
Dual-energy computed tomography could reliably differentiate metastatic from non-metastatic lymph nodes of less than 0.5 cm in patients with papillary thyroid carcinoma
Introduction
Cervical lymph node (LN) and lymph node metastasis (LNM) is extremely common in patients with papillary thyroid carcinoma (PTC), with an incidence of 30–80% (1,2). Lymph nodes (LNs) with a short diameter of >0.5 cm can be easily detected by computed tomography (CT) (3), especially when the diameter is >1 cm. However, according to current literature, the detection of LNs <0.5 cm using traditional examination methods is challenging. The risk of recurrence of LNM varies from 4% with metastatic LNs <0.2 cm to 27–32% with metastatic LNs >3 cm (4,5). A large, population-based study showed that there was an association between the metastatic LN ratio and disease-specific mortality in 60–75% of patients with PTC (6). After LN dissection, the 5-year survival rate can reach 100%, and the 20-year survival rate can exceed 90% (7). Therefore, the treatment regimen is influenced by whether metastatic LNs are present or absent (8).
According to the American Thyroid Association (ATA) statement on preoperative imaging for thyroid cancer surgery, ultrasound (US) stands as the key imaging modality for the assessment of thyroid cancer (9); however, US can only detect 20–31% of patients with central cervical LNM (10-13), whereas the rate of detection for lateral cervical LNM is 70–93.8% (11,12). US is also greatly affected by the operators’ experience level and manipulation (14-16). For PTC patients, the spatial resolution and contrast resolution of traditional CT scans are not high enough for cervical LNs to be accurately detected, as these LNs cannot be easily distinguished from accompanying blood vessels, especially when they measure <0.5 cm (17,18). Diffusion weighted imaging (DWI) has high diagnostic value for distinguishing metastatic and non-metastatic LNs, with an accuracy rate of 91.0–94.3% (19). However, the apparent diffusion coefficient (ADC) value does not apply to cystic degeneration and necrosis in LNs (20-22), especially in the investigation of LNs with a diameter <0.5 cm.
In recent years, dual-energy computed tomography (DECT) has been widely used in the prediction of numerous diseases, such as adherent perinephric fat (23), microthrombosis associated with COVID-19 pneumonia (24), detection of pulmonary emboli (25), and detection of lumbar disk herniation (26). Besides, DECT has been used to predict LNM in some cancers, including lung cancer (19), gastrointestinal tumor (27), breast cancer (28), biliary tract cancer (29), and hepatocellular carcinoma (30). According to the Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for persistent/recurrent and metastatic differentiated thyroid cancer (version 2018) (31), when there are suspected local lesions, neck US, enhanced CT, or contrast magnetic resonance imaging (MRI) are juxtaposed as a level I–2A recommendation, which is the highest recommendation. Additionally, in the National Comprehensive Cancer Network (NCCN) guidelines (version 2018), for papillary carcinoma or suspicion of papillary carcinoma, the use of CT/MRI with contrast was suggested for fixed, bulky, or substernal lesions. This supports the idea that enhanced CT is not a contraindication for patients with PTC. Moreover, CT examination can more accurately determine the extent of LNM, thus playing a vital role in the planning of surgical procedures. Details on the specific reasons for applying DECT in the current study are displayed in Appendix 1. However, there may be concerns regarding the use of iodinated contrast agents, which can compete with iodine-131 (131I) and interfere with subsequent postoperative radioactive iodine (RAI) therapy. In fact, a potential delay in RAI treatment is not harmful, for the reasons detailed in Appendix 1. Meanwhile, DECT can not only obtain more parameters than ordinary enhanced CT, but it also has a significantly lower radiation dose, which has been verified in previous studies (32,33). A study conducted by Liu et al. suggested that the slope of the energy spectrum curve (λHU) in the venous phase and the normalized iodine concentration (NIC) in the arterial phase are effective parameters for diagnosing LNM in patients with PTC (33). However, to date, no relevant studies on metastatic or non-metastatic LNs with a short diameter of <0.5 cm in patients with PTC have been performed.
In the current study, we hypothesized that DECT could distinguish metastatic LNs from non-metastatic LNs with a short diameter <0.5 cm in patients with PTC. Therefore, the main purpose of this study was to investigate the usefulness of quantitative DECT parameters in the preoperative diagnosis of cervical small LNM in patients with PTC.
