The effects of antiepileptic drugs on cognitive functional magnetic resonance imaging
Introduction
The adverse cognitive effects of antiepileptic drugs (AEDs) are well known and may affect many cognitive and behavioral domains. Usually, these collateral effects have been associated with high dosages and polytherapy (1,2). To investigate the effects of AEDs on brain function (and dysfunction) functional magnetic resonance imaging (fMRI) can be used as a research method given the possibilities to explore the ability of drugs to modify brain networks and circuits (1,3). These fMRI studies are called pharmaco-MRI (ph-MRI) and have been more commonly applied to investigate pharmacological effects and interactions in other central nervous system (CNS) diseases (i.e., depression, anxiety disorders and schizophrenia) rather than in epilepsy.
fMRI relies on the blood oxygenation level-dependent (BOLD) signal changes to indirectly find neuronal activity in brain regions related to tasks or stimuli (4). The brain corresponds to approximately 2% of the body weight, but uses about 20% of the glucose and oxygen, which are not stored in the brain. Therefore, a constant supply must exist. Since in normal conditions the energy production depends mainly on the oxidative metabolism of oxygen, with increased synaptic activity there is a greater demand for glucose and oxygen. This generates a local increase in the local cerebral blood flow (CBF), cerebral blood volume (CBV) and cerebral metabolic rate of oxygen consumption (CMRO2). A complex interplay between the variations of CBF, CBV and CMRO2 gives rise to a transient increase in the local concentration of oxyhemoglobin relative to deoxyhemoglobin. This decreases the inhomogeneities in the magnetic field inside the voxel, because oxyhemoglobin (oxygenated hemoglobin) is diamagnetic and deoxyhemoglobin (deoxygenated hemoglobin) is paramagnetic. Thus, when the MRI image is T2*-weighted, the signal intensity is increased. With the aid of statistical tools these voxels can be detected. After an event, the BOLD signal peaks at about 3-6 s and returns to baseline after approximately 20-30 s. These changes are usually modeled with the hemodynamic response function with one or two gamma-variate functions.
In task-related fMRI the subject performs a task (e.g., cognitive, finger tapping, button press) or is presented stimuli (e.g., checkerboard flicker) alternately with another condition, typically a rest period (5,6). Although there is no formal definition of rest, usually the subject performs a control task (e.g., just lying still inside the scanner) or is presented a control stimulus. During the so-called resting-state fMRI (rs-fMRI) the subject lies in the scanner either with eyes open or closed and performs no specific task (7). Analysis of task-related fMRI is normally performed with general linear model (GLM). Some popular approaches in rs-fMRI statistical analysis include seed-based analysis and independent component analysis (ICA). To study the resting-state networks, graph theory can be used. Since complex networks can be found in whole brain studies using GLM, it may be more advantageous to perform region-of-interest (ROI) analyses when there is hypothesis about specific regions related to the drug effect. In this type of study care should be taken to define the region for each subject and to perform the appropriate signal processing in order to extract comparable time series (in percentage for example).
FMRI has no proven risk of biological harm and uses no ionizing radiation. It has been widely used to find the neurophysiological correlates of specific behavior and stimuli in different situations. For clinical purposes fMRI can be employed, for example, to map the eloquent cortex in presurgical evaluation (8) and to compare brain function in normal and pathological situations (9). The interpretation of the results in pathological conditions must be considered with care because the neurovascular coupling may be impaired due to the illness.
In the specific case of epilepsy, fMRI is used to map the eloquent cortex before surgery and to find the hemodynamic correlates of epileptiform discharges. For the latter case, normally electroencephalogram (EEG) and fMRI are simultaneously recorded (10). Since the most common form of epilepsy is mesial temporal lobe epilepsy (TLE), which affects the hippocampus (11), cognitive fMRI studies investigate primarily memory impairment, but little is known about the cognitive effects of AED (5,12). The changes in behaviors are not clear even for drugs like L-dopa, whose molecular mechanism of action is well understood (13).
