
A novel 2-deoxy-2-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT)-based nomogram to predict lymph node metastasis in early stage uterine cervical squamous cell cancer
Introduction
Uterine cervical cancer is one of the most common and lethal malignancies among women worldwide (1). The preferred treatment for clinical early-stage patients is radical surgery with individualized adjuvant treatment. About 15–20% of clinical early-stage patients had lymph node metastasis (2), one of the major prognostic factors and influential for postoperative treatment strategy (3-5). In 2018, the International Federation of Gynecology and Obstetrics (FIGO) staging for cervical cancer was revised, and retroperitoneal lymph node metastases came to be regarded as stage IIIC (6). Thus, an accurate and effective preoperative diagnostic approach for pelvic lymph node (PLN) metastases is urgently required.
Several imaging modalities have been used for preoperative assessment of PLN involvement in cervical cancer and 2-deoxy-2-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) scan is more sensitive than CT and magnetic resonance imaging (MRI) (7-13). However, the PET/CT scan’s diagnostic performance is inconsistent and unsatisfactory, with a sensitivity of 48.6–82% (7-12). Although several studies have added radiomics for PLN metastasis prediction in cervical cancer, the complexity and technological immaturity limited its clinical application (14-16). Thus, we aimed to explore a simple and effective PET/CT scan-based predictive model for PLN metastasis of clinically early-stage cervical cancer.
Since most cervical cancers are squamous cell carcinomas, we analyzed this homologous group of patients. Our study demonstrated a PET/CT scan-based nomogram for preoperative identification of PLN metastasis in early-stage squamous cervical cancer.
Methods
Clinical characteristics
The ethics committee approved this study of Fudan University Shanghai Cancer Center. We retrospectively enrolled 351 uterine cervical squamous cell cancer patients who underwent PET/CT before radical surgery between 2010 and 2017. All patients were diagnosed with FIGO IB–IIA stage (version 2009). According to the World Health Organization (WHO) criteria, the pathological diagnoses were reviewed by two experienced gynecologic pathologists. Immunohistochemical (IHC) staining was performed for Ki-67 (Roche, Basel, Switzerland), Cyclin D1 (DakoCytomation, Glostrup, Denmark), and P53 (DakoCytomation, Glostrup, Denmark) using a Ventana Benchmark XT autostainer (Ventana Medical Systems Inc., Tucson, AZ, USA).
A total of 241 patients between Jan 2010 and July 2015 and 110 patients between Aug 2015 and Dec 2017 were separately assigned into training and external validation cohorts. Patient characteristics including age, FIGO stage, preoperative squamous cell carcinoma antigen (SCCA) level, therapy, tumor diameter, and pathological PLN metastasis were obtained from the medical records.
18F-FDG PET/CT procedure and interpretation
The 18F-FDG was produced automatically by cyclotron (Siemens CTI RDS Eclipse ST, Knoxville, TN, USA) using the Explora FDG4 module in our center, and the radiochemical purity was >95%. All patients fasted ≥6 h before the injection of 18F-FDG. Venous blood glucose levels were maintained <10 mmol/L in our center. Patients were injected with 7.4 MBq/kg (0.2 mCi/kg) 18F-FDG and required to be quiet for approximately 1 h before scanning. Images were obtained on a Siemens biograph 16HR PET/CT scanner (Knoxville, TN, USA). The transaxial intrinsic spatial resolution was 4.1 mm (full width at half-maximum) in the view center. CT scanning (120 kV, 80–250 mA, pitch 3.6, rotation time 0.5) from the proximal thighs to the head was first performed for data acquisition, followed closely by a PET emission scan. The acquisition time was 2–3 min/bed. The PET image data sets were iteratively reconstructed using the attenuation correction of CT data, and the infused images were displayed on a workstation.