Methods
Patient population
This retrospective study was approved by the ethics committee of the Tianjin First Central Hospital (2018N131KY), and the requirement for written informed consent was waived due to its retrospective nature. Between May 2016 and June 2018, 165 patients (29 male, mean age, 48.97±17.30 years; 136 female, mean age, 49.48±12.41 years) suspected as thyroid carcinoma by US were initially consecutively selected. The patients underwent DECT to rule out or confirm the presence of metastatic LNs (according to the CSCO guidelines, version 2018) (31). The inclusion criteria were: (I) >18 years old; (II) PTC confirmed by postoperative pathology; and (III) no history of radiotherapy to the neck. The investigated LNs with a short diameter <0.5 cm was included for further analysis. Other inclusion criteria are listed in Appendix 1. The exclusion criteria included: (I) patients who did not receive surgical treatment; (II) patients whose postoperative pathology confirmed a nodular goiter, adenoma, thyroiditis, or other benign lesion; (III) patients whose pathology confirmed medullary thyroid carcinoma (MTC) or follicular thyroid cancer (FTC); (IV) patients with incomplete information, and (V) patients with history of neck cancer other than PTC (i.e., pharyngeal carcinoma, neck squamous cell carcinoma, and nasopharyngeal carcinoma). Eventually, 52 patients comprising 11 males (mean age, 43.00±15.22 years) and 41 females (mean age, 44.68±10.36 years) were included. The detailed inclusion and exclusion criteria are shown in Figure S1.
The shortest diameter of the largest layer of a LN and quantitative parameters, including iodine concentration (IC), NIC, and λHU in the arterial and the venous phases, which was derived from DECT, were measured for all of the LNs of the enrolled participants. Distinct from the experimental method of Liu et al. (33), our study referred to Park’s method (34) to solve the problems of LNs between the pathological results and DECT. The final histopathologic reports of the surgical neck dissection samples served as the gold standard for LNM. All cervical levels in a neck dissection specimen were labeled based on the newest cervical LN partition standard (35), which was published by the official journal of the European Society of Radiotherapy & Oncology (ESTRO)-Radiotherapy & Oncology (Green Skin Magazine) in November 2013. Cervical LN levels were assigned as non-metastatic, metastatic, or mixed after correlation with the histopathologic reports. If pathological results showed metastasis in all LNs in a subregion, the level was classified as metastatic. If the pathological results showed no metastasis in any of the LNs in a subregion, the level was classified as non-metastatic. If both benign and metastatic LNs were found in a subregion, the level was classified as mixed. The levels with mixed LNs were ruled out, and only levels with non-metastatic or metastatic LNs were included, regardless of the diameter of the LNs. Finally, only LNs with a diameter <0.5 cm measured by DECT were included, and LNs ≥0.5 cm were excluded (Figures S2,S3). Subsequently, the results of the histopathologic assignment were used to match and label LNs on DECT. An LN confirmed by ultrasound-fine needle aspiration biopsy (US-FNAB) was chosen and labeled on DECT images by matching the images and reports of DECT, US, and the final pathologic examination (site-specific matching, 23 LNs). The LNs were assigned as non-metastatic or metastatic according to the postoperative pathological results and labeled as surgical-level matching (336 LNs). LNs with a diameter ≥0.5 cm and those obscured due to artifacts on the iodine map were also excluded (Figure 1). The specific lesion measurement method is presented in the supplementary material as legends (Figures S4,S5,S6). The specific DECT diagnostic criteria of LNM <0.5 cm in patients with PTC are detailed in Appendix 1 and Figure S7.
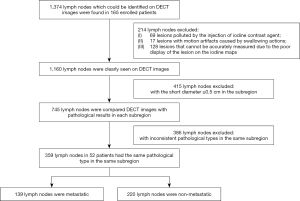
Image acquisition, processing, and analyzing
All images were obtained using a 64 multi-detector row CT scanner (Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany) with dual-phase contrast-enhanced CT. The detailed CT protocol is provided in Appendix 1.