The ph-MRI studies assess the effect of pharmacological agents and the sources of data include drug-related brain changes or behavioral effects. The knowledge about the effect of drugs in fMRI is of great importance in the clinical application of fMRI. For the presurgical mapping of eloquent cortex and the localization of the seizure onset zone in epilepsy, drug-related changes should be separated from the effect of the disease (1). A comprehensive description of technical and methodological details of ph-MRI studies has been published by Mehta and O’Daly (13).
Cognitive ph-MRI studies usually compare the brain signal during task in placebo and drug conditions. As in conventional fMRI a univariate approach and graph theory can be used. When the drug effect in the functional network is studied, more distributed influences of the AEDs in the functional connectome can be identified (14).
One of the difficulties in the application of pharmacological studies in fMRI is the low signal change related to the drug. This can be assessed through a change in a task effect, that is, an interaction effect instead of a main effect. For example, one could compare the brain activation pattern in a cognitive paradigm for a drug and a placebo condition. Another problem is the influence of the pharmacological agent both in the neuronal and vascular level (13).
Pharmacological fMRI studies with AEDs are difficult to perform, because usually patients are already under medication. Therefore, participants are not recruited prior to the administration of the AED, making it more complex to control for effects such as genetic polymorphisms, family history of psychiatric or neurological illness, and concomitant use of other AED, especially because it is common that epilepsy patients are under polytherapy. Since AEDs are part of the epilepsy treatment, the studies normally use cross-sectional designs. To investigate the effect of AEDs all variability introduced by the subjects themselves must be reduced. Hence, one of the challenges in fMRI studies with AEDs is to find a reasonable number of patients with similar characteristics (e.g., gender, age, seizure type) that are under the same medication. Another difficulty is the control condition, because epilepsy itself may affect the fMRI results rather than only the AEDs. Generally in pharmacological studies a placebo is administered, but it may not be possible in epilepsy because AEDs are controlling the seizures. Comparing different doses of the same medication could be one alternative, or at least this could be entered as a nuisance variable when the patient medication profile does not match perfectly. Nevertheless, it may not be sufficient to control for the dose because there is variability between subjects in absorption of some AEDs (15). Possible additional confounding variables include the medication effects (e.g., heart rate) and patient characteristics (e.g., age of epilepsy onset). If the study is not well designed, there may not be enough statistical power to identify cognitive networks affected by the AEDs. Despite all these limitations, well-designed ph-MRI may provide an opportunity to investigate changes in brain functional networks associated with drug administration, as well as the interaction between drug, behavioral changes and brain activity. Furthermore, it allows the detection and characterization of changes of brain function during specific tasks modulated/induced by drugs (1).
The same mechanisms responsible for the seizure control by the AEDs may be also involved in the cognitive problems, in that the AEDs reduce neuronal activation. By decreasing the likelihood of excessive firing, the frequency of seizures is reduced. However, other mechanisms may be involved, since even AEDs that boost the GABAergic system cause only small cognitive impairment, such as gabapentin and tiagabine (16).
Here we review the studies that investigated the effects of AEDs on cognitive fMRI.
AEDs and cognitive fMRI studies
Topiramate (TPM)
TPM is a broad-spectrum AED indicated for the treatment of epilepsy, as well as other neurological and psychiatric diseases, including migraine headaches. Neurocognitive dysfunction has been associated to TPM, affecting individuals with epilepsy or migraine as well as healthy controls (17-19). It is important to note that cognitive impairment is an important factor for discontinuation of TPM (20,21) and can be identified by neuropsychological tests even in those individuals who do not report cognitive decline (21,22). Divergent results have been described regarding the impact of rapid titration (23-25) and dose effect on cognition (18,26), as some studies observed adverse effects even at low doses (19,27) whilst others confirmed the persistence of dysfunction on steady-state period (17,18,28).
