Non-invasive detection of intracranial pressure related to the optic nerve
Introduction
Intracranial pressure (ICP) refers to the pressure generated by the contents of the cranial cavity [brain tissue, blood, and cerebrospinal fluid (CSF)] on the wall of the cranial cavity. ICP is usually measured in millimeter of mercury and centimeter water column, and normal ICP in supine adults is 7–15, 5–15, or 0–15 mmHg in the literature. Intraocular pressure (IOP) is the pressure exerted by the eye’s contents on the wall and the interaction between the contents of the eyeball. IOP is usually measured in millimeter of mercury. The normal IOP range is from 10–21 mmHg. IOP and ICP are 2 sets of pressure systems that are interrelated and relatively independent. Through the circulation of the aqueous humor and CSF, ICP and IOP are relatively stable (1).
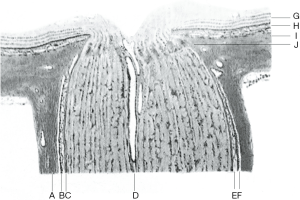
The optic nerve fiber belongs to a part of the central nervous system. It passes through the posterior scleral foramen, where the inner 1/3 of the sclera and the choroid from the lamina cribrosa (LC), and the outer 2/3 of the sclera evolves into the dura. The optic nerve is a part of the alba extending outward from the brain fiber and representing retinal ganglion cells (2,3). The posterior segment of the optic nerve is covered with 3 layers of meninges. The outer layer is the dura mater, the middle layer is the arachnoid mater, and the inner layer is the pia mater. There are 2 spaces between the 3 layers of the tunica vaginalis; the subdural space and the subarachnoid space (Figure 1). The front end of the 2 spaces ends at the back of the eyeball, forming a blind duct, and communicates directly with the brain space backward, full of CSF. The increase in ICP can be transmitted to the optic nerve sheath (ONS) space around the optic nerve through the CSF of the subarachnoid space so that the pressure of the latter also increases, resulting in the phenomenon of tortuosity and diameter increase (4). ONS dilation has been indicated to be an early indicator of ICP increase, so measuring the ONS diameter (ONSD) can detect whether there is an ICP increase and can be used to monitor changes in this increase (5).
Examinations of increased ICP are mainly used in cases of intracranial hemorrhage, trauma, brain injury, hydrocephalus, and idiopathic high ICP (6,7) and can be used as a prognostic indicator of ischemic encephalopathy after cardiac arrest (8). Reducing ICP has gradually become a focus of interest in recent years. The LC is the weak part of the eyeball’s posterior part, and the IOP and ICP are interrelated at the LC. The pressure gradient across the LC has attracted more and more attention in the diagnosis and treatment of glaucoma. High IOP or low ICP is the pathological basis of optic nerve damage in glaucoma (9). Their detection and judgment are beneficial for glaucoma doctor and neuro-ophthalmologists for the early diagnosis of optic nerve diseases and the timely intervention of optic nerve injuries caused by them (10).
ICP measurement mainly depends on invasive methods, including a lumbar puncture (11), external ventricular drainage (12,13), and intraparenchymal-placed probes (14,15). These are the gold standards of ICP measurement. Invasive ICP measurement is accurate and reliable, but there are many risks, such as bleeding, infection, misplacement, and brain hernia (16-19). Therefore, the development of non-invasive ICP measurement technology for the measurement of ICP can avoid invasive probe placed in the spinal canal, reducing the risk of infection and bleeding. However, existing non-invasive ICP measurement technology cannot measure the ICP quantitatively (i.e., taking centimeter water column and other pressure units as the measurement value); it can only estimate the ICP with the correlation between the intracranial physiological markers, so there is no gold standard (20).
Over the years, non-invasive methods for measuring ICP have emerged, including fundoscopy examinations and optical coherence tomography (OCT) (21), flash visual evoked potential (VEP) (22), IOP and venous ophthalmodynamometry (23), tympanic membrane displacement (24), electroencephalogram (25), retinal blood pressure (26), blood flow velocity pulsatility index (27), and cerebral blood flow (28). Due to the large variation and difficulty in implementation, some of these methods are gradually reduced, however, there are several non-invasive methods to detect ICP, which are considered to be more useful. They are closely related to the optic nerve.
As an important link between ICP and IOP, optic nerve can reflect the balance between them. The optic nerve can be used as an important part of ICP measurement; however, the imbalance between ICP and IOP will also affect the optic nerve. In the present study, we review the non-invasive ICP detection related to the optic nerve; the principles, similarities, and differences of these methods; and the current research progress.
Ocular ultrasound
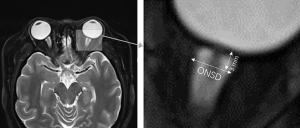
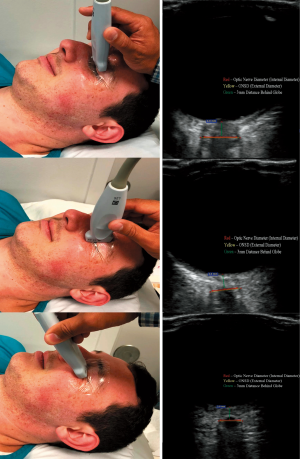
Ocular ultrasound is a non-invasive measurement of ICP. The method is to measure the ONSD 3 mm behind the eyeball when the optic nerve ultrasound shows clear and uniform low reflection band extending backward from the base, and the ONS parallel shows bilateral thin and weak echo lines (29). Each eye needs to measure multiple angles and then average them (30) (Figure 2). Amini et al. found that in patients with non-traumatic intracranial hypertension, ONSD was associated with increased ICP, which could be detected when ICP was increased by 5 mmHg, with high sensitivity and specificity, and increased ICP up to 15 mmHg could be detected all the time (32). Previous studies have suggested that ultrasonic detection of the ONSD to estimate ICP is limited to the increase of ICP, but it is not associated with reduced ICP (33). However, recent studies have shown that ultrasound can also detect a decrease in ICP (34). Also, it has been reported that the sensitivity of ultrasound measurement of the ONSD is >90%, and ultrasound can be carried out quickly at the bedside of patients at any time, feasible, simple, and easy to learn, and has obvious advantages in the examination of patients with brain injury (35-37).