Region of interest (ROI) segmentation and consistency analysis
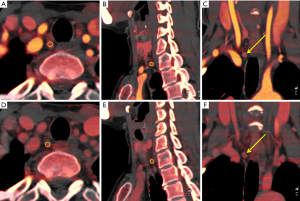
All of the LNs were evaluated by two radiologists with proficiency in head and neck disease and 13 and 15 years of experience, respectively. The two radiologists were blinded to one another and the final diagnosis. Each ROI from three different adjacent slices of the neck was drawn to measure the IC of each LN. The ROI was placed in the solid part with an area >2 mm2 including the entire LNs (Figure 2). The criteria for ROI selection are detailed in Appendix 1. The average value from three measurements was taken for final evaluation. An ROI with an area of 20 mm2 was drawn on the ipsilateral common carotid artery (CCA) as a reference. One week later, SPSS software version 22.0 (SPSS Inc., IBM Corp., Chicago, IL, USA) was used to randomly select case samples; 14% of data was randomly selected, and 50 LNs were retested. Intra- and inter-observer consistency analyses were performed to confirm the measurement consistency. The measured parameters included the maximal short axial diameter of the LNs, IC of the LNs, and the CCA in the arterial and the venous phases. The NIC was obtained using the following formula: NIC = ICLN/ICCCA. The “Mono Energetic” program was selected for energy spectrum analysis, the energy spectrum Hounsfield Unit (HU), which was defined as the difference between the CT value at 100 keV and that at 140 keV, was calculated as follows: λHU = (HU 100 keV – HU 140 keV)/40, where HU 100 keV stood for the CT value measured on 100 keV images and HU 140 keV represented the CT value measured on 140 keV images.
Statistical analysis
The IBM software SPSS statistics version 22.0 was used for statistical analysis, and GraphPad prism version 7.04 (GraphPad software, San Diego, CA, USA) and MedCalc version 18.2.1 (MedCalc Software Ltd., Ostend, Belgium) were used to draw graphs. A consistency test was performed to test the agreement of quantitative parameters between the two readers. An intraclass correlation coefficient (ICC) of <0.4 showed a diagnostic test to have poor consistency, while a diagnostic test with an ICC value of >0.75 was considered to have good consistency. The Kolmogorov-Smirnov (K-S) test method was used to test for the normal distribution of continuous variables of quantitative parameters. If the quantitative parameter fitted the normal distribution, mean ± standard deviation was used to describe it and t-test was used to compare the difference between metastatic and non-metastatic LNs. If it did not fit the normal distribution, the median (range) and Mann-Whitney U tests were used. The diagnostic ability of the short diameter and quantitative parameters were evaluated by receiver operating characteristic (ROC) curve analysis, and the optimal threshold was selected using the Youden index method. The area under the curve (AUC) of parameters was compared using the z-test. According to the current study design, multiple LNs of the same patient were measured, which could have resulted in a problem with correlation that introduced bias to estimates. To control for this potential issue, a generalized estimating equation (GEE) model with log links with robust variance was used to examine the association of the selected variables with LNM. This statistical method accounted for the correlation between the repeated measures within a participant. The GEE parameter estimates were expressed as the coefficients (β), odds ratio (OR), and the 95% confidence intervals (CIs). The multicollinearity of the multivariate model was assessed using the tolerance and variance inflation factor. The test level was set at P=0.05.
Results
General information
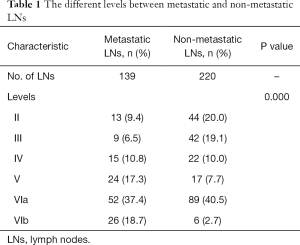
The demographic, clinical, and pathological characteristics of the 52 patients included in this study are shown in Table S1. A total of 1,374 LNs distributed in all the subregions could be identified on the DECT images of the enrolled patients. A total of 1,160 (87.9%) LNs were seen on DECT without artifacts. Among them, the DECT images of 745 (64.2%) LNs with a short diameter <0.5 cm and pathological results in each level were compared. Finally, a total of 359 (27.2%) small LNs including 139 metastatic and 220 non-metastatic LNs from 52 patients were included and analyzed according to the pathological results after LN dissection. All 359 LNs were located in level II, III, IV, V, VIa, and VIb, respectively. Detailed clinical information of the LNs is shown in Table 1 and Table S2, Appendix 1, and Figures S8,S9.
Full table
Result of consistency analysis
The imaging characteristics of DECT were basically consistent between the two radiologists. The inter-observer and intra-observer consistency analysis for all the parameters was >0.75, which equated to good consistency (Appendix 1, Table S3 and Figure S10).
Comparison of imaging parameters between metastatic and non-metastatic LNs
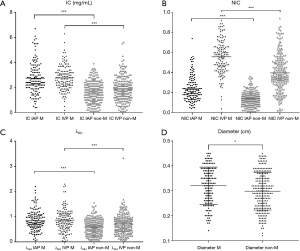
The quantitative parameters of metastatic and non-metastatic LNs are listed in Table 2. The diameter, IC in the arterial phase, NIC in the arterial phase, λHU in the arterial phase, IC in the venous phase, NIC in the venous phase, and λHU in the venous phase of metastatic LNs were increased compared to those of non-metastatic LNs (P=0.000–0.007) (Table 2, Figure 3).