All PET/CT parameters, such as PLN in PET/CT, PLN diameter, and maximum standardized uptake value (nSUVmax), were analyzed and interpreted by two experienced nuclear medicine physicians independently and blindly without knowledge of pathological PLN involvement. A consensus was reached in cases of a discrepancy. A multimodality computer platform (Syngo; Siemens, Knoxville, TN, USA) was used to analyze the 18F-FDG PET/CT images. Lymph nodes shown in CT scan with or without FDG uptake in PET scan were both defined as positive lymph nodes in PET/CT. The nSUVmax was obtained by placing a spheroid-shaped volume of interest (VOI) within the PLN. The diameter of PLN was recorded as the minor axis.
Statistical analysis
Statistical analyses were carried out using SPSS statistical software (version 21.0; IBM Inc., Armonk, NY, USA) and R 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria) with the rms statistical packages. Continuous variables were described as medians with ranges, and categorical variables were described as frequencies with percentages. The chi-squared (χ2) or Fisher’s exact test was used for univariate analyses and the correlations between histopathology and nSUVmax and PLN metastasis. Logistic regression was performed for multivariate analyses, and a nomogram was built based on the training cohort of 241 patients. The performance of the nomogram was assessed internally and externally by discrimination and calibration. The concordance index (C-index) was used to determine the discriminative ability by calculating the area under the receiver operating characteristics curve (AUC-ROC). The calibration of the model was performed by comparing the predicted and actual probability of PLN metastasis. For internal validation, the nomogram was subjected to 1,000 bootstrap resamples in a training cohort, and the model was applied to the validation cohort for external validation. We also analyzed the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and PET/CT accuracy alone and nomogram in each cohort. All statistical tests were two-sided, and P<0.05 was considered statistically significant.
Results
Patient characteristics
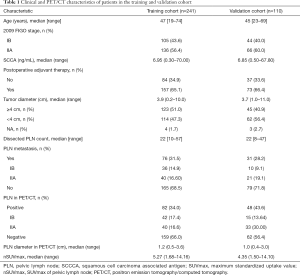
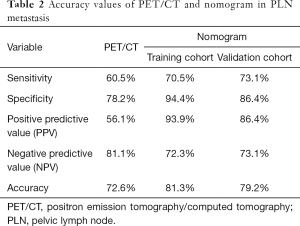
Patient characteristics are described in Table 1. In the training cohort, the median age was 47 [19–74] years old. A total of 105 (43.6%) patients were stage IB, and the other 136 (56.4%) patients were stage IIA. The median of preoperative SCCA was 6.95 (0.30–70.00) ng/mL. Over half (123/241) of the patients had tumor diameters ≥4 cm. All patients underwent PLN dissection during operation. The median of PLN count was 22 [10–57] in the training cohort and 22 [8–47] in the validation cohort, respectively. A total of 82 patients had positive PLNs in the preoperative PET/CT scan, with median diameters of 1.2 (0.5–3.6) cm. The median of nSUVmax was 5.27 (1.68–14.16). Among them, 46 (56.1%) were pathologically confirmed, while 30 (18.9%) PET/CT scan-negative patients had PLN metastasis. The sensitivity, specificity, PPV, NPV, and accuracy of PET/CT for PLN metastasis were 60.5%, 78.2%, 56.1%, 81.1%, and 72.6%, respectively (Table 2).
Full table
Full table
In the validation cohort of 110 patients, the medians (ranges) of age and SCCA were 45 [23–69] years old and 6.85 (0.50–67.80) ng/mL, respectively. Some 44 (40.0%) patients were of 2009 FIGO stage IB, and the others were stage IIA. Another 48 (43.6%) patients detected positive PLNs in PET/CT. The median (range) of nSUVmax and diameters of positive PLNs were 4.35 (1.50-14.10) and 1.0 (0.4–3.0) cm, respectively. However, the actual pathological PLN metastasis rate was only 28.2%.
