MRI assessment of cardiac tumours: part 2, spectrum of appearances of histologically malignant lesions and tumour mimics
Introduction
Cardiac magnetic resonance imaging (MRI) is considered the reference standard technique for characterization of a suspected cardiac mass. It provides an unrestricted field of view, high temporal resolution (30-50 ms) and non-invasive tissue characterization based on multi-parametric assessment of the chemical micro-environment (1). As such MRI is extremely helpful for diagnosis, assessment of the functional impact of a lesion, treatment planning and post treatment follow-up (2,3). We present a two-part review of the role of cardiac MRI in the assessment of cardiac tumours. Part 1 focuses on specific cardiac MRI techniques, protocol design and the appearance of histologically benign tumours. This article, part 2 covers histologically malignant tumours including cardiac metastases and also reviews the MRI appearance of some potential tumour mimics.
Sarcomas account for the vast majority of histologically malignant primary tumours of the heart (4-6). By comparison, metastatic involvement of the heart is 100-500 times more frequent than primary tumours (7). Cardiac tumours are frequently asymptomatic and often discovered incidentally during evaluation of an unrelated problem or physical finding. Symptoms and signs depend on the size and location of the tumour, haemodynamic effects (chamber obstruction or embolization) and interference with cardiac conduction (heart blocks or cardiac arrhythmias).
Malignant primary cardiac tumours
Sarcomas account for around 95% of all primary malignant cardiac tumours with lymphoma, and primary pericardial mesothelioma making up most of the remainder of cases. Although there are a few sporadic reports of long term survival the prognosis of primary cardiac tumours is dire with a median survival from several large series in the order of 6-12 months (5,6). Currently there is no TNM staging classification system for malignant cardiac tumours and treatment pathways and algorithms are not well defined. Complete surgical resection is rarely possible as most cases are at an advanced stage with extensive local infiltration by the time of first clinical presentation and diagnosis however some patients may gain symptomatic benefit with a de-bulking surgical procedure in combination with adjuvant chemotherapy (5,6).
General imaging findings which favour a malignant over a benign cardiac tumour are presented in Table 1.
Sarcomas
Sarcomas are the second most common primary cardiac tumours after myxoma (9). They represent a diverse group of mesenchymal tumours with broad histological subtypes of angiosarcoma, sarcoma of myo- or fibroblastic differentiation and rhabdomyosarcoma.
Angiosarcoma
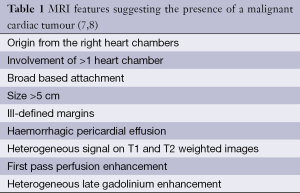
Angiosarcoma is the most common primary malignant cardiac tumour. It is a very aggressive infiltrative lesion with an origin from mesenchymal angioblasts (10). Histologically it is composed of irregularly shaped vascular channels lined by anaplastic epithelial cells with large areas of intra-tumoural haemorrhage and necrosis (11). There is a strong male predominance and a peak incidence in the fourth decade. Approximately 75% of angiosarcomas arise within the right atrium, typically filling this chamber with infiltration into adjacent structures such as the tricuspid valve, right coronary artery, right ventricle and pericardium. A large haemorrhagic pericardial effusion is frequently present (12). At time of clinical presentation the tumour is usually at an advanced unresectable stage with distant metastases present in up to 89% of cases; most often to mediastinal lymph nodes, lung, liver and bone (13). Clinical symptoms usually relate to cardiac tamponade or right heart failure secondary to intracavitary obstruction. Prognosis is extremely poor with very few patients surviving more than 12-month even with surgical debulking and aggressive chemotherapy (13).
MRI demonstrates a large infiltrative mass with heterogeneous signal intensity. T1-weighted images show tumour tissue as predominantly isointense to myocardium with areas of high T1 signal change reflecting intratumoural haemorrhage and areas of signal void reflecting blood flow within vascular channels (14). A large mixed signal pericardial effusion reflecting blood products is invariably present. Angiosarcoma appears predominantly high signal on T2-weighted images (Figure 1). First pass and LGE is avid and may predominate along prominent vascular channels to give a characteristic “sunray” pattern (15).
