et al. showed that ultrasound molecular imaging signal substantially increased when normal mammary tissue progressed through precursor lesions to invasive breast cancer using ultrasound and VEGFR2-targeted microbubbles in a transgenic mouse model (37). Pyszet al. showed that VEGFR2-targeted ultrasound imaging can visualize up to submillimeter sized foci of pancreatic cancer in a transgenic mouse model, indicating that ultrasonic molecular imaging could be used for early pancreatic cancer detection when the tumor is still at a very early stage (38). Ultrasound is already among the first line imaging modalities for organs such as the mammary glands and the pancreas; it can therefore be envisioned to eventually integrate an ultrasound-based molecular imaging approach for earlier detection of these and other cancers in the clinic. Ultrasound molecular imaging is particularly advantageous for this purpose because of the safety and lack of ionizing radiation, which allows repeated screening examinations of for example high risk patients without putting the patients at additional risk by the ultrasound exam. In addition to the general angiogenesis markers such as VEGFR2 or integrins, cancer specific molecular markers differentially expressed on the neovasculature of various cancer types have been discovered though techniques like DNA microarray analysis and mass spectrometry and were subsequently validated through immunohistochemical analyses. Ongoing research explores how ultrasound contrast agents targeted at cancer specific molecular markers may further improve diagnostic accuracy of ultrasonic molecular imaging in earlier cancer detection.
Several preclinical trials have also investigated whether ultrasound molecular imaging can be used to monitor the response of malignancies to treatment including anti-angiogenic therapies and radiotherapy. Clinically this may be relevant for early assessment of whether a particular treatment approach is effective in cancer patients or if an alternative strategy may be attempted instead. Korpanty et al. showed that following treatment with the anti-VEGF antibody (bevacizumab), there was decreased accumulation of anti-VEGFR2 targeted microbubbles in orthotopic pancreatic cancer-bearing mice. Similarly, Pysz et al. demonstrated that VEGFR2-targeted microbubbles could be used to evaluate the early response to anti-VEGF therapy in a human colon cancer xenograft model in mice (10). Not only was there a significant decrease in microbubble accumulation in treated mice in that study, but there were also ultrasound molecular imaging signal changes prior to any morphological alterations, i.e. changes in tumor size, suggesting that ultrasonic molecular imaging may be useful for assessing early treatment response before anatomical changes become apparent (10). Palmowski et al. showed that targeted microbubble agents against αvβ3 integrin could be used to monitor the effects of radiation therapy with carbon ions on molecular marker expressions in a prostate cancer model in rats. Interestingly, three days after radiation therapy αvβ3 integrin expression increased in this cancer model (39). This quantitative molecular information provided by ultrasound could become helpful for tailoring treatment strategies according to the molecular profile of tumors following one therapy (e.g., initial radiation therapy) with an individualized timing of one or more subsequent anticancer therapies (e.g., anti-angiogenic drug following radiation therapy).
Multimodality imaging using microbubbles and nanoparticles
Another area of active research has been aimed at developing multimodality agents, i.e. contrast micro and nanoparticles that can be detected both by ultrasound and by another imaging modality. The most feasible combination is that between ultrasound and photoacoustic imaging (PAI) since both can be integrated into one imaging machine, thereby allowing acquisition of both ultrasonic and PAI information during one imaging exam. PAI is an emerging modality within the realm of molecular imaging. It is based on the principle that objects illuminated and energized by light (e.g., lasers) emit detectable sound waves (principle of “light in” and “ultrasound out”) (40). While the phenomenon was discovered over 100 years ago by Alexander Graham Bell, it has only become employed within biomedical imaging within the past decade. With PAI, small dyes and nanoparticles that absorb light can be used to target extravascular ligands, due to their small size and stability. The multitude of potential clinical applications of PAI alone are reviewed in detail elsewhere (41).
