Optical cryoimaging of mitochondrial redox state in bronchopulmonary-dysplasia injury models in mice lungs
Introduction
Bronchopulmonary dysplasia (BPD) is a chronic lung condition that affects premature infants who receive supplemental oxygen (hyperoxia) or ventilator support for long periods of time. Studies have shown that arrest of alveolar development is a hallmark of BPD caused by either oxygen or mechanical ventilation. We have observed significant abnormalities in lungs prepared from Bcl-2 null mice perhaps as a result of increased oxidative stress and reduced angiogenesis (1). Our hypothesis is that oxidative stress plays a key role in the development of vascular dysfunction associated with BPD. We used optical cryoimaging to investigate the mitochondrial redox state of the tissue related to oxidative stress and pathogenesis of BPD.
Anatomical and functional information of tissue can be obtained by fluorescence imaging techniques via intrinsic fluorophores or exogenous tagged proteins (2) and are used to probe tissue redox state and energy homeostasis in various organs with a high sensitivity and specificity for discriminating between diseased and non-diseased tissue (3). These fluorescence images are able to monitor tissue metabolic state, as an indicator of cellular oxygen consumption (4,5).
NADH and FAD (oxidized form of FADH2), two mitochondrial metabolic coenzymes and the primary electron carriers in oxidative phosphorylation, are intrinsic fluorophores and can be monitored using fluorescent imaging. It has been shown that the ratio of these fluorophores, NADH/FAD, called the mitochondrial redox ratio (RR) (6-9), is a marker of the mitochondrial redox and metabolic state of tissue and a change in RR is an index of a change in lung tissue bioenergetics. Here we tested whether deficiency in Bcl-2, an anti-apoptotic protein with important role in angiogenesis (10), results in increased oxidative stress and attenuation of lung angiogenesis contributing to PBD.
Methods
As our injury model, lungs from three groups of mice were studied: Bcl-2 +/+, Bcl-2 -/- (global Bcl-2 null) and Bcl-2 VE-cad (Bcl-2 only deleted in the endothelium). Bcl-2 +/+ lungs were used as control and Bcl-2 VE-cad and Bcl-2 -/- mice were used as potential models of BPD. The mice were sacrificed at 3 weeks of age.
Lung tissue metabolic state was preserved by rapid freezing, immediately after harvesting the tissue, in chilled isopentane (2-methyl butane, Fisher Scientific, IL) within liquid nitrogen (LN2, –196 °C) then embedded in a customized fluorescent-free black mounting medium for cryo fluorescence imaging.
Cryoimaging or Low-temperature fluorescence imaging provides 3-D fluorescence images of cryopreserved intact organs. Imaging in lower temperatures (–40 °C) guaranties a higher quantum yield of fluorescence of NADH and FAD as compared to room temperature (11-13). Cryoimager is an automated image acquisition instrument consisted of hardware and software designed to acquire fluorescence images of tissue sections. Image acquisition and the instrument have been previously described (14). Briefly each sample is sequentially sliced using a computer controlled microtome and NADH and FAD fluorescent images is captured for each slice using a CCD camera.
For the Image Processing step, the composite images were created using all the image slices for each lung, for both NADH and FAD signals. The ratio of NADH and FAD (NADH/FAD) was calculated voxel by voxel, using Matlab. For each lung, a histogram of RR values was created, and the mean (or first moment) of this histogram was calculated for the whole volume of the tissue according to Eq. [1].
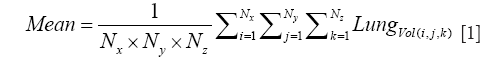
Statistical comparisons were also carried out on a population of N=3 for control and N=3 for Bcl-2 VE-cad and N=4 for control and N=9 for Bcl-2 -/- mice lungs using a one-tailed student’s t-test, with P<0.05 for each group as the criterion for statistical significance.