Sarcomas with myofibroblastic differentiation
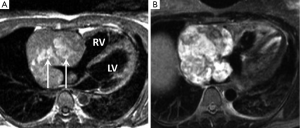
These are a diverse group of tumours many of which contain heterologous elements such as fat and bone. They are subclassified as undifferentiated sarcoma, leiomyosarcoma, fibrosarcoma, liposarcoma and osteosarcoma. Most present in adulthood with a peak incidence between the 3rd and 5th decades (16). Location is most often from the left atrium, typically along its posterior wall with direct infiltration into one or more pulmonary vein ostia (17,18). These tumours tend to be locally aggressive although distant metastases can occur (19). Complete surgical clearance is rarely possible.
MRI features are non-specific and most often those of an infiltrating left atrial lesion with intermediate T1- and high T2-signal intensity with varying amounts of first pass and LGE. The main imaging differential is a broad based (sessile) myxoma with key distinguishing features for sarcoma being extension into the pulmonary veins and breach of the left atrial wall (Figure 2) (20-25).
Rhabdomyosarcoma
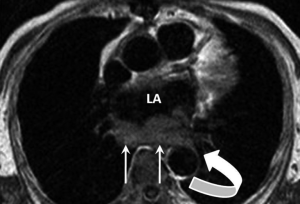
Rhabdomyosarcoma is the commonest primary paediatric cardiac tumour and infrequently occurs in adults. These tumours arise from the myocardium with no chamber predilection. They commonly show areas of necrosis have multiple sites of origin and have a strong tendency for infiltrating into the pericardial space and the valves (18).
Described MRI features are those of a peripherally solid lesion (isointense to myocardium on T1-weighted images) with central necrotic elements (high T2-signal regions). The solid components show LGE and cavitating pulmonary metastases may be present (Figure 3) (18,26).
Primary cardiac lymphoma
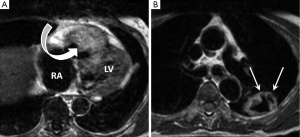
Primary cardiac lymphoma is much less frequent than secondary involvement and is defined as disease confined to the heart or pericardium (11,27). The vast majority are of large B-cell origin and occur in immunocompromised patients (22). They usually arise from the right heart chambers, particularly the right ventricle with an associated pericardial effusion (28). These tumours are usually aggressive but may respond well to monoclonal anti-CD20 antibody (rituximab). MRI features are variable with two main patterns described. The first is of multiple solid appearing myocardial based masses which are isointense on T1 and mildly hyperintense on T2-weighted images, most often occurring within the right ventricle (29). The second is that of diffuse pericardial soft tissue infiltration with a haemorrhagic effusion (Figure 4). Lymphoma tissue often displays heterogeneous LGE (29,30). Absence of necrosis and less frequent valvular involvement in conjunction with a history of immunocompromise suggest the possibility of lymphoma over other primary cardiac tumours.

Primary pericardial malignancy
Pericardial mesothelioma arises from mesothelial cells of the parietal pericardium. It virtually always occurs in patients with a history of asbestos exposure, many of whom with have asbestos related pleural disease (pleural thickening, effusion, plaques). There is progressive encasement of the heart causing breathlessness and chest pain, often with clinical signs of pericardial constriction and/or tamponade (31,32). It is unusual for mesothelioma to directly invade the adjacent myocardium (33,34). Prognosis is usually very poor but surgical resection for localized cases can be curative (35,36). MRI shows multiple coalescing pericardial masses isointense to myocardium on T1 and heterogeneous on T2-weighted images.
Pericardial synovial sarcoma is an extremely rare and high aggressive tumour composed of epithelial and spindle cells. Clinical presentation, prognosis and MRI features are very similar to angiosarcoma appearing as a highly infiltrative multi-lobulated mass with avid first pass and LGE (37).
Cardiac metastasis
Cardiac metastases are 100-500 times more common than primary cardiac malignancy with approximately 12% of oncology patients having myocardial or pericardial spread at autopsy although many remain clinically silent (38). Tumours that most frequently metastasis to heart are bronchogenic, breast, and melanoma (39). The pericardium and epicardium are most frequently involved with seeding occurring via haematogenous spread through the coronary arteries or via lymphatic channels. Direct invasion most often occurs with central bronchogenic tumours or via venous extension into the right atrium (renal cell or hepatocellular carcinoma). Management is mainly palliative with pericardiocentesis or creation of a pericardial window in cases of recurrent pericardial effusion/tamponade (9,39).