PAI lends itself naturally to multimodality imaging with ultrasound, as often the same contrast agent is used for both modalities. Kim et al. developed a novel dual-modality contrast agent for both ultrasound and PAI that was composed of encapsulated-ink poly(lactic-co-glycolic acid) (PLGA) microbubbles and nanobubbles (42). They showed that the agent was detectable by both PAI and US at different concentrations in gelatin phantoms and proposed that one potential application for these PLGA bubbles could be for intraoperative evaluation of the tumor resection margins. The two modalities may complement each other in that they can simultaneously provide anatomical and functional information in tumors. Wilson et al. developed liquid perfluorocarbon nanodroplets with encapsulated plasmonic nanoparticles, which, when exposed to pulsed laser irradiation, vaporized, sending out a strong photoacoustic signal, and then formed into microbubbles detectable by ultrasound (43). As mentioned previously, the ability of these nanoparticle sized contrast agents to extravasate exposes a number of additional exciting possibilities. One can envision these agents being targeted against extraluminal ligands, and then pulsed with laser irradiation to visualize with photoacoustic imaging along with a subsequent ultrasound scan of formed microbubbles.
Ultrasound contrast agents have also been studied for possible combination with magnetic resonance imaging. Microbubbles have some inherent MR signal which is thought to be due to susceptibility differences induced by the gas-liquid interface of microbubbles (44,45). Several groups have further altered microbubbles by embedding magnetic nanoparticles into the bubble shells to make them ferromagnetic. These altered microbubbles may be used as contrast agents for both ultrasound imaging and MRI (46). Ferromagnetic micro- and nanobubbles can also be delivered into desired regions with the guidance of an external magnetic field. This would allow for increased interaction of targeted agents with the area under consideration and may have implications for increasing targeting in particular in high flow vessels such as the aorta or the carotid. Magnetized microbubbles may also have implications for improved drug delivery by attaching an increased number of microbubbles to the vascular wall before sonoporation (see below). Furthermore, multimodality imaging with MR and US contrast agents would have the added benefit of compensating for some of the shortcomings of ultrasound, namely its small field of view and lack of whole body imaging. While ultrasound could be used for assessment of focal functional and molecular changes for example within a tumor, the addition of MR could provide better appreciation for anatomic information regarding involvement of nearby structures or sites of distant metastasis.
Delivering cancer therapeutics
Another interesting area of research involving microbubble and nanoparticle contrast agents has been within the therapeutic realm. The hope is to create multifunctional contrast agents that double both as imaging tools and as vehicles for drug delivery (theranostics). A proposed model envisions the use of microbubbles or nanoparticles to deliver chemotherapeutic drugs or gene therapies directly and specifically to tumor sites using ultrasound. Ultrasound could be used to directly visualize microbubbles bearing chemotherapeutics and then can be used to burst the microbubbles, thus releasing the therapeutic agent directly at the tumor site and increasing penetration into the extravsacualr space through sonoporation (Figure 5). Gao et al. demonstrated that nanobubbles bearing doxorubicin could extravasate through leaky tumor vasculature and coalesce in the extravascular space, thus facilitating robust and long-lasting tumor imaging in breast cancer xenografts (28). They were also able to demonstrate on-demand release of the encapsulated doxorubicin under the action of ultrasound. It is possible to focus ultrasound acoustic energy into millimeter and even sub-millimeter volumes, which further aids precise spatial control of drug delivery (47).
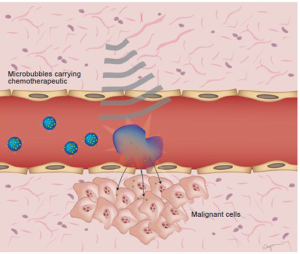
Several groups have also investigated microbubbles and nanoparticles as vehicles for gene therapy (48). Hosseinkhani et al. have shown efficient US-enhanced gene delivery using polyplexes of DNA and cationic-derivatized solid natural polymers in vitro (49,50). In a subsequent study, plasmid carrying agents were injected intravenously followed by insonation with 3 MHz US transdermally, which resulted in in vivo expression of a growth factor and angiogenesis inhibitor, NK4, in mice carrying a subcutaneous lung carcinoma tumor mass (50). Wang et al. investigated whether novel cationic microbubbles which directly bind DNA could also be used for enhance gene delivery compared to microbubbles with a neutral surface charge (51). As a proof of principle, plasmids encoding luciferase were bound onto the surface of cationic microbubbles and after insonation, the amount of luciferase activity was evaluated in vitro as well as within tumor xenografts implanted subcutaneously in nude mice hind limbs (51). When compared with neutral, uncharged plasmid bearing microbubbles, the cationic microbubbles demonstrated a 2.4-3.2 fold greater efficiency in vivo. The promise of microbubbles or nanobubbles for its potential role in targeted drug and gene delivery has stimulated active current research; the review of potential indications and current limitations of ultrasound in the theranostic realms is beyond the scope of this mini-review and have been reviewed elsewhere (52-55).
