Poly Implant Prothèse (PIP) incidence of rupture: a retrospective MR analysis in 64 patients
Introduction
Silicone gel breast implants produced by the French company Poly Implant Prothèse (PIP) containing hydroxypropyl cellulose, also known as M-Implants and Rofil-implants, have been introduced worldwide, since 1991 and approximately 400,000 women may have PIP silicone implants (1). The company went into liquidation in 2010, after the French Medical Device Regulatory Agency (AFSSAPS) revealed that had been using industrial grade silicone, rather than the purer medical grade silicone filler, originally tested for the award of the European commission mark of approval (2).
On December 2011, the French government recommended that 30,000 French women who had PIP breasts implants, should have removed them, due to the high rupture rates. Similarly, in the UK, the Medicines and healthcare products regulatory agency (MHRA) issued a device alert advising clinicians not to use PIP implants; in addition, they stated that the rupture rates was in the order of 1% compared to the rate of 5%, suggested by the French regulator. Furthermore, fears about the increased rupture rates of these implants and risks of toxicity have subsequently led to increased patient anxiety.
To date, few clinical trials in the English literature, have confirmed a higher incidence of rupture of PIP hydrogel implants, compared to other breast implants (3-7).
Contrast enhanced magnetic resonance imaging (MRI) is an established diagnostic tool in the evaluation of the extent of disease in patients with known breast cancer, in monitoring the response to neo-adjuvant therapy and screening of patients at high risk for breast carcinoma (8-11). Among the most important clinical application of breast MRI, there is the assessment of patients after breast augmentation therapy (12-14).
The purpose of this retrospective study was to describe the MRI features of PIP hydrogel implants in a group of 64 patients and to assess the incidence of implants rupture, compared to other clinical trials.
Methods
In this double-center study, we retrospectively reviewed the data sets of 64 consecutive patients (mean age, 43±9 years; age range, 27-65 years), who underwent breast MRI examinations, between January 2008 and October 2013. History from each patient included: age of implant; any symptoms and history of previous implants other than PIP prosthesis.
The most common clinical indication to breast MRI, was re-evaluation of patients with suspected implant rupture on the basis of clinical assessment or after conventional imaging examination (either mammography or ultrasound). All patients had undergone breast operation with bilateral textured cohesive gel PIP implant insertion for aesthetic reasons. The mean time after operation was 8 years (range, 6-14 years). One patient had undergone breast augmentation in Colombia in 2004. The implantation time in this case was 9 years. No patients reported history of direct trauma to their implants.
Breast MR imaging technique
Thirty-three patients underwent MRI examinations, using a 1.5-Tesla superconducting MR system (Gyroscan, Intera, Philips, The Netherlands) with a dedicated surface breast coil. Patient’s positioning was prone, with both breasts hanging into the bilateral surface coil, avoiding any compression during the diagnostic procedure. An unenhanced axial T2-weighted turbo spin-echo sequence (TR/TE, 3,800/120; slice thickness, 3 mm; field of view, 240×200 mm2) was followed by a T2-weighted silicone selected sequence (TR/TE, 5,944/70; slice thickness, 3 mm; field of view, 240×200 mm2); subsequently, an axial T1-weighted fat-suppressed 2D fast spoiled recalled echo sequence (TR/TE, 8.89/1.7; slice thickness, 3 mm; flip angle, 12°; matrix size, 256×256; field of view, 240×200 mm2) was obtained, which was performed before and five times after rapid bolus injection of 0.1 mmol/L of gadopentetate dimeglumine (Magnevist, Bayer Schering Pharma) per kilogram of body weight, at an injection rate of 2.0 mL/s. Image acquisition began immediately after the administration of the contrast material and saline bolus. The total duration of the dynamic study was approximately 6 minutes. Thirty-one patients were studied using a Siemens MR scanner (Magnetom Avanto, Siemens, Forcheim, Germany) with a dedicated coil for prone breast imaging, using the following sequences: coronal T2-weigthed turbo spin-echo (TR/TE, 3,370/129; slice thickness, 3 mm; field of view, 330×240 mm2); axial and sagittal T2-weighted TIRM silicone water saturation with fat suppression (TR/TE, 6,800/85; with selective suppression of water signal, resonance frequency=0; slice thickness, 3 mm; matrix, 192×256; field of view, 240×200 mm2). An axial isotropic T1-weighted spair 3D-VIBE sequence (TR/TE, 4.98/2.39; slice thickness, 3 mm; flip angle, 10°; matrix size, 384×384; field of view, 370×370 mm2) was obtained, which was performed before and five times after the rapid bolus injection of 0.1 mmol/L of gadobenate dimeglumine (Multi Hance, Bracco Italy) per kilogram of body weight, at an injection rate of 2.0 mL/s. Image acquisition began immediately after the administration of the contrast material and saline bolus. Dynamic study duration was approximately 6 minutes. After the examination, subtraction images were obtained by subtraction of the unenhanced images, from the first contrast-enhanced image, on a pixel-by-pixel basis.