Although ONSD is widely used to monitor ICP, its application is still controversial. First, there are different opinions on the method of ultrasound examination. The values measured by ultrasound examination on different planes vary greatly, and the repeatability is poor. Moreover, different probes will also make the ONSD difference (38-40). Some studies have shown that when the eyeball is in the maximum abduction position and the ONSD is reduced by more than 5%, subarachnoid fluid increases (41). Second, the measurement of ONSD requires high-quality ultrasound images (31). When performing ultrasound examination on the same patient, it may appear that high-quality images may be obtained in one view, but not in another view. It is difficult to obtain high-quality images in all directions, and even some patients do not have high-quality images from all directions. If the low-quality image is used to calculate ONSD, we may get the wrong result for ICP, which may be due to the B-wave measurement’s shadow effect in ultrasound (42). Third, the ultrasound characteristics determine that the closer the measurement is to the eyeball, the more artifacts will appear, leading to inaccurate measurement results and significant differences. The final measurement may be the shadow caused by the optic nerve, not the ONS (43).
The measurement results will be biased due to poor interference angle, limited horizontal resolution, and ultrasonic blooming effect. Therefore, when the gain is not significant, the ONSD will appear larger, and when the gain increases, the ONSD decreases (44). At the same time, the measurement results of different ultrasound technicians will be different. In their study, Zoerle et al. found that, after treatment of elevated ICP, the decrease in ICP caused by CSF drainage was not reflected by the corresponding ONSD changes (45). Fourth, the parenchymal thickening of the pia mater and arachnoid sheath caused by optic neuritis, optic glioma, meningioma or leukemia optic nerve infiltration, and other diseases may lead to deviation of ultrasonographic results of ONSD (46,47). Finally, when using ultrasound, its safety needs to be considered. Studies have shown that when ultrasound is used frequently, especially with high-frequency and low-intensity ultrasonic beam absorption, the increase of attenuation will reduce energy, which will be converted into heat, resulting in the emergence of hydroxide and hydrogen-free radicals, which may cause irreversible structure change of the lens, vitreous body, and retina (48,49). To avoid any possible damage, the output of the ultrasonic system should be adjusted according to the ALARA principle (“as low as reasonably achievable”) (29).
Cennamo et al. report that the standardized A-scan can be used to measure the ONSD. When measuring, the A-scan probe was placed at the main position of the lateral canthus of the eye and tilted backward at the orbital level of the optic nerve until the characteristic optic nerve pattern was observed on the echo screen. All measurements were made at a distance of about 2–5 mm behind the globe. When the sound beam was perpendicular to the anterior third of the optic nerve’s surface, the highest bimodal peak corresponding to the arachnoid surface was obtained from each side of the nerve (50). It is difficult to achieve the technology of the A-scan optic nerve, and not every ultrasonic machine has this function (32).
Magnetic resonance imaging (MRI)
MRI is another non-invasive method of ICP measurement that has received significant attention. MRI of the ONSD is similar to that of ultrasound, and the image is relatively clearer. MRI can also exclude ICP elevation caused by other potential reasons when performing optic nerve examination (51). Usually, the neuroimaging manifestations of intracranial hypertension include empty sella, tight subarachnoid spaces, retrobulbar flattening, ONS dilation, cerebellar tonsil herniation, meningocele, transverse venous sinus stenosis, and vertical tortuosity of the optic nerve (52-56). T2-weighted MRI was used to analyze the orbital structure and ONS. Most of the optic nerve reports about ICP came from thin sections of the orbit or volumetric fat-saturated T2-weighted images. The diameter of the axial and coronal positions of the 3-mm retrobulbar were averaged (57,58) (Figure 3). Compared with B-ultrasound, MRI has higher spatial resolution and can distinguish the optic nerve from tunica vaginalis, so the calculation of ONSD is more representative. A study of idiopathic intracranial hypertension (IIH), 5 patients with headache, blurred vision, and bilateral optic disc edema was examined by MRI. The results showed that the diffusion of the optic nerve head in both eyes was limited. After a lumbar puncture, it was confirmed that the CSF opening pressure was >25 cmH2O (59). The reason for this could be the compression of the central retinal artery and vein when ICP increased and may be a manifestation of increased ICP. Alperin et al. used another method to measure ICP, elastic index, which uses MRI to calculate the ratio of pressure to volume change. The principle of this method is that the derivative of volume to pressure decreases with the increase of ICP, and the ratio of the derivative is used to predict ICP (60). MRI-ICP software based on this principle makes calculations easier, and Alperin et al. used this method to estimate craniospinal compliance and improve supine and standing pressures (61). The pressure volume index measured by MRI was proposed to obtain the skull and spinal cord’s compliance index. It was found that the contribution of the spinal canal in IIH was significantly less than that in the normal control group (60% vs. 78%) (62). Moreover, as opposed to other imaging methods, MRI-ICP can also estimate the decrease of ICP. At present, in glaucoma-related studies, MRI-ICP is considered to be the most suitable choice to estimate low or normal ICP (63). However, MRI also has its limitations. It is impractical to transfer a potentially unstable critical patient for a long period, and frequent radiography will result in high costs (64).
Two-depth transcranial Doppler (TD-TCD)
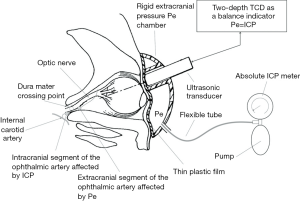
According to the anatomical characteristics of the ophthalmic artery accompanying the optic nerve at the optic canal, and based on the concept of non-invasive arterial blood pressure measurement, the principle of TD-TCD is to use the ophthalmic artery as an ICP sensor to simultaneously measure the blood flow velocity of the intracranial and extracranial segments of the ophthalmic artery to obtain the value of ICP (65) (Figure 4). At present, it is considered to be the only non-invasive method that can accurately measure the absolute value of ICP (66). This method does not need to be calibrated for specific patients, and a specific head frame with an ultrasonic transducer is used to place over the closed eyelid (67). Hamarat et al. used multidepth TCD to pre-locate the depth range of the internal carotid artery edge and simultaneously locate the depth of intracranial segments of the ophthalmic artery extracranial segments of the ophthalmic artery segments to carry out reliable non-invasive ICP measurement (68). TD-TCD or multidepth TCD has high accuracy and reliable measurements and it is suitable for patients without anatomical variation of vascular branches at the optic nerve (>96%) (69). A study of 62 neurology patients showed that the non-invasive TD-TCD device was accurate (standard deviation: 2.19 mmHg) and accurate (mean systematic error: 0.12 mmHg) compared with gold standard CSF pressure measured by lumbar puncture. The mean ICP measured by non-invasive TD-TCD equipment was 12.76 (3.23) mmHg (range, 4.04–23.71) and 13.18 (2.99) mmHg (range, 4.41–24.26) by lumbar puncture. To assess its effectiveness in glaucoma patients, Krakauskaite et al. conducted a pilot study and found that patients with glaucoma had a higher translaminar pressure gradient (TPG) than healthy controls (P<0.001) (66). Also, the decrease of the neuroretinal marginal area was found to be related to the increase of the TPG across the LC in normal tension glaucoma and glaucoma patients (70). However, this method depends on whether the optic nerve’s subarachnoid pathway from the intracranial to intraocular segments is smooth. If the optic canal or the optic canal foramen is blocked by suprasellar meningioma, annular adhesion after tuberculous meningitis, or aneurysm of the optic canal, the accurate ICP value cannot be measured (71). Because of putting pressure on the eyeball, this method is not suitable for patients with ocular trauma, and it also needs to pay attention to the risk of oculocardiac reflex (72). Oculocardiac reflex is the eyeball is mechanically stimulated when the eyeball is removed, compressed, or the eye muscle is stretched, which causes the vagus nerve to be overexcited, leading to arrhythmia, the slower pulse. The reflex arc of the oculocardiac reflex is from the ophthalmic branch of the trigeminal nerve to the pontine nucleus of the trigeminal nerve, and then to the dorsal nucleus of the vagus nerve, leading to the myocardial reaction.