Full table
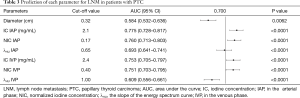
Prediction of each parameter for LNM in patients with PTC
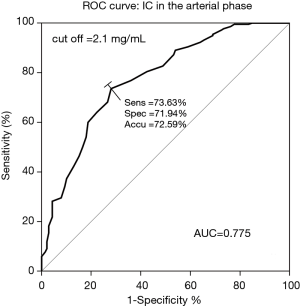
The IC in the arterial phase had the highest AUC (0.775), with a sensitivity, specificity, and accuracy of 73.63%, 71.94%, and 72.59%, respectively. The cut-off value of each quantitative parameter was obtained (Table 3, Figure 4, Figures S11,S12). The differences among the AUC of quantitative parameters are listed in Table S4. The parameters including IC in the arterial phase vs. λHU in the arterial phase (95% CI: 0.042–0.153; P=0.0006), IC in the arterial phase vs. λHU in the venous phase (95% CI: 0.0934–0.237; P<0.0001), IC in the venous phase vs. λHU in the arterial phase (95% CI: 0.00766–0.144; P=0.0292), IC in the venous phase vs. λHU in the venous phase (95% CI: 0.0854–0.201; P<0.0001), NIC in the arterial phase vs. λHU in the arterial phase (95% CI: 0.0215–0.143; P=0.008), NIC in the arterial phase vs. λHU in the venous phase (95% CI: 0.0745–0.225; P=0.0001), NIC in the venous phase vs. λHU in the arterial phase (95% CI: 0.00347–0.143; P=0.0396), NIC in the venous phase vs. λHU in the venous phase (95% CI: 0.0753–0.206; P<0.0001), and λHU in the arterial phase vs. λHU in the venous phase (95% CI: 0.00629–0.129; P=0.0307) all showed statistical significance for differentiation between metastatic and non-metastatic LNs.
Full table
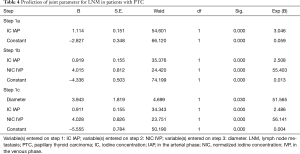
After binary classification logistic regression model analysis, three parameters including diameter, IC in the arterial phase, and NIC in the venous phase were finally included. In other words, the combination of these three parameters was more effective than using any one parameter alone for the prediction of LNM, with an AUC, sensitivity, and specificity were 0.819, 74.10%, and 77.27%, respectively (Table 4, Figure S13).
Full table
GEE estimation of risk factors associated with LNM
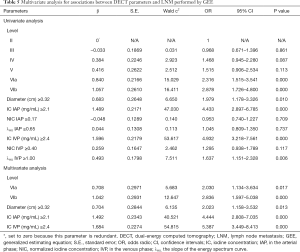
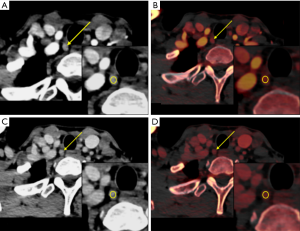
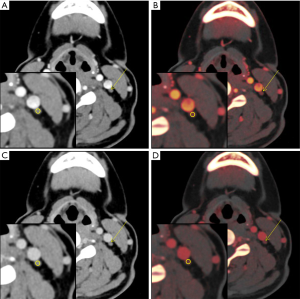
Univariable regression analysis showed that level VIa or VIb, diameter, IC in the arterial phase, IC in the venous phase, and λHU in the venous phase were predictors for predicting LNM (P=0.000–0.010). Further multivariable GEE analysis suggested that among these quantitative parameters, LNs being located in level VIa (OR 2.030, 95% CI: 1.134–3.634, P=0.017) or VIb (OR 2.836, 95% CI: 1.597–5.038, P=0.000), diameter (OR 2.023, 95% CI: 1.158–3.532, P=0.013), IC in the arterial phase (OR 4.444, 95% CI: 2.808–7.035, P=0.000), and IC in the venous phase (OR 5.387, 95% CI: 3.449–8.413, P=0.000) were independent predictors of LNM with a diameter <0.5 cm in patients with PTC. The results of the GEE are shown in Table 5 and two typical examples are shown in Figures 5,6.
Full table
The variance inflation factor of each predictor was <10, and the corresponding tolerance was >0.1; therefore, no multicollinearity among these predictors was noted (Table S5).