Various domains of cognition are affected by TPM according to different studies, including attention, memory, processing speed, slowed thinking and verbal fluency function (24,27,29). Word-finding difficulties have been reported as a frequent complaint from individuals taking TPM (30) and then confirmed by studies which investigate performance on verbal fluency, comparing both the individual performance on and off TPM effect (17,29) as well as by comparing to valproate (VPA) (28) and levetiracetam (LEV) (21). Other studies analyzed verbal processing tests and identified a negative effect of TPM while lamotrigine (LMT) and gabapentin showed a significantly smaller effect (24,25).
Although frequently identified, the pathophysiology of the cognitive dysfunction related to TPM and other AEDs is still poorly understood; therefore some studies have evaluated the impact of AEDs on neurophysiologic measures combined with cognitive tasks. Smith et al. (31) used TPM and LMT as pharmacologic intervention in healthy volunteers to show changes on both EEG and evoked potentials, which were consistent with working memory impairment in those taking TPM. Jung et al. (32) studied the effect of TPM on neuropsychological profile and evented-related potentials in drug naïve patients with epilepsy, and identified reduction of current density of P200 component in parieto-occipital, temporolimbic and dorsolateral prefrontal regions.
Due to the interest in investigating the effects of TPM on brain function, some cross-sectional studies have used language fMRI searching for differences in activations and deactivations in patients (with migraine and epilepsy) taking TPM. De Ciantis et al. (33) performed a language fMRI study (blocked design study alternating periods of rest and periods of silent generation of words beginning with a different input letter presented visually) comparing the performance between patients with migraine (ten subjects, five with cognitive dysfunction) and five healthy controls. They observed that subjects with cognitive dysfunction presented reduced activation of language network, while those without dysfunction displayed a “general overactivation”, suggesting a compensational mechanism. By evaluating patients with mixed causes of epilepsy, Jansen et al. (34) compared five patients using TPM with ten control patients not taking TPM, and performed an fMRI study with a cognitive language task (similar blocked design paradigm alternating rest and periods of covert generation of words). They observed underactivation of the whole brain in the group taking TPM, markedly in the language network.
In another fMRI study of TPM, Szaflarski et al. analyzed patients with TLE, comparing 32 patients and 32 healthy controls. Patients were separated in right TLE (RTLE) (16, eight taking TPM) and left TLE (LTLE) (16, eight taking TPM). All subjects performed a semantic decision and tone decision fMRI language task. The analysis of LTLE patients showed that those taking TPM presented higher activation of left cingulate associated with decreased activation of left superior temporal gyrus. In addition, more LTLE patients taking TPM presented atypical language lateralization than those not taking TPM. Different findings were obtained from the comparison between RTLE patients taking and not taking TPM. RTLE patients taking TPM presented increased activation in the bilateral superior frontal gyrus and left lingual/occipital gyrus, associated with reduced activation in the left inferior frontal gyrus. The examination of effect of TPM dose on BOLD signal revealed positive association in the basal ganglia/anterior thalamus and negative associations in the anterior cingulate cortex and lingual gyrus (35).
We also used language fMRI task to examine the effect of TPM in a cross-sectional analysis of patients with frontal lobe epilepsy (FLE) (8 taking TPM, 27 taking other AEDs and 24 healthy controls), and in a small longitudinal pilot study of six subjects (four patients and two healthy volunteers) (6) (Figure 1). In the longitudinal study the same subject was scanned twice (with and without TPM). In the cross-sectional analysis we observed a reduction of the task-related deactivation of the default mode network (DMN) in patients taking TPM; the longitudinal study confirmed these findings as both single dose (healthy volunteers, 200 mg) and chronic use of TPM were associated with impairment of verbal fluency and disruption of task-related deactivations of DMN.
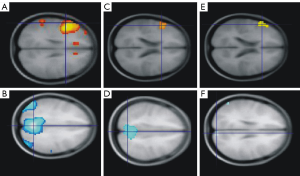
Lamotrigine (LMT)
LMT is an AED also indicated to stabilize mood in both adult and adolescent bipolar disease (ABD) (36). LMT blocks voltage-sensitive sodium channels and does not act directly on N-methyl-D-aspartate (NMDA) receptors. Consequently, under sustained repetitive firing, LMT inhibits the release of glutamate and aspartate, both excitatory neurotransmitters. LMT is well tolerated and can reduce the seizure threshold by at least half in 25-34% of patients (37). Therefore, LMT has a general inhibitory effect on cortical neuronal excitability. In bipolar disorder for example, LMT may have an acute and prophylactic antidepressant activity (38,39), probably reducing cortical excitability in important regions for the pathogenesis of mood disorders through the same mechanisms of seizure control (40).
