The effect of freeze-thawing on magnetic resonance imaging T2* of freshly harvested bovine patellar tendon
Introduction
Musculoskeletal tissue samples procured for research purposes, particular those in preclinical models, are frequently acquired shortly after death. In research studies it is ideal to analyze fresh specimens; however, it is often necessary to freeze samples for evaluation at a later date. Preservation techniques and storage protocols must be compatible with the analytical methods chosen for a particular study. As imaging, gross assessment, mechanical testing, biochemical assessment, and histology are often performed in combination, it is important to account for the effect that a freezing protocol may have on the properties of a specific tissue. For example, freezing of cartilage samples commonly involves use of stabilizing agents such as dimethyl sulfoxide (DMSO), cross linking agents, cationic agents, or protease inhibitors (1). Cross linking agents such as formalin and solvents such as DMSO can prevent proteolysis and formation of ice crystals to preserve accurate protein quantization and histological morphology, respectively; however, if magnetic resonance imaging (MRI) is also performed, these agents will alter T2 values of tissue (1,2). Similarly, cationic agents that prevent leakage of glycosaminoglycans from tissue do so at the expense of neutralizing the fixed charged density, which is the molecular correlate for quantitative T1ρ imaging (3). Optimized freezing protocols for tissues destined for MRI scanning have been created for cartilage preservation using low concentrations of protease inhibitors with short term storage at +4° F or −20° F (1), having a minimal effect on T2 values. A similar protocol for preservation of tendons destined for MRI analysis is not currently available.
Studies in which tendons have been frozen and thawed vary greatly in their methods. Many tendon studies investigating allograft design intentionally destroy the cellular components of the tendon with specific freezing techniques to reduce immunogenicity (4,5). Other studies mention the use of fresh-frozen tendons but the freezing method, pre-treatment conditions, freezing temperature, and thawing protocols differ (4,6-12). Frozen tendon samples have displayed unpredictable alterations of tissue properties; specifically, maximum load, energy of maximum load, maximum stress, ultimate tensile failure and elastic modulus during tensile testing of specimens (4,6,7,10). Tendon properties as assessed using MRI have undergone limited evaluation with respect to the freeze-thaw process (12,13).
Tendons typically exhibit little signal on clinical fast-spin echo (FSE) MRI sequences due to their highly ordered collagen composition, which causes a rapid shortening of T2 relaxation and corresponding loss of signal. Only when the tendon is highly disrupted by edema or hemorrhage will it appear abnormal on FSE images, and subtle injuries may escape detection. Newer techniques, such as ultrashort echo time (UTE) imaging, can visualize tissue species with very short T2 relaxation times, and permits calculation of a reproducible decay constant (T2*). Prolongation of T2* may be used as a biomarker of disruption of highly ordered tissues such as tendons, ligaments, menisci, and periosteum (14-17), and may detect early changes of tendon structural damage. Although UTE T2* has been shown to correlate with disrupted collagen in harvested and in vivo tissue samples (18), the effect of tissue freezing on T2* values has not been completely assessed. In addition, few papers address the effect of freezing on quantitative MRI properties of tendons. Chang et al. described the effect of freeze-thaw cycles on the T2* and T2 values of human Achilles tendons (13). No effect was found with multiple freeze-thaw cycles; however, the sample size was very small (n=4), limited to humans, and the four samples were retrieved from three older female specimens. In contrast, our laboratory frequently performs pre-clinical studies using large animal models from different species and with age variability. Therefore, the purpose of this study was to determine the effect of multiple freeze thaw cycles on T2* values for a sample of juvenile and young adult bovine patellar tendons. The patellar tendon was chosen for investigation since a large portion of tissue would be available for quantitative MRI analysis, and the patellar tendon is used as graft material in human anterior cruciate ligament reconstructive surgery.
Materials and methods
Specimen preparation
Fourteen fresh bovine knees were acquired from a local abattoir within eight hours of death. Four had closed physis (young adult) and ten had open physis (juvenile), but were of market weight. The tendons were inspected visually by a veterinarian for gross evidence of disease or defects. The central patellar tendon was harvested from each knee and wrapped in saline-soaked gauze to maintain hydration. Specimens were refrigerated at 4 °C for 12 hours prior to image acquisition. Initial (pre-frozen) MR imaging was performed within 24 hours of death.