MRI appearances are variable and generally non-specific for the tissue of origin with the exception of melanoma which causing characteristic high T1-signal masses due to the T1 shortening effects of melanin (40,41). Lesions with a haemorrhagic component may also have some areas of high T1 signal secondary to blood breakdown products. Most other metastases usually appear of low signal intensity on T1 and of high signal intensity on T2-weighted imaging and most show prominent LGE (Figure 5) (41). A pericardial effusion will frequently be present.
Potential tumour mimics
A variety of lesions including some anatomical variants can mimic a cardiac tumour of which thrombus often causes the most diagnostic difficulty.
Intracardiac thrombus
Thrombus is by far the most common intracardiac mass and is always one of the main differentials for a suspected primary or secondary cardiac tumour. Thrombi are most often located within the left atrium, especially the appendage and have a particular association with atrial fibrillation. When sited in the left ventricle, they occur as sequelae of myocardial infarction, aneurysmal left ventricular segment or severe ventricular dysfunction. Right heart thrombus is much less common but may be seen in association which central venous catheter lines which can act as a nidus or in cases of severe pectus excavatum deformity.
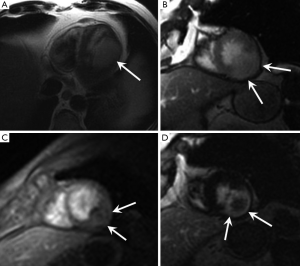
The signal intensity of thrombus on T1 and T2-weighted images varies depending on its chronicity due to various phases of haemoglobin degradation. For example subacute thrombi appear of high signal on T1- and low signal on T2-weighted images due to paramagnetic effects of methaemoglobin whereas organised chronic thrombi will exhibit low signal intensity on both T1- and T2 weighted images due to depleted water content (8). In general contrast enhanced sequences are thought to be more reliable for confident diagnosis. Thrombi do not enhance on dynamic perfusion sequences whereas some tumours with vascularised tissue components will. A more specific feature for thrombi is lack of inversion on LGE sequences performed with a very long inversion time (>500 ms) in comparison with other tissues which will invert (become bright) with this nulling sequence (Figure 6) (42). This technique has proven efficacy for distinguishing thrombi from myxomas (42).
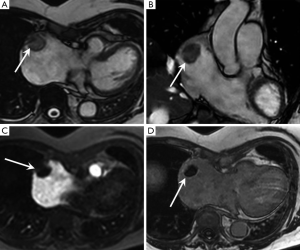
Pericardial cyst
These lesions are benign congenital malformations of pericardium without direct communication with the pericardial space and usually located at the right cardiophrenic angle. They are simple water-based lesions without internal septae. Most are asymptomatic and diagnosed incidentally on chest X-ray and echocardiogram but some may produce symptoms such as chest pain or persistent cough (43).
MRI shows a well-defined, often triangular shaped structure, of uniformly low T1- and high T2-signal intensity without any first pass or LGE. Occasionally high T1- and high T2-signal may be present if they contain proteinaceous material or become secondarily infected.
Bronchogenic cyst
Bronchogenic cysts occur along the differentiating pathway of the trachea and bronchial tree and are thought to represent abnormal budding of ventral foregut tissue (44). They are most often found in the mediastinum, most commonly in the subcarinal or right paratracheal regions. On MRI these lesions are usually round and well circumscribed with variable signal intensity on T1-weighted images depending upon their protein content. T2-weighted images typically show uniformly high signal intensity (45).
Normal intra cardiac structures
Normal intracardiac structures or embryological remnants can sometimes be mistaken for tumours. The crista terminalis is a vertical crest representing the line of fusion between the right atrium and right auricle and can sometimes appear prominent along the posterior wall of the right atrium. Similarly the eustachian valve, right ventricular moderator band and false left ventricle tendon are other structures that can raise suspicion but should not be mistaken for an intracardiac mass (46).
Conclusions
Despite the low incidence of cardiac tumours, prompt evaluation and intervention is crucially important for best outcomes. Although most tumours of the heart are considered histologically benign, there remain significant risks associated with these due to local effects such as interference with conduction pathways and valvular obstruction. Evaluation of a suspected cardiac mass is a frequent and expanding indication for MRI referral due to its superb contrast resolution, multiplanar imaging capabilities and advanced tissue characterization techniques. Radiologists and imaging Cardiologists should be familiar with protocol design and key MRI findings across the spectrum of both benign and malignant cardiac tumours.
Disclosure: The authors declare no conflict of interest.