OCT
OCT is a sensitive and accurate method for detecting optic nerve head lesions. In a cohort study of 104 IIH patients, Vijay et al. found that the central thickness of the optic nerve head examined by OCT was closely related to ICP (right eye: r=0.60, P=0.02; left eye: r=0.73, P=0.002) (21). There was a longitudinal correlation between the central thickness of the optic nerve head and ICP (12 and 24 months)and a positive correlation between the central thickness of the optic nerve head and ICP at all points (73). OCT does not only monitor papillary edema in IIH but also predicts the ICP level non-invasively. The limitation of OCT in the detection of optic edema is that the penetrability of OCT is reduced due to the elevation and edema of the optic nerve, which may hinder the recognition of measurement markers (such as Bruch’s membrane) (74). Also, OCT cannot distinguish whether the decrease of optic disc thickness is due to the axonal loss of retinal ganglion cells caused by optic papilledema or the decrease of ICP (75).
VEP
The correlation between VEP and ICP was found in the 1980s (76). Haredy et al. studied 13 patients with cranial process neuropathy and found that the average latency of patients with elevated ICP (118.7 ms) was longer than that of patients with normal ICP (108.1 ms) and the average amplitude of patients with elevated ICP (12.4 µV) was significantly lower than that of patients with normal ICP (23.3 µV) (P=0.03), indicating the practicability of VEP in monitoring ICP (77). Vieira et al. studied 18 patients with cryptococcal meningitis and found that VEP N2 latency was strongly positively correlated with ICP (r=0.83; 95% CI: 0.60–0.94, P<0.0001) (78). Like OCT, the limitation of VEP is that elevated ICP has a negative effect on the visual pathway (e.g., causing optic atrophy), which will permanently affect the VEP test results, resulting in inaccurate estimation of the ICP (77).
Venous ophthalmodynamometry
Firsching et al. measured the pressure of the central retinal vein in 102 patients and statistically confirmed that the central retinal vein’s pressure was highly correlated with ICP. The increase of central retinal vein pressure indicated that ICP was increased; the probability of occurrence was 84.2%, and 92.8% of patients with normal central retinal vein pressure had normal ICP (23). The limitation of this method was that there was a significant difference between patients (79). Some studies have found that there is only a weak negative correlation between test performance and estimated ICP (80).
Spontaneous retinal venous pulsations
Spontaneous retinal venous pulsations assessed with both fundoscopy and infrared videos can be used to monitor the ICP. D’Antona et al. performed multiple linear regression analyses on 105 patients and confirmed that there was a significant correlation between ICP and spontaneous venous pulsation (r=–9.1; 95% CI: –13.7 to –4.6, P<0.001, adjusted R2=0.42). They found that infrared video assessment of spontaneous retinal vein pulsation was an effective indicator of elevated ICP in patients without papilledema (81). However, this method was limited to patients without papilloma, so its scope of application was limited (82).
Changes in eye tracking
Eye tracking can be used as a non-invasive automatic method to quantify the physiological effects of elevated ICP. Kolecki et al. performed eye tracking in 23 patients with ICP levels ranging from –3 to 30 mmHg 55 times. The results showed that eye-tracking measures related to cranial nerve function decreased linearly with the increase of ICP (P<0.001) (83). The main limitation of the method was the lack of continuous data in patients with high ICP records. Moreover, few patients with elevated ICP opened their eyes long enough for eye tracking to be performed. Also, all patients’ neuropathological status not only leads to an increase in ICP but also to other pathophysiological changes that may affect eye movement (84).
Discussion
ICP assessment plays an important role in the monitoring and early warning of neurological diseases and in determining the occurrence, development, and prognosis of many diseases. The non-invasive, safe, and accurate measurement of ICP has been clinicians’ focus for many years. Because of the optic nerve’s anatomic characteristics, most non-invasive ICP detection methods are focused on the optic nerve and its surroundings. ICP detection has 2 aspects. The first is the increase of ICP, which is suitable for tumor, trauma, hemorrhage, and other brain and spinal cord diseases. The second is that low ICP can be used to evaluate glaucoma in ophthalmology. Also, ophthalmic signs of low ICP have been found in patients with low CSF pressure/volume syndromes, such as spontaneous intracranial hypotension and CSF leaks. For the measurement of the optic nerve, the ONSD is the most commonly used method.
In most cases, the increase of ICP will lead to the transfer of CSF to ONS and increase the ONSD. Similarly, when the ICP decreases, CSF returns to the subarachnoid space, and ONSD decreases accordingly. However, the process mentioned above needs to follow the elastic conservation so that the ONS can return to the original diameter after expansion, which is suitable for the application of ONSD to estimate ICP (85). However, in some cases, such as the rupture of intracranial aneurysms, ICP can suddenly rise, which will cause acute irreversible expansion of the ONS. At this time, the ONSD cannot accurately reflect ICP changes (86). Also, when ICP is elevated for the long term, the ONS will lose its elasticity due to long-term expansion, and the optic nerve edema will continue well after the ICP returns to normal; optic nerve atrophy after the optic nerve edema subsidies will also make the ICP estimation inaccurate. Therefore, the application of the ONSD to evaluate ICP has its limitations (87).