In an fMRI study designed to investigate the role of increased glutamate release induced by ketamine in healthy volunteers, Deakin et al. (41) used LMT due to its properties to inhibit glutamate release. They performed a double-blind, placebo-controlled, randomized, within-subjects design. In the ketamine-LMT experiment they observed that LMT antagonized most of the effects of ketamine. Similarly, Doyle et al. (7) also observed that LMT attenuated the effects of ketamine in a resting state study. In a study of patients with ABD, Pavuluri et al. (36) evaluated 13 patients with a response inhibition task fMRI paradigm before and after treatment (second generation antipsychotics followed by LMT). After treatment they identified a reversion of the dysfunctional neural circuitry observed initially in patients.
Carbamazepine (CBZ)
CBZ is an AED used in patients with localization-related epilepsies. CBZ can also be used in psychiatric diseases, such as acute mania and bipolar disorder, and in patients with chronic neuralgic facial pain and headaches. Its mechanisms of action are still not completely known, and there are few studies about the effects of CBZ in brain metabolism. Jokeit et al. studied 21 patients with refractory TLE, compared to 20 healthy controls. They used a memory retrieval task paradigm for fMRI and observed an inverse relation between CBZ serum level and size of the cluster of activated voxels in mesiotemporal lobes of TLE, possibly associated to reduced cerebral glucose metabolism (42).
Pregabalin (PGB)
PGB is an AED (43) with its primary mechanism of action associated with upregulation of GABA inhibitory activity in addition to a decrease in release of some neurotransmitters, including noradrenaline, glutamate and serotonin. It has also analgesic and anxiolytic properties (44). Due to its anxiolytic properties, Aupperle et al. investigated the effects of PGB on neural activation during an emotional face-matching fMRI task in healthy volunteers (45). Using a double-blind within-subjects design, they observed an attenuation of amygdala activation during fearful face matching and activation of left anterior insula during angry face matching.
Levetiracetam (LEV)
LEV has been recommended to treat focal epilepsies (46) and associated with cognitive profile improvement (47). To evaluate the effect of LEV on activations and deactivations during FMRI working memory tasks, Wandschneider et al. compared retrospectively 59 refractory TLE patients treated with LEV with 48 refractory TLE patients treated without LEV (48). Patients on LEV presented normalization of functional network deactivations in the right temporal lobe in RTLE during visual-spatial task, while LTLE patients presented normalization in the left temporal lobe during verbal task (Figure 2). Additionally, they identified a dose-dependent effect in right hippocampus of RTLE during visual task, as the lower the dose of LEV was associated with the greater atypical activation.
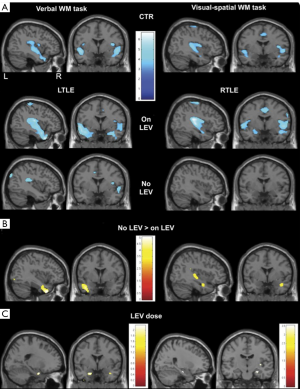
Valproate (VPA)
VPA is also a broad-spectrum AED used not only in epilepsy (49), but also indicated for other neuropsychiatry disorders as bipolar disease (50) and migraine (51). It has been associated with some cognitive impairment in bipolar disease (50), but less likely to cause dysfunction in patients with juvenile myoclonic epilepsy (JME) (5). To investigate the effect of VPA on working memory in JME patients, Vollmar et al. performed an fMRI study and analyzed 21 patients treated with VPA, identifying a dose-dependent normalization of working memory network (positive correlation of VPA dosage with bilateral activations in frontal and parietal areas). Furthermore, they observed a negative correlation between left motor cortex activation and VPA dosage, suggesting a specific effect of this AED in JME.