The correlations between histopathology and nSUVmax, PLN metastasis in the training cohort
There were 162 (67.2%) patients who were non-keratinizing, and 57 (23.7%) were keratinizing squamous cell cancer. After surgery, the median (range) of Ki-67 was 80% (10–95%). Twenty-four (10.0%) of tumors expressed Cyclin D1, and 72 (29.9%) expressed P53 according to postoperative IHC. We further analyzed the correlations between these histopathological indexes and nSUVmax and PLN metastasis. However, none of these variables had associations with nSUVmax or PLN metastasis. These results are shown in Table S1.
Univariate and multivariate analyses for PLN metastasis
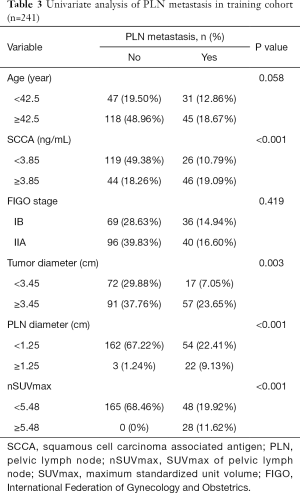
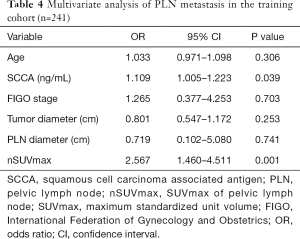
We performed ROC curves to determine optimal cutoff values of continuous parameters regarding PLN metastasis. The cutoffs of SCCA and nSUVmax were 3.85 and 5.48, respectively. In univariate analyses, patients with higher SCCA (P<0.001) and nSUVmax (P<0.001), larger primary tumors (P=0.003), and positive PLN in PET/CT (P<0.001) tended to have a higher probability of PLN metastasis (Table 3). However, in the multivariate analysis, only SCCA [P=0.039, 95% confidence interval (CI): 1.005–1.223] and nSUVmax (P=0.001, 95% CI: 1.460–4.511) were independent predictors for PLN metastasis (Table 4).
Full table
Full table
Performance of the nomogram
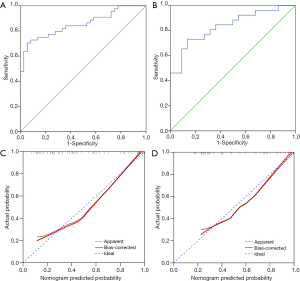
To provide a visualized and individual model for predicting PLN metastasis, we developed a nomogram based on SCCA and nSUVmax, according to the logistic regression analysis (Figure 1). The nomogram was then validated internally and externally.

The nomogram yielded robust discrimination with a C-index of 0.854 (95% CI: 0.772–0.937) and 0.836 (95% CI: 0.723–0.948) in the training and validation cohort, respectively (Figure 2A,B). The calibration curves showed the optimal agreement between the probability of PLN metastasis predicted by the nomogram and the actual probability in both groups (Figure 2C,D). Compared to nSUVmax alone, our nomogram showed elevated sensitivity (70.5%, 73.1% vs. 60.5%), specificity (94.4%, 86.4% vs. 78.2%), and PPV (93.9%, 86.4% vs. 56.1%) in both the training and validation cohorts, while NPV of nomogram decreased in both cohorts (72.3%, 73.1% vs. 81.1%) (Table 2).
Discussion
Our study demonstrated that SCCA and nSUVmax were independent predictors for PLN metastasis of early-stage cervical squamous cell cancer. Based on these 2 parameters, we successfully established a noninvasive and convenient nomogram with improved predictive ability.
Up to 20% of clinical early-stage cervical cancer patients had lymph node metastasis (2). Those patients face undergoing both radical surgery and postoperative chemo-radiation. Thus, the FIGO staging for cervical cancer was revised in 2018, and retroperitoneal lymph node metastases are now regarded as stage IIIC. The treatment strategy has been altered for these patients accordingly (6). Both radiological evaluation and pathological findings could assign the stage in addition to clinical examination and are recorded as stage IIIC (r) or stage IIIC (p), respectively (6). However, diagnostic lymphadenectomy is invasive and may cause short- and long-term complications, and about 80% of clinical early-stage patients might receive little benefit from the procedure (2,12,17,18). Thus, it is crucial to find an accurate and noninvasive radiologic modality for preoperative PLN metastasis assessment.