Breast MR image interpretation
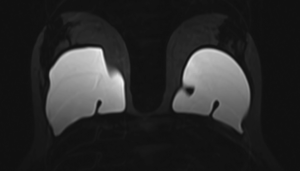
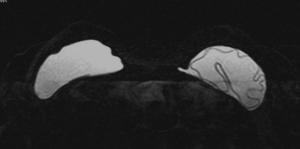
All MR images were retrospectively reviewed by two experienced breast imagers with respectively 16 years (M. I.) and 10 years (B. C.) expertise in breast MR imaging and three groups were identified: group 1, patients with normal breast prosthesis or with signs of mild collapse or coarctation and with no evidence of rupture; group 2, patients with clear signs of intra-capsular rupture, with evidence of silicone within the fibrous capsule or with associated signs, such as the linguine sign (including the sub-capsular lines), the keyhole or noose sign and the droplet sign; and group 3, patients with extra-capsular rupture and evidence of free silicone outside the capsule or peri-prosthetic and or axillary or mediastinal cavity collections (silicomas).
Results
In the overall group of patients, in 43 cases there was a sub-glandular breast implant; in the remaining 21 a sub-pectoral prosthesis. At the time of clinical examination, 41 patients were asymptomatic, 16 complained of breast tenderness and 7 had clinical evidence of rupture. Normal findings were observed in 15 patients and in 26 there was evidence of mild collapse, with associated not significant peri-capsular fluid collections and no evidence of implant rupture (Group 1); in 23 patients, there was suggestion of implant rupture, according to breast MRI, leading to an indication for surgery. In particular, 14 patients (Group 2) showed intra-capsular rupture, with associated evidence of the linguine sign in all cases; the key-hole sign and the droplet sign were observed in 6 cases. In 9 patients (Group 3), there was evidence of extra-capsular rupture, with presence of axillary collections (siliconomas) in 7 cases and peri-prosthetic and mediastinal siliconomas, in 5 cases. The overall rupture rate was of 36% (23 out of 64 patients). No cases of breast implants related cancer were observed in our series. Table 1 summarizes results in patients populations.

Full table
Figure 1 shows an example of a mild implant collapse, without evidence of rupture. Figure 2 shows an example of left intra-capsular implant rupture, with evidence of the linguine sign. Figure 3A,B shows an example of right extra-capsular implant rupture, with evidence of peri-implant silicoma and within the right internal mammary chain. Figure 4 shows an example of right extra-capsular rupture, with spread of silicone within the pectoralis muscle; on the contralateral side there is evidence of intra-capsular rupture, with associated linguine sign.


Discussion
The results of this double-center retrospective study, show an incidence of PIP implants rupture of 36%, compared to other breast implants.
Breast augmentation is one of the western world’s most common cosmetic surgical procedures (7). The majority of breast implantations are done for augmentation purposes and a minority are done for correction of congenital abnormalities (15). Like any medical device, silicone breast implants have a limited product life. This is particularly important considering the young age of the majority of patients. Complications of breast implantation include tenderness, capsular contracture and rupture. Rupture may result from trauma, deterioration of implant shell with time or manufacturing defect. The resulting leaked silicone gel may remain within the scar tissue capsule as an intra-capsular rupture or may migrate outside the capsule but remains in the breast tissue as an extra-capsular rupture (16).
Silicone implants rupture incidence is estimated to be 8% in asymptomatic women and 33% in symptomatic women (17-19). Hölmich et al. in a prospective study of third-generation breast implants, demonstrated on average, 2 per 100 implants rupture per year (20); they also showed that the probability that a device would be intact after augmentation mammoplasty was 98% after 5 years and 83-85% after 10 years. In more recent papers, the reported rupture rate ranged from 8% to 11.1% at 9-11 years, with a rupture rate per patient, ranging from 15.1% to 15.4% (21-23).