References
- Reynen K. Frequency of primary tumors of the heart. Am J Cardiol 1996;77:107. [PubMed]
- Centofanti P, Di Rosa E, Deorsola L, Dato GM, Patanè F, La Torre M, Barbato L, Verzini A, Fortunato G, di Summa M. Primary cardiac tumors: early and late results of surgical treatment in 91 patients. Ann Thorac Surg 1999;68:1236-41. [PubMed]
- Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli LG. Cardiac sarcomas: an update. J Thorac Oncol 2010;5:1483-9. [PubMed]
- Kaminaga T, Takeshita T, Kimura I. Role of magnetic resonance imaging for evaluation of tumors in the cardiac region. Eur Radiol 2003;13 Suppl 4:L1-10.
- Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology 1999;34:295-304. [PubMed]
- Shapira OM, Korach A, Izhar U, Koler T, Wald O, Ayman M, Erez E, Blackmon SH, Reardon MJ. Radical multidisciplinary approach to primary cardiac sarcomas. Eur J Cardiothorac Surg 2013;44:330-5; discussion 335-6.. [PubMed]
- Hoffmann U, Globits S, Schima W, Loewe C, Puig S, Oberhuber G, Frank H. Usefulness of magnetic resonance imaging of cardiac and paracardiac masses. Am J Cardiol 2003;92:890-5. [PubMed]
- Sparrow PJ, Kurian JB, Jones TR, Sivananthan MU. MR imaging of cardiac tumors. Radiographics 2005;25:1255-76. [PubMed]
- Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med 1993;117:1027-31. [PubMed]
- Burke AP, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer 1992;69:387-95. [PubMed]
- Burke A, Virmani R. eds. Tumors of the heart and great vessels. Fascicle 16, 3rd Series. In: Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology; 1996;1-98.
- Best AK, Dobson RL, Ahmad AR. Best cases from the AFIP: cardiac angiosarcoma. Radiographics 2003;23:S141-5. [PubMed]
- Janigan DT, Husain A, Robinson NA. Cardiac angiosarcomas. A review and a case report. Cancer 1986;57:852-9. [PubMed]
- Deetjen AG, Conradi G, Möllmann S, Hamm CW, Dill T. Cardiac angiosarcoma diagnosed and characterized by cardiac magnetic resonance imaging. Cardiol Rev 2006;14:101-3. [PubMed]
- Yahata S, Endo T, Honma H, Ino T, Hayakawa H, Ogawa M, Hayashi H, Kumazaki T. Sunray appearance on enhanced magnetic resonance image of cardiac angiosarcoma with pericardial obliteration. Am Heart J 1994;127:468-71. [PubMed]
- Grizzard JD, Ang GB. Magnetic resonance imaging of pericardial disease and cardiac masses. Magn Reson Imaging Clin N Am 2007;15:579-607. [PubMed]
- Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol (R Coll Radiol) 2007;19:748-56. [PubMed]
- Gilkeson RC, Chiles C. MR evaluation of cardiac and pericardial malignancy. Magn Reson Imaging Clin N Am 2003;11:173-86. [PubMed]
- Yokouchi Y, Hiruta N, Oharaseki T, Ihara F, Oda Y, Ito S, Yamashita H, Ozaki S, Gomi T, Takahashi K. Primary cardiac synovial sarcoma: a case report and literature review. Pathol Int 2011;61:150-5. [PubMed]
- Lazoglu AH, Da Silva MM, Iwahara M, Stelzer P, Marino N, Martinez A, Coplan NL. Primary pericardial sarcoma. Am Heart J 1994;127:453-8. [PubMed]
- Baumgartner RA, Das SK, Shea M, LeMire MS, Gross BH. The role of echocardiography and CT in the diagnosis of cardiac tumors. Int J Card Imaging 1988;3:57-60. [PubMed]
- Araoz PA, Eklund HE, Welch TJ, Breen JF. CT and MR imaging of primary cardiac malignancies Radiographics 1999;19:1421-34. [PubMed]
- Lo FL, Chou YH, Tiu CM, Lan GY, Hwang JH, Chern MS, Teng MM. Primary cardiac leiomyosarcoma: imaging with 2-D echocardiography, electron beam CT and 1.5-Tesla MR. Eur J Radiol 1998;27:72-6. [PubMed]