Image acquisition
All scanning was performed on a clinical 3T scanner (GE Healthcare, Waukesha, WI, USA) with an 8 channel phased-array wrist coil (Invivo, Gainesville, FL, USA). Morphologic multi-planar FSE images were acquired: TE: 24 ms, repetition time (TR): 4,000 ms, receiver bandwidth (RBW): ±50 kHz, acquisition matrix (AM): 512×256-512×384, number of excitations (NEX): 1-2, field-of-view (FOV): 16 cm, slice thickness (ST): 1.0-2.0 mm. Next, axial multi-slice multi-echo two-dimensional UTE images oriented along the length of each tendon were acquired for T2* calculations: TEs =0.05, 5, 10, 15 ms, TR =350 ms, RBW =±62.5 kHz, AM =512×701, NEX =2, flip angle =45°, ST =2 mm, slice spacing =1-2 mm. Following scanning, each tendon was placed in a sealed plastic bag to prevent dehydration, and was frozen at −20 °C for at least 24 hours. Following freezing, each tendon was permitted to fully thaw at room temperature, requiring approximately 6 hours, and the MR imaging protocol was repeated. Each tendon underwent a total of four freeze-thaw cycles following scanning of the fresh tendon sample.
Image analysis
Patellar tendon T2* values were calculated from the UTE images acquired following each freeze-thaw cycle by fitting the TE to the corresponding signal intensity: SI (TE) = So*e(-TE/T2*)+C, where SI (TE) is the signal intensity at echo time TE, So is proportional to apparent proton density, T2* is the inherent transverse relaxation time constant, and C is a constant to account for image noise. Average bulk T2* values from all voxels comprising individual tendons, approximately 15,000 voxels, were generated for statistical analysis.
Statistical analysis
A two-way repeated measures analysis of variance (ANOVA) was performed to determine the effects of specimen age (juvenile or young adult) and freeze thaw cycles on tendon T2* values. A post hoc Student-Newman-Keuls (SNK) test was performed when statistical significance was found. Significance was set at P<0.05.
Results
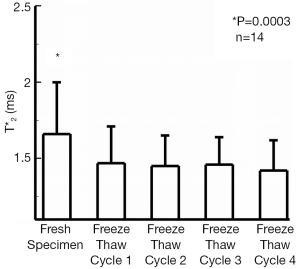
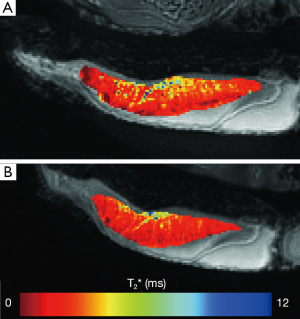
The T2* values of all fresh bovine patellar tendons were significantly longer (1.66±0.35, mean ± st.dev.) than T2* values measured after 1, 2, 3, or 4 freeze-thaw cycles, P=0.0003 (1.47±0.24, 1.45±0.20, 1.46±0.18, and 1.43±0.20 ms respectively). This represented approximately 12% reduction in tendon T2* values after having undergone a single freeze-thaw cycle. T2* values following any number of freeze-thaw cycles were similar to one another (Figure 1). Significant differences of T2* values were found between the young adult specimens, 1.38±0.31 ms, and those from juvenile specimens, 1.72±0.23 ms, P=0.02. The interaction between the two factors of age and freeze-thaw cycle was not significant, P=0.05. The juvenile specimens had a significant shortening of T2* after one freeze thaw cycle, P=0.001, but no changes of T2* were seen following the first freeze-thaw cycle. Fresh young adult specimens tended to have the longer T2* values as compared to T2* following individual the freeze-thaw cycles, but this was not significant, P=0.25. Seven samples demonstrated a slight reduction in T2* from the first to the last freeze-thaw cycle, three demonstrated no change, and four demonstrated a slight prolongation of T2* values. A representative T2* map of a fresh tendon sample and of the same tendon sample following four freeze-thaw cycles is shown in Figure 2.