However, the measurement of ONSD is mainly determined by ultrasound or MRI (35,57). However, they all estimate ICP through onsd on the premise that there is no pathological change in the optic nerve itself. For example, if the patient has optic neuritis or tumor and other diseases that can cause edema, thickening or atrophy of the optic nerve itself, then measuring onsd to estimate ICP will not be accurate (46,71). It is also important to understand the optic nerve changes after ICP increases or decreases and whether the change is immediate or delayed. This also has a great impact on the estimation and management of the disease. If ICP is increased for a long time, the ONS’s elasticity will decrease, and the irreversible damage of the optic nerve will also affect the results of ICP (85,87). Hansen et al. found that when a pressure load of ≥45–55 mmHg is applied, the ONSD no longer reaches its baseline value. Therefore, they proposed that when using ONSD detection to evaluate ICP in neurological intensive care and emergency medicine, the impaired reversibility of the ONSD should be considered after a prolonged duration of intracranial hypertension. A hysteresis model was proposed (88). Some studies have shown that there is an obvious correlation between the ONSD measured by ultrasound and MRI (89-91), but some studies have found that there is almost no consistency between the results of ultrasound and MRI (92). Other studies have shown that increasing the magnetic field intensity of MRI inspection can make the image resolution higher and clearer, which is more conducive to obtaining accurate ONSD values (58). Moreover, these are non-invasive measurements and are not gold standards, so it is unclear which methods can correctly reflect the actual ONSD value. Therefore, other detection methods need to be developed to limit this limitation (Table 1).
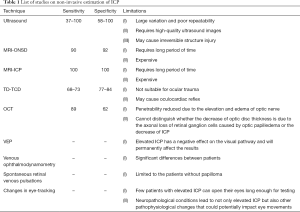
Full table
Studies have shown that ICP and IOP are both dynamic parameters in physiology. Both have circadian rhythm changes and similar and corresponding responses to temperature changes, body position, and chest and abdominal pressure. Nogueira et al. found that, the circadian rhythm pattern of body temperature is associated with further ICP values in patients with severe brain injury receiving hypothermia treatment (Pearson correlation: –0.861, adjusted R2=0.725, P<0.001) (93). Robba et al. prospectively studied 40 patients undergoing peritoneal endoscopic surgery. Three different methods were used for the non-invasive estimation of ICP, with a significant increase in ONSD after pneumonic injection combined with Trendelenburg position (94). The physiological cycle of IOP is clear (95), but the circadian rhythm pattern of ICP is not (96). Whether the changes of ICP and IOP will affect the detection results of the ONSD is still unknown. Liu et al. found that Valsalva maneuver increased ICP transiently, which was significantly higher than IOP (97). The CSF pressure measured during lumbar puncture is the value of lateral lying. A prospective study of 30 patients hospitalized in the neuro-intensive care unit showed a significant increase in ICP when they were placed at 15-degree right and left lateral positions and at a 45-degree right lateral position (98). According to Schwartz et al., ICP measurements in the prone position were significantly higher than those in the lateral position. The mean difference was 2.7 cmH2O (P<0.001) and 1.6 cmH2O (P=0.017) for prone flat and prone tilted, respectively (99). Some scholars found that whether the pressure of CSF in the spinal canal is consistent with that around the optic nerve is still uncertain. CSF around the optic nerve and in the ventricle is connected in the chiasmatic cistern, and there are many membranes in the chiasmatic cistern, which may restrict the free flow of CSF, change the dynamics of CSF, and affect the transmission of pressure (100,101). Also, all the examinations are instantaneous and cannot represent the long-term changes and fluctuations of ICP. Besides, the patient’s general condition may affect the ICP value. Some patients are in good general condition, such as patients with IIH or glaucoma, while others are in poor general condition, such as patients with traumatic brain injury or cerebral hemorrhage.
The above non-invasive ICP measurement methods of the optic nerve and its surrounding tissues are currently the focus of research. So far, MRI-ICP and TD-TCD have been more accurate and closer to invasive detection’s gold standard (64,65,93). Other technologies cannot directly obtain the values, and it is difficult to estimate ICP. Therefore, a conversion and correction formula needs to be developed (102). More in-depth research or more reliable detection methods should be developed to treat and judge ICP as a standard for treatment and prognosis. Non-invasive ICP detection for the optic nerve is the focus of current research but warrants further study. In future research, scholars’ challenges will be how to improve the accuracy and repeatability of detection. Also, the current detection indicators and application parameters are not unified, sample sizes are small, and there is a lack of large-scale, multi-center, and multi-ethnic research data and a lack of long-term tracking change results. Furthermore, due to patients’ different conditions, inspection standards may vary, and the credibility of the results is not high enough to form a suitable conclusion. Future research should consider using larger research populations, obtain more extensive data on ICP, develop more suitable new technology, and provide more help for clinical diagnosis and treatment of neurology and ophthalmic diseases.
Acknowledgments