- Schrem SS, Colvin SB, Weinreb JC, Glassman E, Kronzon I. Metastatic cardiac liposarcoma: diagnosis by transesophageal echocardiography and magnetic resonance imaging. J Am Soc Echocardiogr 1990;3:149-53. [PubMed]
- Yamagishi M, Yamada N, Kuribayashi S. Images in cardiology: Magnetic resonance imaging of cardiac osteosarcoma. Heart 2001;85:311. [PubMed]
- Villacampa VM, Villarreal M, Ros LH, Alvarez R, Cózar M, Fuertes MI. Cardiac rhabdomyosarcoma: diagnosis by MR imaging. Eur Radiol 1999;9:634-7. [PubMed]
- Sütsch G, Jenni R, von Segesser L, Schneider J. Heart tumors: incidence, distribution, diagnosis. Exemplified by 20,305 echocardiographies. Schweiz Med Wochenschr 1991;121:621-9. [PubMed]
- Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics 2000;20:1073-103. [PubMed]
- Ryu SJ, Choi BW, Choe KO. CT and MR findings of primary cardiac lymphoma: report upon 2 cases and review Yonsei Med J 2001;42:451-6. [PubMed]
- Chiles C, Woodard PK, Gutierrez FR, Link KM. Metastatic involvement of the heart and pericardium: CT and MR imaging. Radiographics 2001;21:439-49. [PubMed]
- Gössinger HD, Siostrzonek P, Zangeneh M, Neuhold A, Herold C, Schmoliner R, Laczkovics A, Tscholakoff D, Mösslacher H. Magnetic resonance imaging findings in a patient with pericardial mesothelioma. Am Heart J 1988;115:1321-2. [PubMed]
- Kaul TK, Fields BL, Kahn DR. Primary malignant pericardial mesothelioma: a case report and review. J Cardiovasc Surg (Torino) 1994;35:261-7. [PubMed]
- Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol 2008;9:147-57. [PubMed]
- Loire R, Tabib A. Malignant mesothelioma of the pericardium. An anatomo-clinical study of 10 cases. Arch Mal Coeur Vaiss 1994;87:255-62. [PubMed]
- Eren NT, Akar AR. Primary pericardial mesothelioma. Curr Treat Options Oncol 2002;3:369-73. [PubMed]
- Butz T, Faber L, Langer C, Körfer J, Lindner O, Tannapfel A, Müller KM, Meissner A, Plehn G, Trappe HJ, Horstkotte D, Piper C. Primary malignant pericardial mesothelioma - a rare cause of pericardial effusion and consecutive constrictive pericarditis: a case report. J Med Case Rep 2009;3:9256. [PubMed]
- Ohnishi J, Shiotani H, Ueno H, Fujita N, Matsunaga K. Primary pericardial mesothelioma demonstrated by magnetic resonance imaging. Jpn Circ J 1996;60:898-900. [PubMed]
- Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol 2005;6:219-28. [PubMed]
- Klatt EC, Heitz DR. Cardiac metastases. Cancer 1990;65:1456-9. [PubMed]
- Mousseaux E, Meunier P, Azancott S, Dubayle P, Gaux JC. Cardiac metastatic melanoma investigated by magnetic resonance imaging. Magn Reson Imaging 1998;16:91-5. [PubMed]
- Crean AM, Juli C. Diagnosis of metastatic melanoma to the heart with an intrinsic contrast approach using melanin inversion recovery imaging. J Comput Assist Tomogr 2007;31:924-30. [PubMed]
- Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, Weaver JA, Smedira NG, White RD. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006;152:75-84. [PubMed]
- Feigin DS, Fenoglio JJ, McAllister HA, Madewell JE. Pericardial cysts. A radiologic-pathologic correlation and review. Radiology 1977;125:15-20. [PubMed]
- St-Georges R, Deslauriers J, Duranceau A, Vaillancourt R, Deschamps C, Beauchamp G, Pagé A, Brisson J. Clinical spectrum of bronchogenic cysts of the mediastinum and lung in the adult. Ann Thorac Surg 1991;52:6-13. [PubMed]
- Nakata H, Egashira K, Watanabe H, Nakamura K, Onitsuka H, Murayama S, Murakami J, Masuda K. MRI of bronchogenic cysts. J Comput Assist Tomogr 1993;17:267-70. [PubMed]
- Broderick LS, Brooks GN, Kuhlman JE. Anatomic pitfalls of the heart and pericardium. Radiographics 2005;25:441-53. [PubMed]