
A better understanding of testicular and/or epididymal tuberculosis based on clinical, ultrasonic, computed tomography, and magnetic resonance imaging features at a high-volume institute in the modern era
Introduction
Tuberculosis (TB) is still a major public health burden worldwide, especially in developing countries. There is an estimated 9 million newly affected cases globally each year, and there were almost 1.5 million deaths attributable to TB in 2013 (1). Moreover, there has been a trend of recrudescence in African countries and Eastern Europe in recent decades, which was majorly caused by the acquired immune deficiency syndrome (AIDS) epidemic, drug-resistance TB, and migration patterns (2,3). Immunosuppression attributable to human immunodeficiency virus (HIV) infection raises the risk of extrapulmonary TB infection (4,5). Extrapulmonary TB comprises 10% of TB cases, while genitourinary TB accounts for 30–40% of all extrapulmonary cases (6,7).
Genitourinary TB causes severe complications such as infertility, sexual dysfunction, and renal failure. The most common site of involvement is the epididymis in male patients, resulting from hematogenous spread or retrograde extension from seminal vesicles and the prostate (8). As for testicular involvement, it usually spreads contiguous from the epididymitis, and the hematogenous spread of isolated testicular TB is possible but infrequent (9). Historically, bilateral testis involvement was more common than unilateral involvement; however, unilateral testicular-epididymis TB seems to be more frequent in the modern era (10,11).
The initial presenting symptom of most patients is a painful scrotal mass. It is difficult to differentiate TB from other bacterial infections, granulomatous infection, testicular infarction, and tumors without specific clinical symptoms. However, treatment strategies for these lesions are totally distinct from each other. Furthermore, clinicians lack awareness of testicular and/or epididymal TB, and appropriate treatment can be delayed, leading to 69% of patients diagnosed via unnecessary surgery (12). This study aimed to summarize the clinical and radiological features of testicular and/or epididymal TB at a high-volume institute in west China over the past decade.
Methods
Study population
From 1 January 2008 to 31 December 2019, 69 patients with testicular and/or epididymal TB at the West China Hospital of Sichuan University were recruited for this study. Inclusion criteria were as follows: (I) all patients were diagnosed through the confirmation of Mycobacterium tuberculosis in the histopathology of resected samples during surgery; (II) had undergone preoperative scrotum ultrasonography and/or abdominal and pelvic computed tomography/magnetic resonance imaging (CT/MRI); (III) adequate image quality for analysis. Other sites of TB relied on radiographic evidence or histopathology of resected samples.
All available clinical characteristics were collected along with data concerning age, body temperature, clinical symptoms, T lymphocyte spot test for tuberculosis infection (T-SPOT.TB), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum β-human chorionic gonadotropin (β-HCG), serum alpha fetoprotein (AFP), clinical duration, and TB of other sites. According to our institution’s criteria: ESR >25 mm/h, CRP >5 mg/L, serum β-HCG ≥3.81 mIU/mL, and serum AFP ≥8 ng/mL were considered to be elevated. Furthermore, the individuals were examined by scrotal ultrasonography (n=51), abdominal and pelvic enhanced CT (n=23), and pelvic MRI (n=1), respectively.
The Ethics Committee approved this study of the West China Hospital of Sichuan University. Participant information was anonymized and de-identified before analysis.
Data acquisition and analysis by scrotum ultrasonography, abdominal and pelvic CT/MRI
The scrotal ultrasound device used was LOGIQ 7 (GE Healthcare, Chicago, IL, USA) with linear probes of 7.5–12 MHz with the scrotum settings. The color Doppler scale was set at 3.5 cm/s, with the gain adjusted as needed to avoid aliasing and background noise. The morphology, size, internal echo of testis, scrotum, spermatic cord, and the relative position in the spermatic sac were observed by two dimensional (2D) ultrasound. The testis and epididymis’ blood flow was evaluated by color Doppler flow imaging (CDFI) and compared with the healthy side. The distribution of color blood flow signals was divided into levels 0–III, according to the Adler semiquantitative standard (13). Based on previous studies, the sonography of testicular and/or epididymal TB can be categorized into 4 types: type 1, diffusely enlarged heterogeneously or homogeneously lesion; type 2, nodular enlarged heterogeneously lesion; type 3, multiple small hypoechoic nodules (miliary type); type 4, space-occupying lesions (10,11,14,15).