Conclusions
There is a scarcity of fMRI studies designed to investigate the effects of AEDs on cognition. The complex interaction between disease, polytherapy, comorbidities and genetic aspects may be responsible for the difficulties in performing ph-MRI studies in patients with epilepsy. However, the findings of recent research have provided insightful information about drug-specific effects on cognition and behavior, suggesting that such studies should be encouraged despite the limitations.
Acknowledgements
This study was supported by Brazilian National Council for Scientific and Technological Development (CNPq—313831/2014-9), CEPID-FAPESP: “the Brazilian Institute of Neuroscience and Neurotechnology (BRAINN)”, grant 2013/07559-3.
Disclosure: The authors declare no conflict of interest.
References
- Koepp MJ. Gender and drug effects on neuroimaging in epilepsy. Epilepsia 2011;52 Suppl 4:35-7. [PubMed]
- Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, Resnick T, Benbadis SR. The consequences of refractory epilepsy and its treatment. Epilepsy Behav 2014;37:59-70. [PubMed]
- Nathan PJ, Phan KL, Harmer CJ, Mehta MA, Bullmore ET. Increasing pharmacological knowledge about human neurological and psychiatric disorders through functional neuroimaging and its application in drug discovery. Curr Opin Pharmacol 2014;14:54-61. [PubMed]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA 1992;89:5951-5. [PubMed]
- Vollmar C, O'Muircheartaigh J, Barker GJ, Symms MR, Thompson P, Kumari V, Duncan JS, Janz D, Richardson MP, Koepp MJ. Motor system hyperconnectivity in juvenile myoclonic epilepsy: a cognitive functional magnetic resonance imaging study. Brain 2011;134:1710-9. [PubMed]
- Yasuda CL, Centeno M, Vollmar C, Stretton J, Symms M, Cendes F, Mehta MA, Thompson P, Duncan JS, Koepp MJ. The effect of topiramate on cognitive fMRI. Epilepsy Res 2013;105:250-5. [PubMed]
- Doyle OM, De Simoni S, Schwarz AJ, Brittain C, O'Daly OG, Williams SC, Mehta MA. Quantifying the attenuation of the ketamine pharmacological magnetic resonance imaging response in humans: a validation using antipsychotic and glutamatergic agents. J Pharmacol Exp Ther 2013;345:151-60. [PubMed]
- Jiang Z, Krainik A, David O, Salon C, Troprès I, Hoffmann D, Pannetier N, Barbier EL, Bombìn ER, Warnking J, Pasteris C, Chabardes S, Berger F, Grand S, Segebarth C, Gay E, Le Bas JF. Impaired fMRI activation in patients with primary brain tumors. Neuroimage 2010;52:538-48. [PubMed]
- Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, Ranjeva JP, Guye M. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 2010;81:1147-54. [PubMed]
- Chaudhary UJ, Carmichael DW, Rodionov R, Thornton RC, Bartlett P, Vulliemoz S, Micallef C, McEvoy AW, Diehl B, Walker MC, Duncan JS, Lemieux L. Mapping preictal and ictal haemodynamic networks using video-electroencephalography and functional imaging. Brain 2012;135:3645-63. [PubMed]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, Luo C, Lu G, Chen H. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One 2010;5:e8525. [PubMed]
- Stretton J, Winston G, Sidhu M, Centeno M, Vollmar C, Bonelli S, Symms M, Koepp M, Duncan JS, Thompson PJ. Neural correlates of working memory in Temporal Lobe Epilepsy--an fMRI study. Neuroimage 2012;60:1696-703. [PubMed]
- Mehta MA, O'Daly OG. Pharmacological application of fMRI. Methods Mol Biol 2011;711:551-65. [PubMed]