Discussion
In the current study, we found that the DECT quantitative parameters, especially the combination of diameter, IC in the arterial phase, and NIC in the venous phase, had significantly higher accuracy for preoperative diagnosis of metastatic cervical LNs <0.5 cm in patients with PTC. An IC in the arterial phase ≥2.1 mg/mL was an optimum single parameter. Metastasis could be considered for LNs meeting the following parameters: located in level VIa or VIb, diameter ≥0.32 cm, IC in the arterial phase ≥2.1 mg/mL, and IC in the venous phase ≥2.4 mg/mL.
To date, the majority of studies on the use of DECT in patients with PTC have focused on metastatic LNs >0.5 cm (18,33). A study by Liu et al. (33) showed the best single parameter for the detection of metastatic LNs was λHU in the venous phase, and Zhao et al. (18) showed that the optimal IC diagnostic threshold was 2.56 mg/mL in the arterial phase. These findings differ from our results, which we believe to be due to the following reasons. DECT can obtain characteristic energy spectrum curves of different substances, as well as single energy images within a certain energy range and CT values at any single energy point. Therefore, λHU can reflect the attenuation characteristics of lesions under different energy conditions, and λHU can be used for tissue composition analysis. The iodine map can directly reflect the difference in IC in lesions, especially for LNs <0.5 cm.
In our study, IC, NIC, and λHU of metastatic LNs were higher than that of non-metastatic LNs in both the arterial and the venous phases, which was consistent with the findings of previous studies (18,33). The main reason for this observation was that non-metastatic LNs had a low blood supply, while metastatic LNs had a high blood supply on account of the specific iodine absorption characteristics of the thyroid, even in small LNs.
This study showed that, among all the quantitative parameters, IC in the arterial phase had the highest sensitivity for differentiating metastatic from non-metastatic LNs. The GEE analysis showed that IC in the venous phase ≥2.4 mg/mL was another independent factor affecting LNM. IC has been considered as a direct response to blood flow, and is affected by the number of blood vessels (36). In PTC, normal follicular cells, responsible for thyroid iodine uptake, which exist in benign conditions such as follicular adenoma or nodular hyperplasia, are replaced by cancer cells or fibrotic tissues (37). Therefore, the differences in iodine uptake might lead to significantly different IC values among metastatic and non-metastatic LNs. The specific iodine absorption characteristics of thyroid tissue and the changes in tumor-related vascular patterns in LNs were also found to be correlated with IC (38). However, some scholars (33,39) have reported NIC to be a relatively stable parameter that could reduce differences among patients to a certain extent, and as being superior to IC in diagnostic efficiency. As IC is a direct measurement, we think it is easier to obtain and less likely to be disturbed by extraneous factors, which was consistent with the results of Zhao et al. (18). Moreover, we found that the use of quantitative parameters, especially the combination of diameter, IC in the arterial phase, and NIC in the venous phase, showed a higher AUC compared to the single parameters.
The most common type of metastasis in patients with PTC is lymphatic metastasis (40,41). According to ATA guidelines (42), the first metastatic region was the central region (VI), which was more common than the cervical region of the ipsilateral neck and the paratracheal metastasis. According to the study of Gregoire et al. (35), the majority of the metastatic LNs were around the internal jugular vein, and lymphatic vessels could be transferred to the posterior trigone or mediastinal LNs, but rarely to the submandibular region (Ib) (11,35). In summary, LNs in level VI were more likely to be involved.
The GEE analysis showed that a diameter ≥0.32 cm could be regarded as another independent influencing factor for LNM in patients with PTC. We found that for LNs <0.5 cm, the greater the diameter, the greater the possibility of metastasis.
Our study has five limitations. First, we employed a retrospective study design, and even though strict inclusion and exclusion criteria were adopted, there was still potential participant selection bias. Second, our study lacked a comparison of morphological features of LNs, besides diameter. However, the purpose of this study was not to evaluate the morphological diagnosis of LNs but to evaluate whether the specific parameters of DECT could identify differences in IC between the two groups. Third, considering the vascular spatial heterogeneity in PTC, a possible deviation was that a manual ROI may not represent total tumor vascularity. However, the method of manual ROI placement in the solid part of the LN including the entire LN in this study has been verified as a reliable and efficient measurement, and has been widely used in previous studies (33). Fourth, a preoperative US diagnosis of suspected LNs was not performed. Finally, in our study, the correlation of quantitative parameters with the morphology of LNs was not investigated, as this required one-to-one correspondence between pathological morphology and DECT measurements; however, this will be carried out in subsequent studies.