A total of 23 participants underwent enhanced CT examination, and 1 patient was furtherly evaluated by MRI; 2 experienced radiologists restudied the images. The CT was performed by a 64 multi-detector CT scanner (Philips Brilliance, Philips, Amsterdam, Netherlands) with 120 kV voltage, 210 mA current, and width of collimator 64 mm × 0.635 mm. After performing unenhanced imaging, a bolus of the contrast agent ominipaque (1 mL/kg of body weight) was injected by power injection.
The participant underwent a 3.0 T MRI (Magnetom Skyra, Siemens Healthineers, Erlangen, Germany). He was supine and headfirst for the examination. The scrotum was placed between the thighs and maintained it in a fixed position. The scrotal imaging protocol comprised the following sequence: T1-weighted spin echo and T2-weighted spin echo (axis, coronal, and sagittal position), axial fat-suppressed T2-weighted turbo spin echo. T1-weighted image (T1WI): TR500–600 ms, TE15–20 ms. T2WI: TR37,00–5,500 ms, TE80–100 ms. Slice thickness, 3 mm; gap, 0.6 mm; 24 slices.
The following characteristics were studied: location (left, right, and bilateral), boundary (clear and unclear), the presence of calcification, necrosis, the involvement of adjacent tissue, and CT attenuation values in unenhanced and enhanced CT scan. The density and intensity of the lesion were compared with normal testicular parenchyma. The degree of enhancement was classified as none, slight [0–20 Hounsfield unit (HU)], moderate (>20–40 HU), or significant (>40 HU). The uniformity was described as heterogeneous or homogenous.
Statistical analysis
The software SPSS 19.0 for Windows (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR). The Pearson chi-square or Fisher exact test was used to comparing categorical variables. A P-value <0.05 was considered statistically significant.
Results
Baseline characteristics
The main clinical characteristics of all participants are summarized in Table 1. A total of 69 patients diagnosed with testicular and/or epididymal TB with a median age of 43.5 years (IQR 34.3–53.8) were enrolled. The most common initial presentation was scrotal mass and scrotal pain. Elevated body temperature on admission was recognized in 2 (2.9%) participants, and the others were within the normal range. Also, T-SPOT.TB was negative in 1 (1.4%), positive in 7 (10.1%), and not available in 61 (88.5%) participants. Serum ESR was elevated in 7 (10.1%), normal in 6 (8.7%), and not available in 56 (81.2%) participants. The median clinical duration of all participants was 4 months (IQR 2–12 months). Pulmonary TB or renal TB was recognized in 15 (21.7%) and 11 (15.9%) participants, respectively.
Full table
Features of testicular and/or epididymal TB in sonography
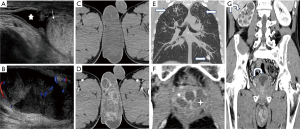
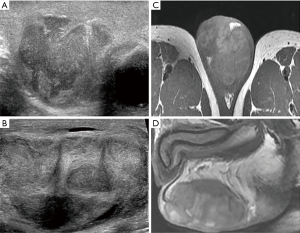
A total of 51 patients underwent scrotal ultrasonic examination, 44 (86.3%) had involvement of the epididymis, and 30 (58.8%) had involvement of testis, as summarized in Table 2. Bilateral involvement of the epididymis or testis was seen in 14 (24.1%) and 9 (30%) participants, respectively. Regarding unilateral lesion, right involvement was prevalent (epididymis, 43.2%; testis, 40.0%). The imaging features of testicular TB and epididymal TB were significantly different (P<0.001). Diffusely enlarged heterogeneously or homogeneously hypoechoic lesion was most common in the epididymis (35, 60.3%), while most of them were heterogeneous (33/35, 94.2%). Miliary type (46.2%) was predominant in testis (Figure 1,2). The nodular enlarged heterogeneously hypoechoic lesion was less common in testicular TB (Figure 3). Space occupying lesions were observed in epididymal TB and testicular TB (Figures 4,5).