Several radiological modalities, such as CT, MRI, and PET/CT, have been suggested for preoperative cervical cancer (19). Previous studies indicated that PET/CT was superior to CT and MRI for retroperitoneal lymph node assessment, with sensitivity and specificity of 48.6–82% and 75–98%, respectively (7-12,20,21). Our study showed a similar diagnostic accuracy of PET/CT scan alone. However, up to 30 (12.4%), PET/CT negative patients had pathological metastatic lymph nodes (LNs), which is consistent with previous reports (20,22). Also, similar to Jung et al.’s findings (23), only 46 (56.1%) PET/CT-positive PLNs were pathologically confirmed. Thus, the discrepancy between the PET/CT scan and pathological findings limited its clinical application. Although recent studies added radiomics to the PET/CT scan to obtain more comprehensive and improved data for PLN metastasis prediction in cervical cancer, the complexity and technological immaturity limited its clinical application (14-16).
We aimed to explore a simple and effective PET/CT scan-based method for PLN metastasis prediction of cervical cancer. Thus, we incorporated preoperative clinical and PET/CT parameters regardless of postoperative histopathologic risks and successfully gleaned an individualized and easy-to-use nomogram for preoperative PLN involvement prediction. Kim et al. (12) also reported a PET/CT based nomogram for PLN involvement prediction in early-stage cervical cancer, with improved sensitivity (72.1%), specificity (84.4%), and a good calibration and discrimination in external validation. These results were consistent with our study; nevertheless, there were still several points of difference between their study and ours. Firstly, since histopathological type is an acknowledged predictive and prognostic factor for cervical cancer, we investigated the most common pathological type, squamous cell cancer, as a homologous group. Moreover, since a considerable proportion of metastatic lymph nodes were <1 cm in diameter for cervical cancer, we enrolled a functional parameter, nSUVmax, to compensate for the diagnostic difficulty. Finally, we provided a much simpler nomogram, including only the preoperative SCCA level and nSUVmax, and yielded a similar predictive efficiency for PLN metastasis in early-stage cervical squamous cancer.
Many studies examining relationships between SUV and expression of different histopathological parameters have reported on uterine cervical cancer (16,24) and other malignancies (25-27). However, different results were obtained among various tumors. Unlike the above studies, our study focused on the FDG uptake of PLN, not the primary tumor. However, none of the squamous type, Ki-67, the expression of Cyclin D1, or P53 had relationships with nSUVmax. Furthermore, the research regarding relationships between histopathological indexes and PLN metastasis in uterine cervical cancer is limited. However, none of these indexes could predict PLN metastasis.
The current study had some limitations. It was a retrospective study with potential recall bias. Also, although we validated this model internally and externally and the model fits well, prospective studies are still required to confirm the predictive value of this nomogram in a multicenter clinical practice setting.
Conclusions
In conclusion, we successfully developed and validated a simple and effective nomogram for preoperative PLN metastasis prediction in early-stage uterine cervical squamous cell cancer. This individualized model improved the predictive ability for PLN metastasis and provided an easy-to-use tool for assigning stage and creating appropriate treatment planning.