Discussion
The current study evaluated the effect of numerous freeze-thaw cycles on the inherent MR parameter T2* of bovine patellar tendon samples. Our study demonstrated a small but significant reduction of tendon T2* values after one initial freeze-thaw cycle, attributable primarily to the specimens from juvenile animals. The magnitude of the reduction was less than 1 ms. Since the only significant difference was found between the fresh and the first freeze-thaw cycle, the difference seen may have been due to loss of free water from the cellular component, which is susceptible to freezing and the formation of ice crystals. Subsequent freeze-thaw cycles would not have a further effect on this lost cellular component explaining the lack of further reduction in T2* values. In this study, we found a significant 12% difference of T2* between the fresh and frozen samples. This corresponds well with previous studies which performed serial scanning of fresh and frozen specimens. To put our results in perspective, previous reports demonstrated changes of T2* on the order of 30-87% due to pathologic states (19). Therefore the small change in T2* that was found in our study, while significant, is smaller than would be anticipated with pathologic changes in tendon.
The effect of freeze-thawing on T2* values has been assessed in just one prior study, of limited sample size, in which no significant change in tendon T2* values were found between fresh and frozen-thawed geriatric, female, human Achilles tendon samples (13). Several differences exist between the current study and the prior study, including: the tendon sample evaluated (Achilles versus patellar), the source species, the age of tissue sample, and the gender. Each of these factors or a combination may explain these results.
The mechanical properties of a tendon are dependent on anatomic location, exercise, and immobilization (20), In addition, analogous tendons in animal models often behave differently in quadrupeds than in humans (21). For example, scar formation is a known result in rotator cuff repair in sheep that does not occur in human repairs (22). As tendon characteristics vary from quadrupeds to humans, it is presumed that T2* values may also be different between species. The average value of the fresh human Achilles samples were recorded at 1.04-1.78 ms with an average of 1.18±0.45 (13), whereas the samples in this study were 1.10 to 2.10 ms with an average of 1.66 ±0.34.
The samples in this study were obtained from relatively young animals as beef cattle are slaughtered commonly before age 3 (juvenile), whereas older culled breeding stock are usually over 3 years old but less than 10 years of age, which is still relatively youthful compared to the tendons from Chang et al. Material and structural properties of the tendon increase from birth through maturity and then decrease from maturity through old age (20,23). Aging tendon tissue has been shown to have an increase in extracellular matrix volume and a decrease of the relative number of cells (24). Other changes include progressive degeneration of the tendinous structures, progressive disruption of the integrity of the attachment of the tendon to the bone by Sharpey’s fibers, loss of staining quality, fragmentation of the tendon, diminished vascularity and diminished fibrocartilage (23). The tenocytes have been shown to also be longer and thinner with reduced protein synthesis, and disoriented collagen fibers as a result of variations in thickness due to an increase in collagen, a decrease in mucopolysaccharides, and a decrease in water content (24). This likely explains the mild elevations of our bulk T2* samples compared to the human Achilles tendons, as young tendons would have a larger cellular component. This may also explain our small but significant drop in relatively young tendons compared to geriatric tendons, as geriatric tendons may not have had a substantial cellular component to lose in the first freeze thaw cycle. Although the T2* value has not been studied in young versus old specimens, differences in articular cartilage T2 values have been shown between children and adolescents and between adolescents and adults. Children with open physis tend to have longer T2 values than adolescents with closed physis (25). After adolescence, the T2 values again prolong with age. The difference seen in this study between tendon T2* values of open and closed physis specimens may reflect the cellular and matrix alterations during development and maturity in which the younger group has lower material and structural properties that increase during maturity, reflected as a small prolongation in T2*. It may be hypothesized that tendons in geriatric cattle may demonstrate a mild reduction in T2* due to loss of these structural and material properties; however, cattle of this age are seldom available.
Finally, tendon structural and mechanical properties have been noted between genders (26,27), including stress, strain, stiffness, and tensile strength and Young’s modulus. The collagen content and collagen cross linking are not different although the total dry mass is reduced in women with a reduction of collagen content per tendon wet weight, resulting in a reduced water content in women (28). This may further explain the differences seen in our study as our samples were unknown gender. Chang et al used solely female tendons.