Full table
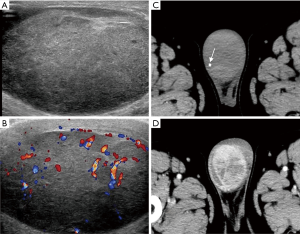
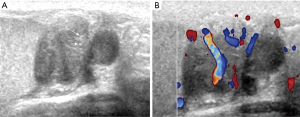
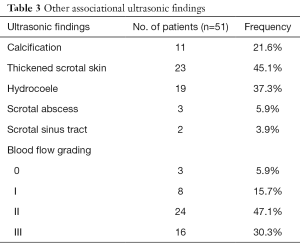
Other ultrasonic associating findings of tubercular orchitis are summarized in Table 3. About half of the participants had thickened scrotal skin (45.1%). Meanwhile, hydrocoele and calcification were common among participants (37.3% and 21.6%, respectively). The CDFI revealed most lesions as moderate with abundant blood flow (grade II and III accounted for 47.1% and 30.3%, respectively).
Full table
CT and MRI features of testicular and/or epididymal TB
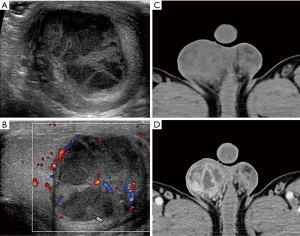
A total of 23 participants underwent enhanced CT examination; the lesions of 13 (56.5%) participants were located in the right side of the scrotum, 7 (30.4%) were located in left, and 3 (13.1%) participants had bilateral lesions. Among 19 participants, 21 space-occupying lesions were observed (19/23, 82.6%), including 5 (5/21, 23.8%) solid masses, 15 (15/21, 71.4%) mixed solid cystic masses, and 1 (1/21, 4.8%) cystic mass. The boundary of most of the lesions was unclear (17/21, 81.0%). After managing contrast agents, all the lesions were heterogeneous enhancement; 19 (19/21, 90.5%) were presented as an annular or multilocular enhancement (Figure 1D,2D,5D). A total of 2 participants manifested with enlarged testis or/and epididymis with inhomogeneous enhancement, and 1 participant presented as the bilateral decreased density of the testes and epididymis without enhancement. Punctate calcification in the testis was recognized in 5 participants.
Pelvic MRI was further performed on 1 participant, demonstrating bilateral scrotal lesions (Figure 3C,D,E). A heterogeneous mass with separation was located on the left side of the scrotum. It was slightly hyperintense on T2-weighted images and hypointense on T1-weighted images, with a size of 7.0 cm × 4.9 cm. The mass was compressing the right testis, and a similar signal nodule sized 2.0 cm × 1.8 cm was observed in the lower part of the mass.
Ultrasonic diagnoses and clinical preoperative diagnoses
A comparison of the ultrasonic and clinical preoperative diagnoses is shown in Table 4. Investigating participants with scrotal ultrasonography led to the main differential diagnoses of TB, space-occupying lesion, and nontuberculous infection (39.2%, 29.4%, and 23.5%, respectively). It was usually challenging to differentiate TB from space-occupying lesions (46.4% vs. 39.1%).