Acknowledgments
Funding: The work was supported by the National Natural Science Foundation of China (81771861, 81971646) and Shanghai Scientific and Technological Innovation Program (18410711200) for Shaoli Song.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-348). The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics committee of Fudan University Shanghai Cancer Center (050432-4-1212B). However, no informed consent was required for patients due to the nature of a retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ferrandina G, Pedone Anchora L, Gallotta V, Fagotti A, Vizza E, Chiantera V, De Iaco P, Ercoli A, Corrado G, Bottoni C, Fanfani F, Scambia G. Can We Define the Risk of Lymph Node Metastasis in Early-Stage Cervical Cancer Patients? A Large-Scale, Retrospective Study. Ann Surg Oncol 2017;24:2311-8. [Crossref] [PubMed]
- Kidd EA, El Naqa I, Siegel BA, Dehdashti F, Grigsby PW. FDG-PET-based prognostic nomograms for locally advanced cervical cancer. Gynecol Oncol 2012;127:136-40. [Crossref] [PubMed]
- Grigsby PW. PET/CT imaging to guide cervical cancer therapy. Future Oncol 2009;5:953-8. [Crossref] [PubMed]
- Gien LT, Covens A. Lymph node assessment in cervical cancer: prognostic and therapeutic implications. J Surg Oncol 2009;99:242-7. [Crossref] [PubMed]
- Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet 2018;143 Suppl 2:22-36. [Crossref] [PubMed]
- Rose PG, Adler LP, Rodriguez M, Faulhaber PF, Abdul-Karim FW, Miraldi F. Positron emission tomography for evaluating para-aortic nodal metastasis in locally advanced cervical cancer before surgical staging: a surgicopathologic study. J Clin Oncol 1999;17:41-5. [Crossref] [PubMed]
- Choi HJ, Roh JW, Seo SS, Lee S, Kim JY, Kim SK, Kang KW, Lee JS, Jeong JY, Park SY. Comparison of the accuracy of magnetic resonance imaging and positron emission tomography/computed tomography in the presurgical detection of lymph node metastases in patients with uterine cervical carcinoma: a prospective study. Cancer 2006;106:914-22. [Crossref] [PubMed]
- Sironi S, Buda A, Picchio M, Perego P, Moreni R, Pellegrino A, Colombo M, Mangioni C, Messa C, Fazio F. Lymph node metastasis in patients with clinical early-stage cervical cancer: detection with integrated FDG PET/CT. Radiology 2006;238:272-9. [Crossref] [PubMed]
- Loft A, Berthelsen AK, Roed H, Ottosen C, Lundvall L, Knudsen J, Nedergaard L, Hojgaard L, Engelholm SA. The diagnostic value of PET/CT scanning in patients with cervical cancer: a prospective study. Gynecol Oncol 2007;106:29-34. [Crossref] [PubMed]
- Choi HJ, Ju W, Myung SK, Kim Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: meta-analysis. Cancer Sci 2010;101:1471-9. [Crossref] [PubMed]
- Kim DY, Shim SH, Kim SO, Lee SW, Park JY, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH. Preoperative nomogram for the identification of lymph node metastasis in early cervical cancer. Br J Cancer 2014;110:34-41. [Crossref] [PubMed]
- Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, Chon HS, Chu C, Clark R, Cohn D, Crispens MA, Damast S, Dorigo O, Eifel PJ, Fisher CM, Frederick P, Gaffney DK, Han E, Huh WK, Lurain JR, Mariani A, Mutch D, Nagel C, Nekhlyudov L, Fader AN, Remmenga SW, Reynolds RK, Tillmanns T, Ueda S, Wyse E, Yashar CM, McMillian NR, Scavone JL. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:64-84. [Crossref] [PubMed]