This study had several limitations. First, only four echoes were used in the current analysis to calculate T2*. Previous studies have acquired more echoes to evaluate short T2 species, at the cost of a prolonged scan time, for example 22 minutes for 11 echoes (19) or 26 minutes for 13 echoes (29). The scanning sequence used in the current study is one which we have used to determine the utility of T2* mapping in the setting of meniscal repair (18), and implements in a clinically feasible scan time of under 10 minutes. Other studies have been successful in correlating T2* with tendon properties when using only two echo time points (30). Second, this study froze specimens to −20 °C. Laboratories frequently freeze tissues and cells lines between −40 to −135 °C to for cryopreservation. The temperature of −20 °C was chosen since this is the common temperature used to preserve samples within our laboratory.
In conclusion, freezing of untreated tendons leads to small but significant reductions in UTE T2* values. The results of our study demonstrate the importance of using uniform (fresh or frozen) tissue samples when assessing UTE T2* and noting that one freeze-thaw cycle may significantly reduce T2* values in young tendon samples.
Acknowledgements
Internal research funds were used in support of this study. We thank Dr. Tony Chen for procurement of the tendon samples. Hospital for Special Surgery MRI Laboratory receives institutional research support from General Electric Healthcare.
Authors’ contributions: SL Pownder, MF Koff and HG Potter designed the overall study, collected and analyzed data, and co-wrote the paper. PH Shah carried out experiments and collected and analyzed data with SL Pownder, MF Koff and HG Potter. All authors discussed and edited the paper. MF Koff supervised this study.
Disclosure: The authors declare no conflict of interest.
References
- Fishbein KW, Gluzband YA, Kaku M, Ambia-Sobhan H, Shapses SA, Yamauchi M, Spencer RG. Effects of formalin fixation and collagen cross-linking on T2 and magnetization transfer in bovine nasal cartilage. Magn Reson Med 2007;57:1000-11. [PubMed]
- Shepherd TM, Thelwall PE, Stanisz GJ, Blackband SJ. Aldehyde fixative solutions alter the water relaxation and diffusion properties of nervous tissue. Magn Reson Med 2009;62:26-34. [PubMed]
- Wheaton AJ, Casey FL, Gougoutas AJ, Dodge GR, Borthakur A, Lonner JH, Schumacher HR, Reddy R. Correlation of T1rho with fixed charge density in cartilage. J Magn Reson Imaging 2004;20:519-25. [PubMed]
- Chen L, Wu Y, Yu J, Jiao Z, Ao Y, Yu C, Wang J, Cui G. Effect of repeated freezing-thawing on the Achilles tendon of rabbits. Knee Surg Sports Traumatol Arthrosc 2011;19:1028-34. [PubMed]
- Burk J, Erbe I, Berner D, Kacza J, Kasper C, Pfeiffer B, Winter K, Brehm W. Freeze-thaw cycles enhance decellularization of large tendons. Tissue Eng Part C Methods 2014;20:276-84. [PubMed]
- Arnout N, Myncke J, Vanlauwe J, Labey L, Lismont D, Bellemans J. The influence of freezing on the tensile strength of tendon grafts: a biomechanical study. Acta Orthop Belg 2013;79:435-43. [PubMed]
- Clavert P, Kempf JF, Bonnomet F, Boutemy P, Marcelin L, Kahn JL. Effects of freezing/thawing on the biomechanical properties of human tendons. Surg Radiol Anat 2001;23:259-62. [PubMed]
- Dargel J, Schmidt-Wiethoff R, Schmidt J, Koebke J. Histomorphology and microradiography of quadriceps tendon-patellar bone grafts in press-fit anterior cruciate ligament reconstruction. J Orthop Res 2005;23:1206-10. [PubMed]
- Halewood C, Hirschmann MT, Newman S, Hleihil J, Chaimski G, Amis AA. The fixation strength of a novel ACL soft-tissue graft fixation device compared with conventional interference screws: a biomechanical study in vitro. Knee Surg Sports Traumatol Arthrosc 2011;19:559-67. [PubMed]