Full table
Definitive diagnosis
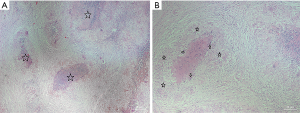
In our study, all participants were diagnosed by confirming Mycobacterium tuberculosis in the histopathology of resected samples during surgery. Well-formed granulomas in a nodular pattern that were recognized through hematoxylin and eosin stain (Figure 6A). Furthermore, central areas of necrosis were surrounded by granulomas composed of numerous epithelioid histiocytes and lymphocytes (Figure 6B). Concurrent involvement of the epididymis and testis was more common than isolated involvement of either (44.9%, 37.7%, and 17.4%, respectively). Meanwhile, right involvement was more common than left and bilateral (44.9%, 34.8%, and 20.3%, respectively).
Discussion
In the past, male genital TB was reported as an uncommon disease (16). Nevertheless, it is reported that the proportion of male genital TB has risen from 5.5% to 25.3% between 2006 and 2019 in some areas around the world (17-19). The underdiagnosis of testicular and/or epididymal TB is of concern, as it can lead to the development of infertility, sexual dysfunction, and other severe complications. Clinicians lack awareness for testicular and/or epididymal TB, which has led to 69% of patients being diagnosed via unnecessary surgery (12). Previous studies have manifested some clinical and ultrasonographic features of testicular and/or epididymal TB. However, these have involved small series of patients, and most were case reports. Some studies were reported a long time ago, while some testicular and/or epididymal TB characteristics may have changed in recent times. Herein, we retrospectively investigated 69 patients from the recent decade, the maximal number to our knowledge. We collected the baseline characteristics, ultrasonic, CT, and MRI features for analysis.
In our study, most participants complained of scrotal mass and scrotal pain on admission. After a careful inquiry about medical history, history, and running a physical examination, we recommend that some laboratory tests are performed selectively, including tumor markers, inflammatory indicators, and indicators associated with TB infection, such as AFP, β-HCG, luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), ESR, CRP, TB antibody, T.SPOT.TB, and so on. Also, imaging examinations are necessary, especially scrotal sonograph. The excellent soft-tissue contrast resolution of MRI may offer more valuable diagnostic information in the differential diagnosis. Biopsy could serve as an alternative diagnostic tool; whether to have it or not depends on the results of the abovementioned examinations. When distinguishing between TB and a tumor has failed, a biopsy is recommended for younger patients with fertility requirements. For elderly patients with no need to preserve fertility and in severe conditions, surgery may be suggested.
This study’s main findings were as follows: firstly, patients with testicular and/or epididymal TB have a relatively long course of the disease (median 4 months, IQR 2–12 months). Painful scrotal mass and flank pain are the most common presenting symptoms. Specifically, specific TB symptoms (fevers, night sweats, weakness, and weight loss) were absent in the present series, for laboratory examinations, T-SPOT. TB may be a valuable and sensitive marker. Secondly, the sonographic patterns of testicular TB and epididymal TB are significantly different. Diffusely enlarged heterogeneous lesions were most common in the epididymis, while miliary types were most often observed in the testis. Thirdly, on enhanced CT, the characteristic manifestation of our participants was annular or multilocular enhancement pattern.
In the study of Kulchavenya et al. (19), the peak age was 40–59 years. The peak age was 43.5 years (IQR, 34.3–53.8 years) in our present study, consisting of previous reports. Suankwan et al. (18) found that patients’ common symptoms include scrotal mass, scrotal pain, urinary tract infection, and nonspecific constitutional symptoms, which was parallel to the findings of our present study.
According to the data available for our study, we found that ESR and CRP were elevated in approximately half of the participants and T-SPOT.TB was positive in the majority of participants (7/8, 87.5%). The T-SPOT.TB test may have the potential as a reliable and sensitive method to recognize testicular and/or epididymal TB from other scrotal lesions. Nevertheless, in consideration of poor accessibility, high fees, false-negative result due to different stages of infection (e.g., specimens obtained before cellular immunity has occurred), and immune system dysfunction/underlying diseases (such as HIV-infection, cancer, infants, and so on), a combination of other examinations is necessary (20).