Obstacles and future directions of ultrasound molecular imaging
Ultrasound molecular imaging is a relatively nascent field, and while there are numerous exciting possibilities there are also a number of obstacles to be overcome. One challenge that remains is designing microbubbles and nanoparticles that bind to their target ligands specifically and with high affinity, such that a small number of target receptors can be detected and imaged. As mentioned previously, one such strategy to improve targeting efficiency has been to attach two different ligands on the microbubble shell (12,13). Recently, triple-targeted microbubbles to αvβ3-integrin, P-selectin, and VEGFR2 have been shown to increase image intensity by 40% compared with single and dual targeted contrast agents when imaging breast cancer tumor-bearing mice (56). Another strategy to improve the interaction between micro or nanobubbles with their target is to apply so-called Radiation Force–Enhanced Targeting, wherein acoustic radiation force of substantial magnitude can be generated even at low acoustic pressures when the ultrasound probe is perpendicular to a vessel (57). This increases the interaction between the targeted contrast agent and the endothelium. A similar strategy has been proposed with the use of an external magnetic field and directing magnetic microbubbles into an area of interest to increase the interaction between targeted agents and their ligands (58).
Another significant challenge with ultrasound molecular imaging is that of distinguishing free versus bound contrast agents. Due to the large number of particles injected intravenously, the proportion of specifically bound microbubbles or nanobubbles to the free bubbles in circulation is very low; the challenge remains distinguishing those bubbles that are bound to the target. Traditionally this has been achieved by acquiring two ultrasound data sets – one following several minutes after contrast injection when microbubbles have been allowed to bind to their target and a second set after a destructive ultrasound pulse when there are only free unbound agents. By subtracting the image intensity of the second set from the first, one is able to determine the amount of bound microbubbles (4,5,59). However, this is time consuming and requires post-processing and thus could hinder the real-time work flow of ultrasound imaging. One proposed technique to avoid this time consuming process is to employ signal processing to differentiate free versus bound contrast agents based on ultrasound. This would allow for more rapid imaging and only one data acquisition set. Zhao et al. showed that circulating and bound microbubbles do indeed have a different echo signature (60). By employing acoustic radiation force and a pulsed sequence to promote adhesion, the presence of adherent agents was able to be detected by the signal change due to targeted microbubble adhesion (61). The group also utilized a spectral high-pass filter, to differentiate bound microbubbles from the background tissue (61). Recently, Pysz et al. have shown that through the use of a fast new algorithm measuring the “dwell time” of microbubbles, near real-time assessment and monitoring of molecularly-attached microbubbles can be accomplished in vivo in a human colon cancer xenografts model in mice (62). This improved work-flow afforded by the fast near real-time attached microbubble assessment tool may facilitate clinical translation of ultrasonic molecular imaging and better integrate this technique into the routine workflow of clinical ultrasound imaging (62).
Another challenge of quantitative ultrasound imaging includes motion-induced artifacts that can substantially influence magnitudes of measured ultrasound imaging signals. Differences ranging between 0.5% to 33.3% in measured tumor vascularity on contrast-enhanced ultrasound with an without motion-compensation were observed in a recent study on human colon cancer xenografts implanted subcutaneously at the back of mice simulating respiratory motion-induced artifacts comparable to those in the liver (63). Using this new motion compensation algorithm integrated into the software of a clinical ultrasound system, a rectangular user-defined motion tracking box with adjustable size and height can be delineated around for example a tumor, and efficient real-time motion compensation can be accomplished using the technique of Sum of Absolute Differences; this new real-time technique makes contrast-enhanced ultrasound more reproducible and accurate compared to non-motion compensated contrast-enhanced ultrasound imaging and it can be readily translated into the clinic since it was developed for a clinical ultrasound system (63). An alternative recent approach utilized an intrinsic landmark-based gating technique (defining for example the skin or the liver capsule as gating landmark for motion compensation) and resulted in substantially lower variation of ultrasonic molecular imaging signal obtained from the liver of healthy and NEMO knockout mice compared to a non-motion compensated approach (64). Overall, these technical developments make ultrasound functional and molecular imaging more user friendly and facilitate eventual clinical translation of this promising imaging approach (63).