- Jung HJ, Vangipuram G, Fisher MB, Yang G, Hsu S, Bianchi J, Ronholdt C, Woo SL. The effects of multiple freeze-thaw cycles on the biomechanical properties of the human bone-patellar tendon-bone allograft. J Orthop Res 2011;29:1193-8. [PubMed]
- Micucci CJ, Frank DA, Kompel J, Muffly M, Demeo PJ, Altman GT. The effect of interference screw diameter on fixation of soft-tissue grafts in anterior cruciate ligament reconstruction. Arthroscopy 2010;26:1105-10. [PubMed]
- van der Made AD, Maas M, Beenen LF, Oostra RJ, Kerkhoffs GM. Postmortem imaging exposed: an aid in MR imaging of musculoskeletal structures. Skeletal Radiol 2013;42:467-72. [PubMed]
- Chang EY, Bae WC, Statum S, Du J, Chung CB. Effects of repetitive freeze-thawing cycles on T2 and T2 of the Achilles tendon. Eur J Radiol 2014;83:349-53. [PubMed]
- Gold GE, Pauly JM, Macovski A, Herfkens RJ. MR spectroscopic imaging of collagen: tendons and knee menisci. Magn Reson Med 1995;34:647-54. [PubMed]
- Rahmer J, Börnert P, Dries SP. Assessment of anterior cruciate ligament reconstruction using 3D ultrashort echo-time MR imaging. J Magn Reson Imaging 2009;29:443-8. [PubMed]
- Reichert IL, Benjamin M, Gatehouse PD, Chappell KE, Holmes J, He T, Bydder GM. Magnetic resonance imaging of periosteum with ultrashort TE pulse sequences. J Magn Reson Imaging 2004;19:99-107. [PubMed]
- Gatehouse PD, Bydder GM. Magnetic resonance imaging of short T2 components in tissue. Clin Radiol 2003;58:1-19. [PubMed]
- Koff MF, Shah P, Pownder S, Romero B, Williams R, Gilbert S, Maher S, Fortier LA, Rodeo SA, Potter HG. Correlation of meniscal T2* with multiphoton microscopy, and change of articular cartilage T2 in an ovine model of meniscal repair. Osteoarthritis Cartilage 2013;21:1083-91. [PubMed]
- Williams A, Qian Y, Golla S, Chu CR. UTE-T2* mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage 2012;20:486-94. [PubMed]
- McCarthy MM, Hannafin JA. The mature athlete: aging tendon and ligament. Sports Health 2014;6:41-8. [PubMed]
- Longo UG, Forriol F, Campi S, Maffulli N, Denaro V. Animal models for translational research on shoulder pathologies: from bench to bedside. Sports Med Arthrosc 2011;19:184-93. [PubMed]
- Turner AS. Experiences with sheep as an animal model for shoulder surgery: strengths and shortcomings. J Shoulder Elbow Surg 2007;16:S158-63. [PubMed]
- Brewer BJ. Aging of the rotator cuff. Am J Sports Med 1979;7:102-10. [PubMed]
- Ippolito E, Natali PG, Postacchini F, Accinni L, De Martino C. Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. J Bone Joint Surg Am 1980;62:583-98. [PubMed]
- Shiraj S, Kim HK, Anton C, Horn PS, Laor T. Spatial variation of T2 relaxation times of patellar cartilage and physeal patency: an in vivo study in children and young adults. AJR Am J Roentgenol 2014;202:W292-7. [PubMed]
- Onambélé GN, Burgess K, Pearson SJ. Gender-specific in vivo measurement of the structural and mechanical properties of the human patellar tendon. J Orthop Res 2007;25:1635-42. [PubMed]
- Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol 2003;88:520-6. [PubMed]
- Lemoine JK, Lee JD, Trappe TA. Impact of sex and chronic resistance training on human patellar tendon dry mass, collagen content, and collagen cross-linking. Am J Physiol Regul Integr Comp Physiol 2009;296:R119-24. [PubMed]
- Pauli C, Bae WC, Lee M, Lotz M, Bydder GM, D'Lima DL, Chung CB, Du J. Ultrashort-echo time MR imaging of the patella with bicomponent analysis: correlation with histopathologic and polarized light microscopic findings. Radiology 2012;264:484-93. [PubMed]
- Biercevicz AM, Murray MM, Walsh EG, Miranda DL, Machan JT, Fleming BC. T2 * MR relaxometry and ligament volume are associated with the structural properties of the healing ACL. J Orthop Res 2014;32:492-9. [PubMed]