Scrotal sonography is considered the best examination to evaluate scrotal lesions due to its high resolution, real-time, and accessibility, especially in developing areas. The imaging features of testicular TB and epididymal TB are significantly different (P<0.001), which has not been disclosed before. The diffusely enlarged heterogeneous lesion is the most common in the epididymis, as is the miliary type in the testis. Notably, 12 (38.7%) of the 31 participants with testicular and epididymal TB presented as diffusely enlarged epididymis and miliary type testis heterogeneously simultaneously. The miliary type has been suggested as characteristic of testicular TB in a previous study (21). Herein, we speculated that the presence of a diffusely enlarged heterogeneous epididymis might also be characteristic of epididymal TB.
Other associating ultrasonic findings included thickened scrotal skin, calcification, hydrocoele, scrotal abscess, and sinus tract. Muttarak reported that thickened scrotal skin is most often seen, which was similar to the findings of our present study (14). Calcification was punctate in our series, while coarse calcification was recognized in the tumor as is reported (22). Salmeron et al. (23) reported that caseous abscess involving the scrotal skin could result in sinus tract formation, and it should be regarded as TB until proven otherwise. Besides, it has been reported that 50% of participants in a series had abscesses or sinus formations (24). Nevertheless, few of our participants had abscesses or sinus formations, which may increase patients visiting their doctors and receiving timely interventions over the recent decade.
It is challenging to differentiate TB from nontuberculous infection with sonography, as there are many similarities between the 2 lesions. However, some differences have been discussed in previously reported cases (10,11,14). Nontuberculosis epididymitis is more likely to be homogeneous enlargement, while TB epididymitis favors a heterogeneously hypoechoic pattern. This is highly in keeping with the findings of the present study. Pathologic findings showed that TB was characterized by granulomatous inflammation accompanied by focal or extensive necrosis. This may account for the heterogeneous echo of testicular and-/or epididymal TB. As mentioned above, patients with testicular and/or epididymal TB have a relatively long disease course. Classic clinical symptoms of inflammation such as the acute onset of scrotal redness and swelling, and high fever, can help distinguish acute nontuberculous infection from TB.
Testicular and/or epididymal TB is often confused with a tumor due to similar age, epidemiologic, and nonspecific symptoms. The differential diagnoses consist of seminoma, nonseminoma (e.g., embryonal carcinoma, teratocarcinoma), and lymphoma (22,23). Seminoma and lymphoma are more frequent and tend to be homogeneous on sonography; the echo of seminomas are similar to that of the normal testis, and the lymphoma usually lower than that. Nonseminoma favors heterogeneous, but it tends to occur in children and adolescents. Besides, epididymal presence in conjunction with a testicular lesion is more inclined to infection than a neoplasm. Bilateral involvement is also a useful differentiating feature.
The CT and MRI manifestations of testicular and/or epididymal TB have not been well studied. They are further performed to reveal the characterization of lesions, especially that of space-occupying lesions. In our participants, lesions were characterized as ill-defined heterogeneous with annular or multilocular enhancement. According to pathologic findings, the typical enhancement manifestation was confirmed to be multiple fusional granulomas with caseous necrosis, surrounded by fibrous connective tissue and chronic inflammatory cells. Intramural septal enhancement is recognized as the characteristic manifestation of seminoma, the scrotum's most prevalent tumor (25). There seems to be an overlap between TB and seminoma. Seminoma often manifests as a well-defined homogenous mass, as the lesion is confined by tunica albuginea; however, the boundary of testicular TB is usually unclear due to adhesion with the surrounding tissue. The excellent soft-tissue contrast resolution of MRI can offer more valuable diagnostic information in the differential diagnosis. Patients with seminoma manifest a slightly lower intensity than normal testicular tissue on T2WI. According to different course stages, a wide variety of intensities on T2WI can be manifested by TB (26).