Funding: This work was supported by grants from the Natural Science Foundation of Xinjiang Province (No. 2020D01A122), the Natural Science Foundation of Liaoning Province (No. 2020-MS-07) and the Natural Science Foundation of Liaoning Province (No. 2020-MS-01).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-1188). The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ficarrotta KR, Passaglia CL. Intracranial pressure modulates aqueous humour dynamics of the eye. J Physiol 2020;598:403-13. [Crossref] [PubMed]
- Lindén C, Qvarlander S, Jóhannesson G, Johansson E, Östlund F, Malm J, Eklund A. Normal-Tension Glaucoma Has Normal Intracranial Pressure: A Prospective Study of Intracranial Pressure and Intraocular Pressure in Different Body Positions. Ophthalmology 2018;125:361-8. [Crossref] [PubMed]
- Chen CL, Bojikian KD, Gupta D, Wen JC, Zhang Q, Xin C, Kono R, Mudumbai RC, Johnstone MA, Chen PP, Wang RK. Optic nerve head perfusion in normal eyes and eyes with glaucoma using optical coherence tomography-based microangiography. Quant Imaging Med Surg 2016;6:125-33. [Crossref] [PubMed]
- Hayreh SS. Pathogenesis of optic disc edema in raised intracranial pressure. Prog Retin Eye Res 2016;50:108-44. [Crossref] [PubMed]
- Maissan IM, Dirven PJ, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg 2015;123:743-7. [Crossref] [PubMed]
- Mangat HS, Chiu YL, Gerber LM, Alimi M, Ghajar J, Härtl R. Hypertonic saline reduces cumulative and daily intracranial pressure burdens after severe traumatic brain injury. J Neurosurg 2015;122:202-10. [Crossref] [PubMed]
- Kawoos U, McCarron RM, Auker CR, Chavko M. Advances in Intracranial Pressure Monitoring and Its Significance in Managing Traumatic Brain Injury. Int J Mol Sci 2015;16:28979-97. [Crossref] [PubMed]
- Reis C, Akyol O, Araujo C, Huang L, Enkhjargal B, Malaguit J, Gospodarev V, Zhang JH. Pathophysiology and the Monitoring Methods for Cardiac Arrest Associated Brain Injury. Int J Mol Sci 2017;18:129. [Crossref] [PubMed]
- Gallina P, Savastano A, Becattini E, Orlandini S, Scollato A, Rizzo S, Carreras G, Di Lorenzo N, Porfirio B. Glaucoma in patients with shunt-treated normal pressure hydrocephalus. J Neurosurg 2018;129:1078-84. [Crossref] [PubMed]
- Siaudvytyte L, Januleviciene I, Daveckaite A, Ragauskas A, Siesky B, Harris A. Neuroretinal rim area and ocular haemodynamic parameters in patients with normal-tension glaucoma with differing intracranial pressures. Br J Ophthalmol 2016;100:1134-8. [Crossref] [PubMed]
- Wang F, Lesser ER, Cutsforth-Gregory JK, Bhatti MT, Kilgore KP, Hodge DO, Graff-Radford J, Petersen RC, Knopman DS, Mielke MM, Lanzino G, Leavitt JA, Chen JJ. Population-Based Evaluation of Lumbar Puncture Opening Pressures. Front Neurol 2019;10:899. [Crossref] [PubMed]
- Bales JW, Bonow RH, Buckley RT, Barber J, Temkin N, Chesnut RM. Primary External Ventricular Drainage Catheter Versus Intraparenchymal ICP Monitoring: Outcome Analysis. Neurocrit Care 2019;31:11-21. [Crossref] [PubMed]
- Sun Z, Wu L, Liu Z, Zhong W, Kou Z, Liu J. Optimizing accuracy of freehand cannulation of the ipsilateral ventricle for intracranial pressure monitoring in patients with brain trauma. Quant Imaging Med Surg 2020;10:2144-56. [Crossref] [PubMed]
- Behfar MH, Abada E, Sydanheimo L, Goldman K, Fleischman AJ, Gupta N, Ukkonen L, Roy S. Inductive passive sensor for intraparenchymal and intraventricular monitoring of intracranial pressure. Annu Int Conf IEEE Eng Med Biol Soc 2016;2016:1950-4. [Crossref] [PubMed]
- Marbacher S, Milavec H, Neuschmelting V, Andereggen L, Erhardt S, Fandino J. Outer skull landmark-based coordinates for measurement of cerebral blood flow and intracranial pressure in rabbits. J Neurosci Methods 2011;201:322-6. [Crossref] [PubMed]
- Rajajee V, Williamson CA, Fontana RJ, Courey AJ, Patil PG. Noninvasive Intracranial Pressure Assessment in Acute Liver Failure. Neurocrit Care 2018;29:280-90. [Crossref] [PubMed]
- Robba C, Cardim D, Tajsic T, Pietersen J, Bulman M, Rasulo F, Bertuetti R, Donnelly J, Xiuyun L, Czosnyka Z, Cabeleira M, Smielewski P, Matta B, Bertuccio A, Czosnyka M. Non-invasive Intracranial Pressure Assessment in Brain Injured Patients Using Ultrasound-Based Methods. Acta Neurochir Suppl 2018;126:69-73. [Crossref] [PubMed]
- Hockel K, Schuhmann MU. ICP Monitoring by Open Extraventricular Drainage: Common Practice but Not Suitable for Advanced Neuromonitoring and Prone to False Negativity. Acta Neurochir Suppl 2018;126:281-6. [Crossref] [PubMed]
- Pitfield AF, Carroll AB, Kissoon N. Emergency management of increased intracranial pressure. Pediatr Emerg Care 2012;28:200-4; quiz 205-7. [Crossref] [PubMed]
- Nag DS, Sahu S, Swain A, Kant S. Intracranial pressure monitoring: Gold standard and recent innovations. World J Clin Cases 2019;7:1535-53. [Crossref] [PubMed]
- Vijay V, Mollan SP, Mitchell JL, Bilton E, Alimajstorovic Z, Markey KA, Fong A, Walker JK, Lyons HS, Yiangou A, Tsermoulas G, Brock K, Sinclair AJ. Using Optical Coherence Tomography as a Surrogate of Measurements of Intracranial Pressure in Idiopathic Intracranial Hypertension. JAMA Ophthalmol 2020;138:1264-71. [Crossref] [PubMed]
- Andersson L, Sjölund J, Nilsson J. Flash visual evoked potentials are unreliable as markers of ICP due to high variability in normal subjects. Acta Neurochir (Wien) 2012;154:121-7. [Crossref] [PubMed]
- Firsching R, Müller C, Pauli SU, Voellger B, Röhl FW, Behrens-Baumann W. Noninvasive assessment of intracranial pressure with venous ophthalmodynamometry. Clinical article. J Neurosurg 2011;115:371-4. [Crossref] [PubMed]
- Gwer S, Sheward V, Birch A, Marchbanks R, Idro R, Newton CR, Kirkham FJ, Lin JP, Lim M. The tympanic membrane displacement analyser for monitoring intracranial pressure in children. Childs Nerv Syst 2013;29:927-33. [Crossref] [PubMed]
- Kristiansson H, Nissborg E, Bartek J Jr, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol 2013;25:372-85. [Crossref] [PubMed]
- Dattilo M, Read AT, Samuels BC, Ethier CR. Detection and characterization of tree shrew retinal venous pulsations: An animal model to study human retinal venous pulsations. Exp Eye Res 2019;185:107689 [Crossref] [PubMed]