- Giessing C, Thiel CM. Pro-cognitive drug effects modulate functional brain network organization. Front Behav Neurosci 2012;6:53. [PubMed]
- Simon C, Stieger B, Kullak-Ublick GA, Fried M, Mueller S, Fritschy JM, Wieser HG, Pauli-Magnus C. Intestinal expression of cytochrome P450 enzymes and ABC transporters and carbamazepine and phenytoin disposition. Acta Neurol Scand 2007;115:232-42. [PubMed]
- Tang V, Warden J, Cullen N, Rutledge E. Topiramate in traumatic brain injury: adverse effects on cognitive function. J Head Trauma Rehabil 2007;22:409-10. [PubMed]
- Thompson PJ, Baxendale SA, Duncan JS, Sander JW. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry 2000;69:636-41. [PubMed]
- Loring DW, Williamson DJ, Meador KJ, Wiegand F, Hulihan J. Topiramate dose effects on cognition: a randomized double-blind study. Neurology 2011;76:131-7. [PubMed]
- Romigi A, Cervellino A, Marciani MG, Izzi F, Massoud R, Corona M, Torelli F, Zannino S, Uasone E, Placidi F. Cognitive and psychiatric effects of topiramate monotherapy in migraine treatment: an open study. Eur J Neurol 2008;15:190-5. [PubMed]
- Tatum WO 4th, French JA, Faught E, Morris GL 3rd, Liporace J, Kanner A, Goff SL, Winters L, Fix A. PADS Investigators. Post-marketing antiepileptic drug survey. Postmarketing experience with topiramate and cognition. Epilepsia 2001;42:1134-40. [PubMed]
- Bootsma HP, Ricker L, Diepman L, Gehring J, Hulsman J, Lambrechts D, Leenen L, Majoie M, Schellekens A, de Krom M, Aldenkamp AP. Long-term effects of levetiracetam and topiramate in clinical practice: A head-to-head comparison. Seizure 2008;17:19-26. [PubMed]
- Kockelmann E, Elger CE, Helmstaedter C. Significant improvement in frontal lobe associated neuropsychological functions after withdrawal of topiramate in epilepsy patients. Epilepsy Res 2003;54:171-8. [PubMed]
- Aldenkamp AP. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology 2000;54:271-2. [PubMed]
- Martin R, Kuzniecky R, Ho S, Hetherington H, Pan J, Sinclair K, Gilliam F, Faught E. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology 1999;52:321-7. [PubMed]
- Meador KJ, Loring DW, Vahle VJ, Ray PG, Werz MA, Fessler AJ, Ogrocki P, Schoenberg MR, Miller JM, Kustra RP. Cognitive and behavioral effects of lamotrigine and topiramate in healthy volunteers. Neurology 2005;64:2108-14. [PubMed]
- Lee HW, Jung DK, Suh CK, Kwon SH, Park SP. Cognitive effects of low-dose topiramate monotherapy in epilepsy patients: A 1-year follow-up. Epilepsy Behav 2006;8:736-41. [PubMed]
- Brandes JL, Saper JR, Diamond M, Couch JR, Lewis DW, Schmitt J, Neto W, Schwabe S, Jacobs D; MIGR-002 Study Group. Topiramate for migraine prevention: a randomized controlled trial. JAMA 2004;291:965-73. [PubMed]
- de Araujo Filho GM, Pascalicchio TF, Lin K, Sousa PS, Yacubian EM. Neuropsychiatric profiles of patients with juvenile myoclonic epilepsy treated with valproate or topiramate. Epilepsy Behav 2006;8:606-9. [PubMed]
- Lee S, Sziklas V, Andermann F, Farnham S, Risse G, Gustafson M, Gates J, Penovich P, Al-Asmi A, Dubeau F, Jones-Gotman M. The effects of adjunctive topiramate on cognitive function in patients with epilepsy. Epilepsia 2003;44:339-47. [PubMed]
- Crawford P. An audit of topiramate use in a general neurology clinic. Seizure 1998;7:207-11. [PubMed]
- Smith ME, Gevins A, McEvoy LK, Meador KJ, Ray PG, Gilliam F. Distinct cognitive neurophysiologic profiles for lamotrigine and topiramate. Epilepsia 2006;47:695-703. [PubMed]