The typical pathological alterations of TB are granulomatous inflammation accompanied by focal or extensive central necrosis. The diffusely enlarged heterogeneous lesions confirmed by sonography to be granulomatous inflammation, caseation necrosis, and fibrosis may result from various pathologic stages of TB. Pathologic findings showed that space-occupying lesions were characterized by multiple fusional granulomas with central caseous necrosis and surrounded by fibrous connective tissue and chronic inflammatory cells. This may also account for annular or multilocular enhancement on enhanced CT.
We summarized the clinical characteristics, ultrasonic, CT, and MRI features, and compared them with pathological manifestations in the maximal number of participants to our knowledge. Some clinical characteristics and radiological features of testicular and/or epididymal TB may have changed in modern times. Our series had some limitations. Firstly, some clinical characteristics (including ESR, CRP, T-SPOT.TB, and others) and imaging examinations were absent in some individuals, and few participants underwent MRI. These limitations were due to the retrospective nature of our study.
Conclusions
In summary, for patients with positive T-SPOT.TB, accompanied by characteristic patterns on scrotal sonography and furtherly confirmed by enhanced CT (annular or multilocular enhancement pattern), clinicians should be very mindful of testicular and/or epididymal TB differentially diagnosing scrotal diseases. A diagnostic model based on clinical symptoms, TB history, laboratory findings (especially T-SPOT.TB), and imaging features should be considered in future research.
Acknowledgments
Funding: National Natural Science Foundation of China (81370855, 81770756, and 81200551) and Sichuan Province Science and Technology Department (2015SZ0230, 2017KJT0034, and 2020YJ0054).
Footnote
Conflicts of Interest. All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-1005). The authors have no conflicts of interest to declare.
Ethical Statement: The Ethics Committee approved the present study of the West China Hospital of Sichuan University. Participant information was anonymized and de-identified before analysis. The informed consent was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dheda K, Barry CE 3rd, Maartens G. Tuberculosis. Lancet 2016;387:1211-26. [Crossref] [PubMed]
- Dadu A, Hovhannesyan A, Ahmedov S, van der Werf MJ, Dara M. Drug-resistant tuberculosis in eastern Europe and central Asia: a time-series analysis of routine surveillance data. Lancet Infect Dis 2020;20:250-8. [Crossref] [PubMed]
- Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med 2020;8:19. [Crossref] [PubMed]
- Qian X, Nguyen DT, Lyu J, Albers AE, Bi X, Graviss EA. Risk factors for extrapulmonary dissemination of tuberculosis and associated mortality during treatment for extrapulmonary tuberculosis. Emerg Microbes Infect 2018;7:102. [Crossref] [PubMed]
- Walzl G, McNerney R, du Plessis N, Bates M, McHugh TD, Chegou NN, Zumla A. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis 2018;18:e199-210. [Crossref] [PubMed]
- Figueiredo AA, Lucon AM. Urogenital tuberculosis: update and review of 8961 cases from the world literature. Rev Urol 2008;10:207-17. [PubMed]
- Kulchavenya E, Kim CS, Bulanova O, Zhukova I. Male genital tuberculosis: epidemiology and diagnostic. World J Urol 2012;30:15-21. [Crossref] [PubMed]