While many preclinical studies have shown encouraging results in ultrasound molecular imaging, until recently targeted ultrasound contrast agents had not been moved into clinical trials due to the difficult approval process for new contrast agents or drugs by agencies such as the US FDA. Most recently, a first exploratory Phase 0 clinical trial in patients with histology proven prostate cancer has been initiated to assess toxicity and efficiency of a clinical grade human VEGFR2-targeted contrast microbubble (BR55) (65). In that trial, patients undergo ultrasound molecular imaging at different time points up to 30 min following intravenous injection of BR55; ultrasound imaging signal in the prostate gland is then scored visually and correlated with human VEGFR2 (KDR) expression as assessed by immunohistochemistry. Experience from this clinical trial will provide information on safety and feasibility of ultrasound molecular imaging in the clinical arena using currently available ultrasound equipment and will help in the design of future clinical trials that explore the potential of this promising technique in other organs beyond the prostate.
Conclusions
Ultrasound molecular imaging has the potential for becoming a powerful tool in many aspects related to cancer imaging and therapy. Multiple preclinical trials have shown that it could play a role in early cancer detection, the characterization and molecular profiling of malignancies, and assessing treatment effects. Additional exciting possibilities relate to the use of targeted and non-targeted contrast agents for drug and gene delivery to ultrasonically accessible tissues. While there are several challenges that must be overcome first, ultrasound molecular imaging employing microbubbles has entered a first clinical trial and it is expected that this promising technique will soon find its clinical niche among other molecular imaging strategies for improved diagnosis and imaging-guided therapy of cancer in patients.
Acknowledgements
This work was supported by the NIH R01 CA155289-01A1 and the Canary Foundation. We would like to thank Amy N. Morris, graphic designer at the Department of Radiology, Stanford University, for her help with figure design.
Disclosure: The authors declare no conflict of interest.
References
- Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology 2010;257:24-39.
- Pysz MA, Willmann JK. Targeted contrast-enhanced ultrasound: an emerging technology in abdominal and pelvic imaging. Gastroenterology 2011;140(3):785-790.
- Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 2006;32:1369-75.
- Deshpande N, Needles A, Willmann JK. Molecular ultrasound imaging: current status and future directions. Clin Radiol 2010;65:567-81.
- Kircher MF, Willmann JK. Molecular Body Imaging: MR Imaging, CT, and US. Part I. Principles. Radiology 2012;263:633-43.
- Kiessling F, Huppert J, Palmowski M. Functional and molecular ultrasound imaging: concepts and contrast agents. Curr Med Chem 2009;16:627-42.
- Gessner R, Dayton PA. Advances in molecular imaging with ultrasound. Mol Imaging 2010;9:117-27.
- Klibanov AL. Preparation of targeted microbubbles: ultrasound contrast agents for molecular imaging. Med Biol Eng Comput 2009;47:875-82.
- Pochon S, Tardy I, Bussat P, et al. BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis. Invest Radiol 2010;45:89-95.
- Pysz MA, Foygel K, Rosenberg J, et al. Antiangiogenic cancer therapy: monitoring with molecular US and a clinically translatable contrast agent (BR55). Radiology. 2010;256:519-27.
- Anderson CR, Rychak JJ, Backer M, et al. scVEGF microbubble ultrasound contrast agents: a novel probe for ultrasound molecular imaging of tumor angiogenesis. Invest Radiol 2010;45:579-85.
- Ferrante EA, Pickard JE, Rychak J, et al. Dual targeting improves microbubble contrast agent adhesion to VCAM-1 and P-selectin under flow. J Control Release 2009;140:100-7.
- Willmann JK, Lutz AM, Paulmurugan R, et al. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology 2008;248:936-44.