- Zweifel C, Czosnyka M, Carrera E, de Riva N, Pickard JD, Smielewski P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery 2012;71:853-61. [Crossref] [PubMed]
- Ruesch A, Yang J, Schmitt S, Acharya D, Smith MA, Kainerstorfer JM. Estimating intracranial pressure using pulsatile cerebral blood flow measured with diffuse correlation spectroscopy. Biomed Opt Express 2020;11:1462-76. [Crossref] [PubMed]
- Kishk NA, Ebraheim AM. Accuracy and safety of B-scan optic nerve ultrasonography to predict increased intracranial pressure in idiopathic intracranial hypertension. Neuroradiol J 2019;32:225-6. [Crossref] [PubMed]
- Robba C, Santori G, Czosnyka M, Corradi F, Bragazzi N, Padayachy L, Taccone FS, Citerio G. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: a systematic review and meta-analysis. Intensive Care Med 2018;44:1284-94. [Crossref] [PubMed]
- Raval R, Shen J, Lau D, Ferguson N, Kelly T, Daniels J, Dorotta I, Ramsingh D. Comparison of Three Point-of-Care Ultrasound Views and MRI Measurements for Optic Nerve Sheath Diameter: A Prospective Validity Study. Neurocrit Care 2020;33:173-81. [Crossref] [PubMed]
- Amini A, Kariman H, Arhami Dolatabadi A, Hatamabadi HR, Derakhshanfar H, Mansouri B, Safari S, Eqtesadi R. Use of the sonographic diameter of optic nerve sheath to estimate intracranial pressure. Am J Emerg Med 2013;31:236-9. [Crossref] [PubMed]
- De Bernardo M, Vitiello L, Rosa N. A-scan ultrasonography and optic nerve sheath diameter assessment during acute elevations in intra-abdominal pressure. Surgery 2020;167:1023-4. [Crossref] [PubMed]
- Fichtner J, Ulrich CT, Fung C, Knüppel C, Veitweber M, Jilch A, Schucht P, Ertl M, Schömig B, Gralla J. Z’Graggen WJ, Bernasconi C, Mattle HP, Schlachetzki F, Raabe A, Beck J. Management of spontaneous intracranial hypotension - Transorbital ultrasound as discriminator. J Neurol Neurosurg Psychiatry 2016;87:650-5. [Crossref] [PubMed]
- Strumwasser A, Kwan RO, Yeung L, Miraflor E, Ereso A, Castro-Moure F, Patel A, Sadjadi J, Victorino GP. Sonographic optic nerve sheath diameter as an estimate of intracranial pressure in adult trauma. J Surg Res 2011;170:265-71. [Crossref] [PubMed]
- Liu D, Li Z, Zhang X, Zhao L, Jia J, Sun F, Wang Y, Ma D, Wei W. Assessment of intracranial pressure with ultrasonographic retrobulbar optic nerve sheath diameter measurement. BMC Neurol 2017;17:188. [Crossref] [PubMed]
- Lochner P, Czosnyka M, Naldi A, Lyros E, Pelosi P, Mathur S, Fassbender K, Robba C. Optic nerve sheath diameter: present and future perspectives for neurologists and critical care physicians. Neurol Sci 2019;40:2447-57. [Crossref] [PubMed]
- Kishk NA, Ebraheim AM, Ashour AS, Badr NM, Eshra MA. Optic nerve sonographic examination to predict raised intracranial pressure in idiopathic intracranial hypertension: The cut-off points. Neuroradiol J 2018;31:490-5. [Crossref] [PubMed]
- Wang N, Xie X, Yang D, Xian J, Li Y, Ren R, Peng X, Jonas JB, Weinreb RN. Orbital cerebrospinal fluid space in glaucoma: the Beijing intracranial and intraocular pressure (iCOP) study. Ophthalmology 2012;119:2065-73.e1. [Crossref] [PubMed]
- Wang J, Li K, Li H, Ji C, Wu Z, Chen H, Chen B. Ultrasonographic optic nerve sheath diameter correlation with ICP and accuracy as a tool for noninvasive surrogate ICP measurement in patients with decompressive craniotomy. J Neurosurg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Zhang X, Medow JE, Iskandar BJ, Wang F, Shokoueinejad M, Koueik J, Webster JG. Invasive and noninvasive means of measuring intracranial pressure: a review. Physiol Meas 2017;38:R143-82. [Crossref] [PubMed]
- Liu D, Michon J. Measurement of the subarachnoid pressure of the optic nerve in human subjects. Am J Ophthalmol 1995;119:81-5. [Crossref] [PubMed]
- Liu D, Kahn M. Measurement and relationship of subarachnoid pressure of the optic nerve to intracranial pressures in fresh cadavers. Am J Ophthalmol 1993;116:548-56. [Crossref] [PubMed]
- De Bernardo M, Vitiello L, Rosa N. Sonographic evaluation of optic nerve sheath diameter in idiopathic intracranial hypertension. J Clin Neurosci 2020;73:331-2. [Crossref] [PubMed]
- Zoerle T, Caccioppola A, D’Angelo E, Carbonara M, Conte G, Avignone S, Zanier ER, Birg T, Ortolano F, Triulzi F, Stocchetti N. Optic Nerve Sheath Diameter is not Related to Intracranial Pressure in Subarachnoid Hemorrhage Patients. Neurocrit Care 2020;33:491-8. [Crossref] [PubMed]
- Ganslandt O, Mourtzoukos S, Stadlbauer A, Sommer B, Rammensee R. Evaluation of a novel noninvasive ICP monitoring device in patients undergoing invasive ICP monitoring: preliminary results. J Neurosurg 2018;128:1653-60. [Crossref] [PubMed]
- Meeker AR, Ko MW, Carruth BP, Strumpf KB, Bersani TA. Diagnosis of optic nerve sheath meningioma during optic nerve sheath decompression. Orbit 2017;36:35-8. [Crossref] [PubMed]
- Modrzejewska M. Guidelines for ultrasound examination in ophthalmology. Part III: Color Doppler ultrasonography. J Ultrason 2019;19:128-36. [Crossref] [PubMed]
- King RL, Liu Y, Harris GR. Quantification of Temperature Rise within the Lens of the Porcine Eye Caused by Ultrasound Insonation. Ultrasound Med Biol 2017;43:476-81. [Crossref] [PubMed]
- Cennamo G, Montorio D, Breve MA, Brescia Morra V, Menna F, Cennamo G. Evaluation of optic nerve subarachnoid space in primary open angle glaucoma using ultrasound examination. PLoS One 2018;13:e0208064 [Crossref] [PubMed]
- Saindane AM, Qiu D, Oshinski JN, Newman NJ, Biousse V, Bruce BB, Holbrook JF, Dale BM, Zhong X. Noninvasive Assessment of Intracranial Pressure Status in Idiopathic Intracranial Hypertension Using Displacement Encoding with Stimulated Echoes (DENSE) MRI: A Prospective Patient Study with Contemporaneous CSF Pressure Correlation. AJNR Am J Neuroradiol 2018;39:311-6. [Crossref] [PubMed]
- Yuh EL, Dillon WP. Intracranial hypotension and intracranial hypertension. Neuroimaging Clin N Am 2010;20:597-617. [Crossref] [PubMed]