- Jung KY, Cho JW, Joo EY, Kim SH, Choi KM, Chin J, Park KW, Hong SB. Cognitive effects of topiramate revealed by standardised low-resolution brain electromagnetic tomography (sLORETA) of event-related potentials. Clin Neurophysiol 2010;121:1494-501. [PubMed]
- De Ciantis A, Muti M, Piccolini C, Principi M, Di Renzo A, De Ciantis R, Frondizi D, Iannone G, Ottaviano P, Piccirilli M. A functional MRI study of language disturbances in subjects with migraine headache during treatment with topiramate. Neurol Sci 2008;29 Suppl 1:S141-3. [PubMed]
- Jansen JF, Aldenkamp AP, Marian Majoie HJ, Reijs RP, de Krom MC, Hofman PA, Eline Kooi M, Nicolay K, Backes WH. Functional MRI reveals declined prefrontal cortex activation in patients with epilepsy on topiramate therapy. Epilepsy Behav 2006;9:181-5. [PubMed]
- Szaflarski JP, Allendorfer JB. Topiramate and its effect on fMRI of language in patients with right or left temporal lobe epilepsy. Epilepsy Behav 2012;24:74-80. [PubMed]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J Clin Psychiatry 2010;71:1526-34. [PubMed]
- Messenheimer JA. Lamotrigine. Epilepsia 1995;36 Suppl 2:S87-94. [PubMed]
- Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88. [PubMed]
- Frye MA, Ketter TA, Kimbrell TA, Dunn RT, Speer AM, Osuch EA, Luckenbaugh DA, Cora-Ocatelli G, Leverich GS, Post RM. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14. [PubMed]
- Li X, Tenebäck CC, Nahas Z, Kozel FA, Large C, Cohn J, Bohning DE, George MS. Interleaved transcranial magnetic stimulation/functional MRI confirms that lamotrigine inhibits cortical excitability in healthy young men. Neuropsychopharmacology 2004;29:1395-407. [PubMed]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry 2008;65:154-64. [PubMed]
- Jokeit H, Okujava M, Woermann FG. Carbamazepine reduces memory induced activation of mesial temporal lobe structures: a pharmacological fMRI-study. BMC Neurol 2001;1:6. [PubMed]
- Zaccara G, Almas M, Pitman V, Knapp L, Posner H. Efficacy and safety of pregabalin versus levetiracetam as adjunctive therapy in patients with partial seizures: a randomized, double-blind, noninferiority trial. Epilepsia 2014;55:1048-57. [PubMed]
- French JA, Kugler AR, Robbins JL, Knapp LE, Garofalo EA. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology 2003;60:1631-7. [PubMed]
- Aupperle RL, Tankersley D, Ravindran LN, Flagan T, Stein NR, Stein MB, Paulus MP. Pregabalin effects on neural response to emotional faces. Front Hum Neurosci 2012;6:42. [PubMed]
- Shorvon SD, Löwenthal A, Janz D, Bielen E, Loiseau P. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group. Epilepsia 2000;41:1179-86. [PubMed]
- Helmstaedter C, Witt JA. The effects of levetiracetam on cognition: a non-interventional surveillance study. Epilepsy Behav 2008;13:642-9. [PubMed]
- Wandschneider B, Stretton J, Sidhu M, Centeno M, Kozák LR, Symms M, Thompson PJ, Duncan JS, Koepp MJ. Levetiracetam reduces abnormal network activations in temporal lobe epilepsy. Neurology 2014;83:1508-12. [PubMed]
- Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 2002;16:669-94. [PubMed]
- Muralidharan K, Kozicky JM, Bücker J, Silveira LE, Torres IJ, Yatham LN. Are cognitive deficits similar in remitted early bipolar I disorder patients treated with lithium or valproate? Data from the STOP-EM study. Eur Neuropsychopharmacol 2015;25:223-30. [PubMed]
- Mulleners WM, McCrory DC, Linde M. Antiepileptics in migraine prophylaxis: An updated Cochrane review. Cephalalgia 2015;35:51-62. [PubMed]