- Frinking PJ, Bouakaz A, Kirkhorn J, et al. Ultrasound contrast imaging: current and new potential methods. Ultrasound Med Biol 2000;26:965-75.
- Forsberg F, Shi WT, Goldberg BB. Subharmonic imaging of contrast agents Ultrasonics 2000;38:93-8.
- Schneider M, Anantharam B, Arditi M, et al. BR38, a new ultrasound blood pool agent. Invest Radiol 2011;46:486-94.
- Burns PN, Wilson SR, Simpson DH. Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol 2000;35:58-71.
- Klibanov AL, Rasche PT, Hughes MS, et al. Detection of individual microbubbles of ultrasound contrast agents: imaging of free-floating and targeted bubbles. Invest Radiol 2004;39:187-95.
- Miller DL, Pislaru SV, Greenleaf JE. Sonoporation: mechanical DNA delivery by ultrasonic cavitation. Somat Cell Mol Genet 2002;27:115-34.
- Collis J, Manasseh R, Liovic P, et al. Cavitation microstreaming and stress fields created by microbubbles. Ultrasonics 2010;50:273-9.
- Kimmel E. Cavitation bioeffects. Crit Rev Biomed Eng 2006;34:105-61.
- Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release 2001;74:47-61.
- Patel DN, Bloch SH, Dayton PA, et al. Acoustic signatures of submicron contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control 2004;51:293-301.
- Patel D, Dayton P, Gut J, et al. Optical and acoustical interrogation of submicron contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control 2002;49:1641-51.
- Wisner ER, Ferrara KW, Short RE, et al. Sentinel node detection using contrast-enhanced power Doppler ultrasound lymphography. Invest Radiol 2003;38:358-65.
- Yin T, Wang P, Zheng R, et al. Nanobubbles for enhanced ultrasound imaging of tumors. Int J Nanomedicine 2012;7:895-904.
- Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst 2007;99:1095-106.
- Gao Z, Kennedy AM, Christensen DA, et al. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics 2008;48:260-70.
- Manne U, Srivastava RG, Srivastava S. Recent advances in biomarkers for cancer diagnosis and treatment. Drug Discov Today 2005;10:965-76.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70.
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27-31.
- Lutz AM, Willmann JK, Drescher CW, et al. Early diagnosis of ovarian carcinoma: is a solution in sight? Radiology 2011;259:329-45.
- Rychak JJ, Graba J, Cheung AM, et al. Microultrasound molecular imaging of vascular endothelial growth factor receptor 2 in a mouse model of tumor angiogenesis. Mol Imaging 2007;6:289-96.
- Ellegala DB, Leong-Poi H, Carpenter JE, et al. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation 2003;108:336-41.
- Lee DJ, Lyshchik A, Huamani J, et al. Relationship between retention of a vascular endothelial growth factor receptor 2 (VEGFR2)-targeted ultrasonographic contrast agent and the level of VEGFR2 expression in an in vivo breast cancer model. J Ultrasound Med 2008;27:855-66.
- Willmann JK, Kimura RH, Deshpande N, et al. Targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin-binding knottin peptides. J Nucl Med 2010;51:433-40.
- Bachawal S Jensen K, Foygel K, Tranquart F, et al. Targeted contrast-enhanced ultrasound imaging using KDR-targeted microbubbles for early breast cancer detection in a transgenic mouse model. World Molecular Imaging Congress Annual Meeting, San Diego, CA. 2011;(Abstract).
- Pysz MA, Seeley S, Foygel K, et al. Early Detection of Pancreatic Cancer in Transgenic Mice with Ultrasonic Molecular Imaging and VEGFR2-targeted Microbubbles. American Association for Cancer Research Annual Meeting 2012;(Abstract).
- Palmowski M, Peschke P, Huppert J, et al. Molecular ultrasound imaging of early vascular response in prostate tumors irradiated with carbon ions. Neoplasia 2009;11:856-63.
- Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol 2010;65:500-16.
- Mallidi S, Luke GP, Emelianov S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol 2011;29:213-21.
- Kim C, Qin R, Xu JS, et al. Multifunctional microbubbles and nanobubbles for photoacoustic and ultrasound imaging. J Biomed Opt 2010;15:010510.