- Holbrook J, Saindane AM. Imaging of Intracranial Pressure Disorders. Neurosurgery 2017;80:341-54. [Crossref] [PubMed]
- Vaghela V, Hingwala DR, Kapilamoorthy TR, Kesavadas C, Thomas B. Spontaneous intracranial hypo and hypertensions: an imaging review. Neurol India 2011;59:506-12. [Crossref] [PubMed]
- Agid R, Farb RI, Willinsky RA, Mikulis DJ, Tomlinson G. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology 2006;48:521-7. [Crossref] [PubMed]
- Bidot S, Saindane AM, Peragallo JH, Bruce BB, Newman NJ, Biousse V. Brain Imaging in Idiopathic Intracranial Hypertension. J Neuroophthalmol 2015;35:400-11. [Crossref] [PubMed]
- Kim DH, Jun JS, Kim R. Measurement of the Optic Nerve Sheath Diameter with Magnetic Resonance Imaging and Its Association with Eyeball Diameter in Healthy Adults. J Clin Neurol 2018;14:345-50. [Crossref] [PubMed]
- Geeraerts T, Newcombe VF, Coles JP, Abate MG, Perkes IE, Hutchinson PJ, Outtrim JG, Chatfield DA, Menon DK. Use of T2-weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit Care 2008;12:R114. [Crossref] [PubMed]
- Nagarajan E, Digala LP, Sivaraman M, Bollu PC. Is Magnetic Resonance Imaging Diffusion Restriction of the Optic Disc Head a New Marker for Idiopathic Intracranial Hypertension? J Neurosci Rural Pract 2020;11:170-4. [Crossref] [PubMed]
- Alperin N, Bagci AM. Spaceflight-Induced Visual Impairment and Globe Deformations in Astronauts Are Linked to Orbital Cerebrospinal Fluid Volume Increase. Acta Neurochir Suppl 2018;126:215-9. [Crossref] [PubMed]
- Alperin N, Lam BL, Tain RW, Ranganathan S, Letzing M, Bloom M, Alexander B, Aroucha PR, Sklar E. Evidence for altered spinal canal compliance and cerebral venous drainage in untreated idiopathic intracranial hypertension. Acta Neurochir Suppl 2012;114:201-5. [Crossref] [PubMed]
- Tain RW, Bagci AM, Lam BL, Sklar EM, Ertl-Wagner B, Alperin N. Determination of cranio-spinal canal compliance distribution by MRI: Methodology and early application in idiopathic intracranial hypertension. J Magn Reson Imaging 2011;34:1397-404. [Crossref] [PubMed]
- Tatewaki Y, Mutoh T, Omodaka K, Thyreau B, Matsudaira I, Furukawa H, Yamada K, Kunitoki K, Kawashima R, Nakazawa T, Taki Y. Morphological prediction of glaucoma by quantitative analyses of ocular shape and volume using 3-dimensional T2-weighted MR images. Sci Rep 2019;9:15148. [Crossref] [PubMed]
- Burman R, Shah AH, Benveniste R, Jimsheleishvili G, Lee SH, Loewenstein D, Alperin N. Comparing invasive with MRI-derived intracranial pressure measurements in healthy elderly and brain trauma cases: A pilot study. J Magn Reson Imaging 2019;50:975-81. [Crossref] [PubMed]
- Ragauskas A, Bartusis L, Piper I, Zakelis R, Matijosaitis V, Petrikonis K, Rastenyte D. Improved diagnostic value of a TCD-based non-invasive ICP measurement method compared with the sonographic ONSD method for detecting elevated intracranial pressure. Neurol Res 2014;36:607-14. [Crossref] [PubMed]
- Krakauskaite S, Petkus V, Bartusis L, Zakelis R, Chomskis R, Preiksaitis A, Ragauskas A, Matijosaitis V, Petrikonis K, Rastenyte D. Accuracy, Precision, Sensitivity, and Specificity of Noninvasive ICP Absolute Value Measurements. Acta Neurochir Suppl 2016;122:317-21. [Crossref] [PubMed]
- Koskinen LD, Malm J, Zakelis R, Bartusis L, Ragauskas A, Eklund A. Can intracranial pressure be measured non-invasively bedside using a two-depth Doppler-technique? J Clin Monit Comput 2017;31:459-67. [Crossref] [PubMed]
- Hamarat Y, Deimantavicius M, Kalvaitis E, Siaudvytyte L, Januleviciene I, Zakelis R, Bartusis L. Location of the internal carotid artery and ophthalmic artery segments for non-invasive intracranial pressure measurement by multi-depth TCD. Libyan J Med 2017;12:1384290 [Crossref] [PubMed]
- Ragauskas A, Matijosaitis V, Zakelis R, Petrikonis K, Rastenyte D, Piper I, Daubaris G. Clinical assessment of noninvasive intracranial pressure absolute value measurement method. Neurology 2012;78:1684-91. [Crossref] [PubMed]
- Siaudvytyte L, Januleviciene I, Ragauskas A, Bartusis L, Meiliuniene I, Siesky B, Harris A. The difference in translaminar pressure gradient and neuroretinal rim area in glaucoma and healthy subjects. J Ophthalmol 2014;2014:937360 [Crossref] [PubMed]
- Xu W, Gerety P, Aleman T, Swanson J, Taylor J. Noninvasive methods of detecting increased intracranial pressure. Childs Nerv Syst 2016;32:1371-86. [Crossref] [PubMed]
- Hamarat Y, Bartusis L, Deimantavicius M, Siaudvytyte L, Januleviciene I, Ragauskas A, Bershad EM, Fandino J, Kienzler J, Remonda E, Matijosaitis V, Rastenyte D, Petrikonis K, Berskiene K, Zakelis R. Graphical and statistical analyses of the oculocardiac reflex during a non-invasive intracranial pressure measurement. PLoS One 2018;13:e0196155 [Crossref] [PubMed]
- Scott CJ, Kardon RH, Lee AG, Frisén L, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol 2010;128:705-11. [Crossref] [PubMed]
- Aojula A, Mollan SP, Horsburgh J, Yiangou A, Markey KA, Mitchell JL, Scotton WJ, Keane PA, Sinclair AJ. Segmentation error in spectral domain optical coherence tomography measures of the retinal nerve fibre layer thickness in idiopathic intracranial hypertension. BMC Ophthalmol 2018;17:257. [Crossref] [PubMed]
- Wang JK, Kardon RH, Kupersmith MJ, Garvin MK. Automated quantification of volumetric optic disc swelling in papilledema using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2012;53:4069-75. [Crossref] [PubMed]
- York DH, Pulliam MW, Rosenfeld JG, Watts C. Relationship between visual evoked potentials and intracranial pressure. J Neurosurg 1981;55:909-16. [Crossref] [PubMed]
- Haredy MM, Liasis A, Fu V, Davis A, Pollack IF, Losee JE, Saied S, Nischal KK, Goldstein JA. Serial Visual Evoked Potentials in Patients with Craniosynostosis and Invasive Intracranial Pressure Monitoring. Plast Reconstr Surg 2019;144:446e-52e. [Crossref] [PubMed]