Results
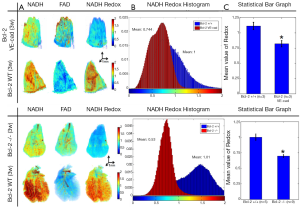
Figure 1A shows the 3-D rendering of NADH and FAD fluorescence signals and their ratio (RR = NADH/FAD) from representative lungs of each of the three groups (Bcl-2 VE-cad vs. Bcl-2 +/+ on top and Bcl-2 -/- vs. Bcl-2 +/+ on bottom). As expected, mice with global Bcl-2 null and Bcl-2 VE-cad showed a decreased NADH signal and an increased FAD signal and as a result decreased RR in respect to the control mice (26% decrease for Bcl-2 VE-cad and 47% for Bcl-2 -/-) which implies generation of more ROS and oxidative stress. Bcl-2 -/- mice also had more ROS and oxidative stress compared with Bcl-2 VE-cad. Figure 1B shows histograms of RR for the lungs. The mean values of these histograms suggest a more reduced mitochondrial redox state for Bcl-2 +/+ lung, and more oxidized mitochondrial redox state for both Bcl-2 VE-cad and Bcl-2 -/-. Figure 1C shows the average ± SE (standard errors) of the mean values of the RR histograms for the three groups of mice, which shows a significant decrease (P<0.021) in the NADH redox in Bcl-2 VE-cad and Bcl-2 -/- lungs.
Discussion and conclusions
We have previously demonstrated the utility of cryoimaging for evaluating the redox status of tissue mitochondrial coenzymes NADH and FAD in intact lungs in another model of BPD (combining injuries due to ventilation with elevated oxygen concentration and bacterial infection) (15). We have shown that the RR, NADH/FAD, is an index of lung tissue mitochondrial redox state, and is an important determinant of mitochondrial bioenergetics. Here we have shown that mice lacking Bcl-2 demonstrate increased oxidative stress as seen in BPD phenotype. Bcl-2 is a key mediator of downstream events that occur in response to both pro- and anti-angiogenic factors, including VEGF and thrombospondin-1 (TSP1), respectively (16). The important role Bcl-2 plays during angiogenesis is demonstrated by the inability of Bcl-2 -/- endothelial cells to undergo capillary morphogenesis and sprouting angiogenesis (17).
A clearer understanding of mitochondrial dysfunction and the role Bcl-2 plays in this process is critical for elucidating the role of mitochondrial bioenergetics in pulmonary developmental arrest and can be further used in prevention of BPD-like injuries. Our studies show that deficiency of Bcl-2 in the endothelium is only partially responsible for increased oxidative stress. The identity of additional cellular components to increased oxidative stress in the global null mice awaits further investigation.
Other endogenous fluorophores in the tissue including collagen and elastin would not be expected to contribute in variations of mitochondrial redox state (18,19). Contribution of cytosolic NADPH, which has the same fluorescence characteristics as NADH, to the NADH fluorescent signal is considered to be small (20) since its concentration and quantum yield is much smaller than NADH (21,22).
NADH and FAD data provide information regarding tissue redox and mitochondrial bioenergetics, a truer and more sensitive early measure of organ function. Because NADH and FAD signals can be detected through fiber optic probes placed on the surface of the lung, RR data could be obtained either intraoperatively or through tube thoracostomies (frequently placed for clinical indications in patients with severe lung injury). Our studies support the capacity of fluorescence imaging to detect pulmonary oxidative injury, and set the stage for in vivo studies and further translation to clinical arenas.
Acknowledgements
This work was supported in part by UWM RGI 8, UWM-UW-Madison intercampus grant and HL116530, RC4 EY021357, P30 EY016665, R21 EY023024, and an unrestricted departmental award from Research to Prevent Blindness. NS is a recipient of research Award from American Diabetes Association, 1-10-B-160 and Retina Research Foundation. CMS is a recipient of Retina Research Foundation-Daneil M. Albert Chair.
Disclosure: The authors declare no conflict of interest.
References
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723-9. [PubMed]
- Vo-Dinh T. eds. Biomedical Photonics Handbook. USA: CRC Press, 2000.