- Wang T, Gao T, Yang J, Yan X, Wang Y, Zhou X, Tian J, Huang L, Zhang M. Preoperative prediction of pelvic lymph nodes metastasis in early-stage cervical cancer using radiomics nomogram developed based on T2-weighted MRI and diffusion-weighted imaging. Eur J Radiol 2019;114:128-35. [Crossref] [PubMed]
- Kan Y, Dong D, Zhang Y, Jiang W, Zhao N, Han L, Fang M, Zang Y, Hu C, Tian J, Li C, Luo Y. Radiomic signature as a predictive factor for lymph node metastasis in early-stage cervical cancer. J Magn Reson Imaging 2019;49:304-10. [Crossref] [PubMed]
- Li K, Sun H, Lu Z, Xin J, Zhang L, Guo Y, Guo Q. Value of [(18)F]FDG PET radiomic features and VEGF expression in predicting pelvic lymphatic metastasis and their potential relationship in early-stage cervical squamous cell carcinoma. Eur J Radiol 2018;106:160-6. [Crossref] [PubMed]
- Macdonald MC, Tidy JA. Can We Be Less Radical with Surgery for Early Cervical Cancer? Curr Oncol Rep 2016;18:16. [Crossref] [PubMed]
- Kadkhodayan S, Hasanzadeh M, Treglia G, Azad A, Yousefi Z, Zarifmahmoudi L, Sadeghi R. Sentinel node biopsy for lymph nodal staging of uterine cervix cancer: a systematic review and meta-analysis of the pertinent literature. Eur J Surg Oncol 2015;41:1-20. [Crossref] [PubMed]
- Lee SI, Atri M. 2018 FIGO Staging System for Uterine Cervical Cancer: Enter Cross-sectional Imaging. Radiology 2019;292:15-24. [Crossref] [PubMed]
- Du R, Li L, Ma S, Tan X, Zhong S, Wu M. Lymph nodes metastasis in cervical cancer: Incidences, risk factors, consequences and imaging evaluations. Asia Pac J Clin Oncol 2018;14:e380-e385. [Crossref] [PubMed]
- Brunette LL, Bonyadlou S, Ji L, Groshen S, Shuster D, Mehta A, Sposto R, Matsuo K, Lin YG, Roman LD. Predictive Value of FDG PET/CT to Detect Lymph Node Metastases in Cervical Cancer. Clin Nucl Med 2018;43:793-801. [Crossref] [PubMed]
- Daraï E, Rouzier R, Ballester M, Barranger E, Coutant C. Sentinel lymph node biopsy in gynaecological cancers: the importance of micrometastases in cervical cancer. Surg Oncol 2008;17:227-35. [Crossref] [PubMed]
- Jung W, Park KR, Lee KJ, Kim K, Lee J, Jeong S, Kim YJ, Kim J, Yoon HJ, Kang BC, Koo HS, Sung SH, Cho MS, Park S. Value of imaging study in predicting pelvic lymph node metastases of uterine cervical cancer. Radiat Oncol J 2017;35:340-8. [Crossref] [PubMed]
- Surov A, Meyer HJ, Höhn AK, Schob S, Winter K, Sabri O, Purz S. Metabolo-volumetric parameters of 18F-FDG-PET can predict expression of EGFR and HIF 1alpha in uterine cervical cancer. Cancer Biomark 2019;24:135-40. [Crossref] [PubMed]
- Grönroos TJ, Lehtiö K, Söderström KO, Kronqvist P, Laine J, Eskola O, Viljanen T, Grénman R, Solin O, Minn H. Hypoxia, blood flow and metabolism in squamous-cell carcinoma of the head and neck: correlations between multiple immunohistochemical parameters and PET. BMC Cancer 2014;14:876. [Crossref] [PubMed]
- Surov A, Meyer HJ, Höhn AK, Winter K, Sabri O, Purz S. Associations Between [(18)F]FDG-PET and Complex Histopathological Parameters Including Tumor Cell Count and Expression of KI 67, EGFR, VEGF, HIF-1α, and p53 in Head and Neck Squamous Cell Carcinoma. Mol Imaging Biol 2019;21:368-74. [Crossref] [PubMed]
- Surov A, Meyer HJ, Wienke A. Standardized Uptake Values Derived from 18F-FDG PET May Predict Lung Cancer Microvessel Density and Expression of KI 67, VEGF, and HIF-1α but Not Expression of Cyclin D1, PCNA, EGFR, PD L1, and p53. Contrast Media Mol Imaging 2018;2018:9257929. [Crossref] [PubMed]