Conclusions
An IC in the arterial phase ≥2.1 mg/mL in cervical LNs with a short diameter <0.5 cm suggested the possibility of metastasis in preoperative DECT examination of patients with PTC. DECT had a higher ability to identify metastasis when the following combination of parameters was taken into consideration: level VIa or VIb, diameter ≥0.32 cm, IC in the arterial phase ≥2.1 mg/mL, and IC in the venous phase ≥2.4 mg/mL.
Acknowledgments
The authors thank: Jianhua Gu, MD, of the Department of General Surgery, Tianjin First Central Hospital for patient recruitment and guidance of clinical work; Wen Shen, MD, of the Department of Radiology, Tianjin First Central Hospital for image acquisition; and Xi Zhao, Senior engineer at Siemens, for their support of dual-energy computed tomography image post-processing.
Funding: This study was supported by the National Natural Science Foundation of China (NSFC, 81871342 to Shuang Xia, 81971585 to Guolin Ma), and the National Key Research and Development Project (2019YFC0120903 to Shuang Xia, 2020YFC2003903 to Guolin Ma).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-846). The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the ethics committee of the Tianjin First Central Hospital (2018N131KY), and the requirement for written informed consent was waived due to its retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carling T, Carty SE, Ciarleglio MM, Cooper DS, Doherty GM, Kim LT, Kloos RT, Mazzaferri EL Sr, Peduzzi PN, Roman SA, Sippel RS, Sosa JA, Stack BC Jr, Steward DL, Tufano RP, Tuttle RM, Udelsman R. American Thyroid Association design and feasibility of a prospective randomized controlled trial of prophylactic central lymph node dissection for papillary thyroid carcinoma. Thyroid 2012;22:237-44. [Crossref] [PubMed]
- Lesnik D, Cunnane ME, Zurakowski D, Acar GO, Ecevit C, Mace A, Kamani D, Randolph GW. Papillary thyroid carcinoma nodal surgery directed by a preoperative radiographic map utilizing CT scan and ultrasound in all primary and reoperative patients. Head Neck 2014;36:191-202. [Crossref] [PubMed]
- Vogl TJ, Schulz B, Bauer RW, Stover T, Sader R, Tawfik AM. Dual-energy CT applications in head and neck imaging. AJR Am J Roentgenol 2012;199:S34-9. [Crossref] [PubMed]
- Mansour J, Sagiv D, Alon E, Talmi Y. Prognostic value of lymph node ratio in metastatic papillary thyroid carcinoma. J Laryngol Otol 2018;132:8-13. [Crossref] [PubMed]
- Lin JD, Hsueh C, Chao TC. Early recurrence of papillary and follicular thyroid carcinoma predicts a worse outcome. Thyroid 2009;19:1053-9. [Crossref] [PubMed]
- Park JH, Yoon JH. Lobectomy in patients with differentiated thyroid cancer: indications and follow-up. Endocr Relat Cancer 2019;26:R381-93. [Crossref] [PubMed]
- Randolph GW, Duh QY, Heller KS. LiVolsi VA, Mandel SJ, Steward DL, Tufano RP, Tuttle RM. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012;22:1144-52. [Crossref] [PubMed]
- Smith VA, Sessions RB, Lentsch EJ. Cervical lymph node metastasis and papillary thyroid carcinoma: does the compartment involved affect survival? Experience from the SEER database. J Surg Oncol 2012;106:357-62. [Crossref] [PubMed]
- Yeh MW, Bauer AJ, Bernet VA, Ferris RL, Loevner LA, Mandel SJ, Orloff LA, Randolph GW, Steward DL. American Thyroid Association statement on preoperative imaging for thyroid cancer surgery. Thyroid 2015;25:3-14. [Crossref] [PubMed]
- Xu SY, Yao JJ, Zhou W, Chen L, Zhan WW. Clinical characteristics and ultrasonographic features for predicting central lymph node metastasis in clinically node-negative papillary thyroid carcinoma without capsule invasion. Head Neck 2019;41:3984-91. [Crossref] [PubMed]
- Lee DW, Ji YB, Sung ES, Park JS, Lee YJ, Park DW, Tae K. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol 2013;39:191-6. [Crossref] [PubMed]
- Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope 2011;121:487-91. [Crossref] [PubMed]
- Khokhar MT, Day KM, Sangal RB, Ahmedli NN, Pisharodi LR, Beland MD, Monchik JM. Preoperative High-Resolution Ultrasound for the Assessment of Malignant Central Compartment Lymph Nodes in Papillary Thyroid Cancer. Thyroid 2015;25:1351-4. [Crossref] [PubMed]
- Han Z, Lei Z, Li M, Luo D, Ding J. Differential diagnosis value of the ultrasound gray scale ratio for papillary thyroid microcarcinomas and micronodular goiters. Quant Imaging Med Surg 2018;8:507-13. [Crossref] [PubMed]
- Cheng SCH, Ahuja AT, Ying M. Quantification of intranodal vascularity by computer pixel-counting method enhances the accuracy of ultrasound in distinguishing metastatic and tuberculous cervical lymph nodes. Quant Imaging Med Surg 2019;9:1773-80. [Crossref] [PubMed]
- Tam AA, Kaya C, Ucler R, Dirikoc A, Ersoy R, Cakir B. Correlation of normal thyroid ultrasonography with thyroid tests. Quant Imaging Med Surg 2015;5:569-74. [PubMed]
- Liu C, Chen S, Yang Y, Shao D, Peng W, Wang Y, Chen Y, Wang Y. The value of the computer-aided diagnosis system for thyroid lesions based on computed tomography images. Quant Imaging Med Surg 2019;9:642-53. [Crossref] [PubMed]
- Zhao Y, Li X, Li L, Wang X, Lin M, Zhao X, Luo D, Li J. Preliminary study on the diagnostic value of single-source dual-energy CT in diagnosing cervical lymph node metastasis of thyroid carcinoma. J Thorac Dis 2017;9:4758-66. [Crossref] [PubMed]
- Kato H, Kanematsu M, Kato Z, Teramoto T, Mizuta K, Aoki M, Makita H, Kato K. Necrotic cervical nodes: usefulness of diffusion-weighted MR imaging in the differentiation of suppurative lymphadenitis from malignancy. Eur J Radiol 2013;82:e28-35. [Crossref] [PubMed]
- Zhou L, Wang JZ, Wang JT, Wu YJ, Chen H, Wang WB, Cao F, Cheng GX. Correlation analysis of MR/CT on colorectal cancer lymph node metastasis characteristics and prognosis. Eur Rev Med Pharmacol Sci 2017;21:1219-25. [PubMed]
- Zhang H, Zhang C, Zheng Z, Ye F, Liu Y, Zou S, Zhou C. Chemical shift effect predicting lymph node status in rectal cancer using high-resolution MR imaging with node-for-node matched histopathological validation. Eur Radiol 2017;27:3845-55. [Crossref] [PubMed]
- Sun H, Zhou J, Liu K, Shen T, Wang X, Wang X. Pancreatic neuroendocrine tumors: MR imaging features preoperatively predict lymph node metastasis. Abdom Radiol (NY) 2019;44:1000-9. [Crossref] [PubMed]
- Xie T, Li Y, He G, Zhang Z, Shi Q, Cheng G. The influence of liver fat deposition on the quantification of the liver-iron fraction using fast-kilovolt-peak switching dual-energy CT imaging and material decomposition technique: an in vitro experimental study. Quant Imaging Med Surg 2019;9:654-61. [Crossref] [PubMed]
- Grillet F, Busse-Cote A, Calame P, Behr J, Delabrousse E, Aubry S. COVID-19 pneumonia: microvascular disease revealed on pulmonary dual-energy computed tomography angiography. Quant Imaging Med Surg 2020;10:1852-62. [Crossref] [PubMed]
- Weidman EK, Plodkowski AJ, Halpenny DF, Hayes SA, Perez-Johnston R, Zheng J, Moskowitz C, Ginsberg MS, Dual-Energy CT. Angiography for Detection of Pulmonary Emboli: Incremental Benefit of Iodine Maps. Radiology 2018;289:546-53. [Crossref] [PubMed]
- Booz C, Noske J, Martin SS, Albrecht MH, Yel I, Lenga L, Gruber-Rouh T, Eichler K, D'Angelo T, Vogl TJ, Wichmann JL. Virtual Noncalcium Dual-Energy CT: Detection of Lumbar Disk Herniation in Comparison with Standard Gray-scale CT. Radiology 2019;290:446-55. [Crossref] [PubMed]
- Li J, Fang M, Wang R, Dong D, Tian J, Liang P, Liu J, Gao J. Diagnostic accuracy of dual-energy CT-based nomograms to predict lymph node metastasis in gastric cancer. Eur Radiol 2018;28:5241-9. [Crossref] [PubMed]