- Vieira MA, Cavalcanti Mdo A, Costa DL, Eulálio KD, Vale OC, Vieira CP, Costa CH. Visual evoked potentials show strong positive association with intracranial pressure in patients with cryptococcal meningitis. Arq Neuropsiquiatr 2015;73:309-13. [Crossref] [PubMed]
- Wong SH, White RP. The clinical validity of the spontaneous retinal venous pulsation. J Neuroophthalmol 2013;33:17-20. [Crossref] [PubMed]
- Querfurth HW, Lieberman P, Arms S, Mundell S, Bennett M, van Horne C. Ophthalmodynamometry for ICP prediction and pilot test on Mt. Everest. BMC Neurol 2010;10:106. [Crossref] [PubMed]
- D’Antona L, McHugh JA, Ricciardi F, Thorne LW, Matharu MS, Watkins LD, Toma AK, Bremner FD. Association of Intracranial Pressure and Spontaneous Retinal Venous Pulsation. JAMA Neurol 2019;76:1502-5. [Crossref] [PubMed]
- McHugh JA. DʼAntona L, Toma AK, Bremner FD. Spontaneous Venous Pulsations Detected With Infrared Videography. J Neuroophthalmol 2020;40:174-7. [Crossref] [PubMed]
- Kolecki R, Dammavalam V, Bin Zahid A, Hubbard M, Choudhry O, Reyes M, Han B, Wang T, Papas PV, Adem A, North E, Gilbertson DT, Kondziolka D, Huang JH, Huang PP, Samadani U. Elevated intracranial pressure and reversible eye-tracking changes detected while viewing a film clip. J Neurosurg 2018;128:811-8. [Crossref] [PubMed]
- Samadani U, Farooq S, Ritlop R, Warren F, Reyes M, Lamm E, Alex A, Nehrbass E, Kolecki R, Jureller M, Schneider J, Chen A, Shi C, Mendhiratta N, Huang JH, Qian M, Kwak R, Mikheev A, Rusinek H, George A, Fergus R, Kondziolka D, Huang PP, Smith RT. Detection of third and sixth cranial nerve palsies with a novel method for eye tracking while watching a short film clip. J Neurosurg 2015;122:707-20. [Crossref] [PubMed]
- Rajajee V, Fletcher JJ, Rochlen LR, Jacobs TL. Comparison of accuracy of optic nerve ultrasound for the detection of intracranial hypertension in the setting of acutely fluctuating vs stable intracranial pressure: post-hoc analysis of data from a prospective, blinded single center study. Crit Care 2012;16:R79. [Crossref] [PubMed]
- Bäuerle J, Niesen WD, Egger K, Buttler KJ, Reinhard M. Enlarged Optic Nerve Sheath in Aneurysmal Subarachnoid Hemorrhage despite Normal Intracranial Pressure. J Neuroimaging 2016;26:194-6. [Crossref] [PubMed]
- Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care 2011;15:506-15. [Crossref] [PubMed]
- Hansen HC, Lagrèze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure - an experimental ultrasound study. Acta Ophthalmol 2011;89:e528-32. [Crossref] [PubMed]
- Raffiz M, Abdullah JM. Optic nerve sheath diameter measurement: a means of detecting raised ICP in adult traumatic and non-traumatic neurosurgical patients. Am J Emerg Med 2017;35:150-3. [Crossref] [PubMed]
- Steinborn M, Fiegler J, Ruedisser K, Hapfelmeier A, Denne C, Macdonald E, Hahn H. Measurement of the Optic Nerve Sheath Diameter in Children: Comparison Between Transbulbar Sonography and Magnetic Resonance Imaging. Ultraschall Med 2012;33:569-73. [Crossref] [PubMed]
- Shirodkar CG, Munta K, Rao SM, Mahesh MU. Correlation of measurement of optic nerve sheath diameter using ultrasound with magnetic resonance imaging. Indian J Crit Care Med 2015;19:466-70. [Crossref] [PubMed]
- Steinborn M, Fiegler J, Kraus V, Denne C, Hapfelmeier A, Wurzinger L, Hahn H. High resolution ultrasound and magnetic resonance imaging of the optic nerve and the optic nerve sheath: anatomic correlation and clinical importance. Ultraschall Med 2011;32:608-13. [Crossref] [PubMed]
- Nogueira AB, Annen E, Boss O, Farokhzad F, Sikorski C, Keller E. Temperature variability in the day-night cycle is associated with further intracranial pressure during therapeutic hypothermia. J Transl Med 2017;15:170. [Crossref] [PubMed]
- Robba C, Cardim D, Donnelly J, Bertuccio A, Bacigaluppi S, Bragazzi N, Cabella B, Liu X, Matta B, Lattuada M, Czosnyka M. Effects of pneumoperitoneum and Trendelenburg position on intracranial pressure assessed using different non-invasive methods. Br J Anaesth 2016;117:783-91. [Crossref] [PubMed]
- Cutolo CA, De Moraes CG, Liebmann JM, Mansouri K, Traverso CE, Ritch R. The Effect of Therapeutic IOP-lowering Interventions on the 24-hour Ocular Dimensional Profile Recorded With a Sensing Contact Lens. J Glaucoma 2019;28:252-7. [Crossref] [PubMed]
- Lin JS, Liu JH. Circadian variations in intracranial pressure and translaminar pressure difference in Sprague-Dawley rats. Invest Ophthalmol Vis Sci 2010;51:5739-43. [Crossref] [PubMed]
- Liu H, Cao X, Zhang M, He M, Li M, Song Y, Dong Z, Yu S. A case report of cough headache with transient elevation of intracranial pressure and bilateral internal jugular vein valve incompetence: A primary or secondary headache? Cephalalgia 2018;38:600-3. [Crossref] [PubMed]
- Uğraş G, Yüksel S, Temiz Z, Eroğlu S, Şirin K. Effects of Different Head-of-Bed Elevations and Body Positions on Intracranial Pressure and Cerebral Perfusion Pressure in Neurosurgical Patients. J Neurosci Nurs 2018;50:247-51. [Crossref] [PubMed]
- Schwartz KM, Luetmer PH, Hunt CH, Kotsenas AL, Diehn FE, Eckel LJ, Black DF, Lehman VT, Lindell EP. Position-related variability of CSF opening pressure measurements. AJNR Am J Neuroradiol 2013;34:904-7. [Crossref] [PubMed]
- Vinje V, Eklund A, Mardal KA, Rognes ME, Støverud KH. Intracranial pressure elevation alters CSF clearance pathways. Fluids Barriers CNS 2020;17:29. [Crossref] [PubMed]
- Long J, Lin H, Cao G, Wang MZ, Huang XJ, Xia J, Sun Z. Relationship between intracranial pressure and phase-contrast cine MRI-derived measures of cerebrospinal fluid parameters in communicating hydrocephalus. Quant Imaging Med Surg 2019;9:1413-20. [Crossref] [PubMed]
- Price DA, Grzybowski A, Eikenberry J, Januleviciene I, Verticchio Vercellin AC, Mathew S, Siesky B, Harris A. Review of non-invasive intracranial pressure measurement techniques for ophthalmology applications. Br J Ophthalmol 2020;104:887-92. [Crossref] [PubMed]