- Ramanujam N, Richards-Kortum R, Thomsen S, Mahadevan-Jansen A, Follen M, Chance B. Low Temperature Fluorescence Imaging of Freeze-trapped Human Cervical Tissues. Opt Express 2001;8:335-43. [PubMed]
- Chance B, Williams GR. A method for the localization of sites for oxidative phosphorylation. Nature 1955;176:250-4. [PubMed]
- Mayevsky A. Brain NADH redox state monitored in vivo by fiber optic surface fluorometry. Brain Res 1984;319:49-68. [PubMed]
- Matsubara M, Ranji M, Leshnower BG, Noma M, Ratcliffe SJ, Chance B, Gorman RC, Gorman JH 3rd. In vivo fluorometric assessment of cyclosporine on mitochondrial function during myocardial ischemia and reperfusion. Ann Thorac Surg 2010;89:1532-7. [PubMed]
- Ranji M, Matsubara M, Leshnower BG, Hinmon RH, Jaggard DL, Chance B, Gorman RC, Gorman Iii JH. Quantifying acute myocardial injury using ratiometric fluorometry. IEEE Trans Biomed Eng 2009;56:1556-63. [PubMed]
- Chance B, Schoener B, Oshino R, Itshak F, Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J Biol Chem 1979;254:4764-71. [PubMed]
- Chance B, Lee CP, Schoener B. High and low energy states of cytochromes. II. In submitochondrial particles. J Biol Chem 1966;241:4574-6. [PubMed]
- Fisher AB. Intermediary metabolism of the lung. Environ Health Perspect 1984;55:149-58. [PubMed]
- Mayevsky A, Chance B. Oxidation-reduction states of NADH in vivo: from animals to clinical use. Mitochondrion 2007;7:330-9. [PubMed]
- Kelly JJ, Ewen JR, Bernard SL, Glenny RW, Barlow CH. Regional blood flow measurements from fluorescent microsphere images using an Imaging CryoMicrotome. Rev Sci Instrum 2000;71:228-34.
- Quistorff B, Haselgrove JC, Chance B. High spatial resolution readout of 3-D metabolic organ structure: an automated, low-temperature redox ratio-scanning instrument. Anal Biochem 1985;148:389-400. [PubMed]
- Sepehr R, Staniszewski K, Maleki S, Jacobs ER, Audi S, Ranji M. Optical imaging of tissue mitochondrial redox state in intact rat lungs in two models of pulmonary oxidative stress. J Biomed Opt 2012;17:046010. [PubMed]
- Sepehr R, Audi SH, Maleki S, Staniszewski K, Eis AL, Konduri GG, Ranji M. Optical imaging of lipopolysaccharide-induced oxidative stress in acute lung injury from hyperoxia and sepsis. J Innov Opt Health Sci 2013;6:1350017. [PubMed]
- Dias S, Shmelkov SV, Lam G, Rafii S. VEGF(165) promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood 2002;99:2532-40. [PubMed]
- Kondo S, Tang Y, Scheef EA, Sheibani N, Sorenson CM. Attenuation of retinal endothelial cell migration and capillary morphogenesis in the absence of bcl-2. Am J Physiol Cell Physiol 2008;294:C1521-30. [PubMed]
- Georgakoudi I, Jacobson BC, Müller MG, Sheets EE, Badizadegan K, Carr-Locke DL, Crum CP, Boone CW, Dasari RR, Van Dam J, Feld MS. NAD(P)H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes. Cancer Res 2002;62:682-7. [PubMed]
- Ramanujam N, Mitchell MF, Mahadevan A, Warren S, Thomsen S, Silva E, Richards-Kortum R. In vivo diagnosis of cervical intraepithelial neoplasia using 337-nm-excited laser-induced fluorescence. Proc Natl Acad Sci U S A 1994;91:10193-7. [PubMed]
- Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. Science 1962;137:499-508. [PubMed]
- Klaidman LK, Leung AC, Adams JD Jr. High-performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regions. Anal Biochem 1995;228:312-7. [PubMed]
- Avi-Dor Y, Olson JM, Doherty MD, Kaplan NO. Fluorescence of pyridine nucleotides in mitochondria. J Biol Chem 1962;237:2377-83.