- Yadav S, Singh P, Hemal A, Kumar R. Genital tuberculosis: current status of diagnosis and management. Transl Androl Urol 2017;6:222-33. [Crossref] [PubMed]
- Sah SP, Bhadani PP, Regmi R, Tewari A, Raj GA. Fine needle aspiration cytology of tubercular epididymitis and epididymo-orchitis. Acta Cytol 2006;50:243-9. [Crossref] [PubMed]
- Kim SH, Pollack HM, Cho KS, Pollack MS, Han MC. Tuberculous epididymitis and epididymo-orchitis: sonographic findings. J Urol 1993;150:81-4. [Crossref] [PubMed]
- Chung JJ, Kim MJ, Lee T, Yoo HS, Lee JT. Sonographic findings in tuberculous epididymitis and epididymo-orchitis. J Clin Ultrasound 1997;25:390-4. [Crossref] [PubMed]
- Lee IK, Yang WC, Liu JW. Scrotal tuberculosis in adult patients: a 10-year clinical experience. Am J Trop Med Hyg 2007;77:714-8. [Crossref] [PubMed]
- Adler DD, Carson PL, Rubin JM, Quinn-Reid D. Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings. Ultrasound Med Biol 1990;16:553-9. [Crossref] [PubMed]
- Muttarak M, Peh WC, Lojanapiwat B, Chaiwun B. Tuberculous epididymitis and epididymo-orchitis: sonographic appearances. AJR Am J Roentgenol 2001;176:1459-66. [Crossref] [PubMed]
- Abraham S, Izaguirre Anariba DE, Dua K, Mir M, Ankireddypalli A. A case of testicular tuberculosis mimicking malignancy in a healthy young man. Ther Adv Infect Dis 2016;3:110-3. [Crossref] [PubMed]
- Gómez García I, Gómez Mampaso E, Burgos Revilla J, Molina MR, Sampietro Crespo A, Buitrago LA, Gómez Rodríguez A, Baquero F. Tuberculous orchiepididymitis during 1978-2003 period: review of 34 cases and role of 16S rRNA amplification. Urology 2010;76:776-81. [Crossref] [PubMed]
- Kulchavenya E, Khomyakov V. Male genital tuberculosis in Siberians. World J Urol 2006;24:74-8. [Crossref] [PubMed]
- Suankwan U, Larbcharoensub N, Viseshsindh W, Wiratkapun C, Chalermsanyakorn P. A clinicopathologic study of tuberculous epididymo-orchitis in Thailand. Southeast Asian J Trop Med Public Health 2012;43:951-8. [PubMed]
- Kulchavenya E, Kholtobin D, Shevchenko S. Challenges in urogenital tuberculosis. World J Urol 2020;38:89-94. [Crossref] [PubMed]
- Di L, Li Y. The risk factor of false-negative and false-positive for T-SPOT.TB in active tuberculosis. J Clin Lab Anal 2018;32:e22273 [Crossref] [PubMed]
- Drudi FM, Laghi A, Iannicelli E, Di Nardo R, Occhiato R, Poggi R, Marchese F. Tubercular epididymitis and orchitis: US patterns. Eur Radiol 1997;7:1076-8. [Crossref] [PubMed]
- Cheng L, Albers P, Berney DM, Feldman DR, Daugaard G, Gilligan T, Looijenga L. Testicular cancer. Nat Rev Dis Primers 2018;4:29. [Crossref] [PubMed]
- Salmeron I, Ramirez-Escobar MA, Puertas F, Marcos R, Garcia-Marcos F, Sanchez R. Granulomatous epididymo-orchitis: sonographic features and clinical outcome in brucellosis, tuberculosis and idiopathic granulomatous epididymo-orchitis. J Urol 1998;159:1954-7. [Crossref] [PubMed]
- Borthwick WM. The pathogenesis of tuberculous epididymitis. Edinb Med J 1946;53:55-70. [PubMed]
- Tsili AC, Tsampoulas C, Giannakopoulos X, Stefanou D, Alamanos Y, Sofikitis N, Efremidis SC. MRI in the histologic characterization of testicular neoplasms. AJR Am J Roentgenol 2007;189:W331-7 [Crossref] [PubMed]
- Mohrs OK, Thoms H, Egner T, Brunier A, Eiers M, Kauczor HU, Hallscheidt P. MRI of patients with suspected scrotal or testicular lesions: diagnostic value in daily practice. AJR Am J Roentgenol 2012;199:609-15. [Crossref] [PubMed]