- Wilson K, Homan K, Emelianov S. Biomedical photoacoustics beyond thermal expansion using triggered nanodroplet vaporization for contrast-enhanced imaging. Nat Commun 2012;3:618.
- Ueguchi T, Tanaka Y, Hamada S, et al. Air microbubbles as MR susceptibility contrast agent at 1.5 Tesla. Magn Reson Med Sci 2006;5:147-50.
- Dharmakumar R, Plewes DB, Wright GA. On the parameters affecting the sensitivity of MR measures of pressure with microbubbles. Magn Reson Med 2002;47:264-73.
- Yang F, Li Y, Chen Z, et al. Superparamagnetic iron oxide nanoparticle-embedded encapsulated microbubbles as dual contrast agents of magnetic resonance and ultrasound imaging. Biomaterials 2009;30:3882-90.
- Willmann JK, van Bruggen N, Dinkelborg LM, et al. Molecular imaging in drug development. Nat Rev Drug Discov 2008;7:591-607.
- Unger EC, McCreery TP, Sweitzer RH. Ultrasound enhances gene expression of liposomal transfection. Invest Radiol 1997;32:723-7.
- Hosseinkhani H, Aoyama T, Ogawa O, et al. Ultrasound enhancement of in vitro transfection of plasmid DNA by a cationized gelatin. J Drug Target 2002;10:193-204.
- Hosseinkhani H, Kushibiki T, Matsumoto K, et al. Enhanced suppression of tumor growth using a combination of NK4 plasmid DNA-PEG engrafted cationized dextran complex and ultrasound irradiation. Cancer Gene Ther 2006;13:479-89.
- Wang DS, Panje C, Pysz MA, et al. Cationic versus neutral microbubbles for ultrasound-mediated gene delivery in cancer. Radiology 2012. DOI 10.1148/radiol.12112368.
- Cavalieri F, Zhou M, Ashokkumar M. The design of multifunctional microbubbles for ultrasound image-guided cancer therapy. Curr Top Med Chem 2010;10:1198-210.
- Li SD, Huang L. Gene therapy progress and prospects: non-viral gene therapy by systemic delivery. Gene Ther 2006;13:1313-9.
- Newman CM, Bettinger T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther 2007;14:465-75.
- Caskey CF, Hu X, Ferrara KW. Leveraging the power of ultrasound for therapeutic design and optimization. J Control Release 2011;156:297-306.
- Warram JM, Sorace AG, Saini R, et al. A triple-targeted ultrasound contrast agent provides improved localization to tumor vasculature. J Ultrasound Med 2011;30:921-31.
- Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J Acoust Soc Am 2002;112:2183-92.
- Cai X, Yang F, Gu N. Applications of magnetic microbubbles for theranostics. Theranostics 2012;2:103-12.
- Hwang M, Lyshchik A, Fleischer AC. Molecular sonography with targeted microbubbles: current investigations and potential applications. Ultrasound Q 2010;26:75-82.
- Zhao S, Kruse DE, Ferrara KW, et al. Acoustic response from adherent targeted contrast agents. J Acoust Soc Am 2006;120:EL63-9.
- Zhao S, Kruse DE, Ferrara KW, et al. Selective imaging of adherent targeted ultrasound contrast agents. Phys Med Biol 2007;52:2055-72.
- Pysz MA, Guracar I, Liu T, et al. Fast Microbubble Dwell-Time based Ultrasonic Molecular Imaging Approach for Quantification and Monitoring of Angiogenesis in Cancer, QIMS 2012. DOI 10.3978/j.issn.2223-4292. 2012.06.05.
- Pysz MA, Guracar I, Foygel K, et al. Quantitative assessment of tumor angiogenesis using real-time motion-compensated contrast-enhanced ultrasound imaging. Angiogenesis 2012. DOI:10.1007/s10456-012-9271-3.
- Grouls C, Hatting M, Tardy I, et al. Development and validation of an intrinsic landmark-based gating protocol applicable for functional and molecular ultrasound imaging. Eur Radiol 2012. [Epub ahead of print].
- Available online: http://wwwclinicaltrialsgov/ct2/show/NCT01253213