- Zhang X, Zheng C, Yang Z, Cheng Z, Deng H, Chen M, Duan X, Mao J, Shen J. Axillary Sentinel Lymph Nodes in Breast Cancer: Quantitative Evaluation at Dual-Energy CT. Radiology 2018;289:337-46. [Crossref] [PubMed]
- Ji GW, Zhang YD, Zhang H, Zhu FP, Wang K, Xia YX, Zhang YD, Jiang WJ, Li XC, Wang XH. Biliary Tract Cancer at CT: A Radiomics-based Model to Predict Lymph Node Metastasis and Survival Outcomes. Radiology 2019;290:90-8. [Crossref] [PubMed]
- Zeng YR, Yang QH, Liu QY, Min J, Li HG, Liu ZF, Li JX. Dual energy computed tomography for detection of metastatic lymph nodes in patients with hepatocellular carcinoma. World J Gastroenterol 2019;25:1986-96. [Crossref] [PubMed]
- Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, Dickson P, Duh QY, Ehya H, Goldner W, Haymart M, Hoh C, Hunt JP, Iagaru A, Kandeel F, Kopp P, Lamonica DM, McIver B, Raeburn CD, Ridge JA, Ringel MD, Scheri RP, Shah JP, Sippel R, Smallridge RC, Sturgeon C, Wang TN, Wirth LJ, Wong RJ, Johnson-Chilla A, Hoffmann KG, Gurski LA. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J Natl Compr Canc Netw 2018;16:1429-40. [Crossref] [PubMed]
- Rizzo S, Radice D, Femia M, De Marco P, Origgi D, Preda L, Barberis M, Vigorito R, Mauri G, Mauro A, Bellomi M. Metastatic and non-metastatic lymph nodes: quantification and different distribution of iodine uptake assessed by dual-energy CT. Eur Radiol 2018;28:760-9. [Crossref] [PubMed]
- Liu X, Ouyang D, Li H, Zhang R, Lv Y, Yang A, Xie C. Papillary thyroid cancer: dual-energy spectral CT quantitative parameters for preoperative diagnosis of metastasis to the cervical lymph nodes. Radiology 2015;275:167-76. [Crossref] [PubMed]
- Park JE, Lee JH, Ryu KH, Park HS, Chung MS, Kim HW, Choi YJ, Baek JH. Improved Diagnostic Accuracy Using Arterial Phase CT for Lateral Cervical Lymph Node Metastasis from Papillary Thyroid Cancer. AJNR Am J Neuroradiol 2017;38:782-8. [Crossref] [PubMed]
- Gregoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, Lee A, Le QT, Maingon P, Nutting C, O'Sullivan B, Porceddu SV, Lengele B. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol 2014;110:172-81. [Crossref] [PubMed]
- Tawfik AM, Michael Bucher A, Vogl TJ. Dual-Energy Computed Tomography Applications for the Evaluation of Cervical Lymphadenopathy. Neuroimaging Clin N Am 2017;27:461-8. [Crossref] [PubMed]
- Galrao AL, Camargo RY, Friguglietti CU, Moraes L, Cerutti JM, Serrano-Nascimento C, Suzuki MF, Medeiros-Neto G, Rubio IG. Hypermethylation of a New Distal Sodium/Iodide Symporter (NIS) enhancer (NDE) is associated with reduced NIS expression in thyroid tumors. J Clin Endocrinol Metab 2014;99:E944-52. [Crossref] [PubMed]
- Vergez S, Sarini J, Percodani J, Serrano E, Caron P. Lymph node management in clinically node-negative patients with papillary thyroid carcinoma. Eur J Surg Oncol 2010;36:777-82. [Crossref] [PubMed]
- Tawfik AM, Razek AA, Kerl JM, Nour-Eldin NE, Bauer R, Vogl TJ. Comparison of dual-energy CT-derived iodine content and iodine overlay of normal, inflammatory and metastatic squamous cell carcinoma cervical lymph nodes. Eur Radiol 2014;24:574-80. [Crossref] [PubMed]
- Mamelle E, Borget I, Leboulleux S, Mirghani H, Suarez C, Pellitteri PK, Shaha AR, Hamoir M, Robbins KT, Khafif A, Rodrigo JP, Silver CE, Rinaldo A, Ferlito A, Hartl DM. Impact of prophylactic central neck dissection on oncologic outcomes of papillary thyroid carcinoma: a review. Eur Arch Otorhinolaryngol 2015;272:1577-86. [Crossref] [PubMed]
- Li L, Wang Y, Luo DH, Zhao YF, Lin M, Guo W, Hu L, Zhou CW, Zhao XM. Diagnostic value of single-source dual-energy spectral computed tomography for papillary thyroid microcarcinomas. J Xray Sci Technol 2017;25:793-802. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]