In 2006, early reports were published on the unusual rupture rates of PIP implants and mechanical testing of the PIP implants, demonstrated the implants were more susceptible to rupture and more of an irritant potential, in contrast to the conventional silicone implants. The PIP Company went into liquidation in 2010 after the AFSSAPS revealed that had been using non-medical grade silicone filler and on December 2011, consequently, French government advised removal of all PIP implants. Official chemical analysis has recently revealed, that the filler of the PIP implant contains a higher proportion of low molecular weight silicone, compared to a medical-grade product, and it is this, that is responsible of the early shell weakening and rupture (2).
In a recent paper, Berry reported a PIP implant rupture rate of 15.9-33.8%; however, these results were obtained from explantation data based on less than 10% (42 patients) of the original cohort of 453 women who had PIP implants in their series (7). In a previous work by Maijers et al. (6), the authors studied 224 PIP implants in 112 women; in particular, the authors compared two cohort of patients, respectively from 2000 (using medical grade silicone) and 2001 (using non medical grade silicone), with surgery as end point in all patients. The authors found a higher incidence of extra-capsular rupture (overall rupture prevalence of 24%), compared to other implants; although no significant difference was observed in rupture rates between the two cohorts.
Oulharj et al. in the largest published series to date, conducted a retrospective study to define the rupture rate of PIP implants and the complications that arise. A total of 828 PIP breast implants were removed in 455 patients. The rate of ruptured implants was 7.73% (64/828), corresponding to 11.6% of patients. A peri-prosthetic effusion was associated with rupture in 44% of cases. Peri-prosthetic capsule biopsy demonstrated the presence of a foreign body, which seemed to be silicone, in 26% of cases and the presence of inflammation in 13% of cases. A statistically significant difference was found between the rates of rupture for texturised implants as compared to the smooth-surfaced implants. These authors advice a preventive explantation of PIP breast implants that is justified given the high failure rate and patients’ exposure to silicone gel that do not comply with European community standards in the absence of rupture, through the early perspiration of implants (24).
Quaba et al. have shown higher rates of PIP implant failure; these authors observed a total of 144 ruptured implants removed from 119 patients, giving a rupture rate of 35.2% per patient and 21.3% per implant, over a mean implantation period of 7.8 years (5). Our data in agreement with this latter study, confirm a high incidence of PIP implants rupture (36%) compared to other breast implants, although the populations are different. The final expert report from the department of health (DoH) recognize the possibility that PIP implants are 2 to 6 times more likely to rupture compared to other implants, with the events occurring within the first 5 years (25).
Another important finding of our study, is the observation of a high incidence of spread of silicone to axillary nodes, compared to other cohesive gel implants; in a previous study, no evidence of gel migration was observed in a series of 106 patients (17); in a recent pictorial review, on ruptured PIP breast implant, Helyar et al. have shown several cases of silicone adenitis (18); in our study we observed an overall incidence of silicone axillary spread, as assessed by MRI of 11% (7 out of 64 patients). In addition, we also observed in 5 out of 64 (8%) patients, migration of silicone into the anterior mediastinal and thoracic cavity. Despite recent publications of sporadical cases of anaplastic large cell lymphoma in breast implants (26,27), no such cases of breast implants related cancer, were observed in our series. In agreement with our results, recent studies carried out by the AFSSAPS, have allayed any fears of toxicity, of the silicone gel filler used by PIP (28).
To the best of our knowledge, no study has been published so far, in the English literature, reporting the incidence of PIP implants failure in Italy, as assessed by MRI.
Limitations of this study include the small number of patients recruited and the limited follow up period after breast augmentation therapy of 8 years; further multi-center studies in larger patients population, are warranted to confirm our results and more in general to define a correct strategy for the long-term assessment and management of silicone breast implant ruptures.
Conclusions
In conclusion, physicians should be aware of the possibility to encounter in their clinical practice, patients who had undergone breast augmentation with PIP hydrogel implants. The higher failure rate and incidence of local signs in patients carrying PIP implant, as shown in this study, accentuates the importance of performance and interpretation of breast MR examinations by radiologists with high expertise and training in breast MR imaging and reinforces the need for appropriate counselling and investigations of patients with such implants, to avoid diagnostic mistakes and eventually possible legal issues.
Acknowledgements
The authors sincerely thank Guglielmo Caprio, Francesco De Biase, Lucia Di Marzo, Gennaro Fraia, Fabio Garbino and Graciana Diez-Roux for their valuable technical assistance, in making this study possible.
Disclosure: The authors declare no conflict of interest.
References
- PIP breast implants latest from the NHS. NHS Choices. Available online: http://www.nhs.uk/news/2012/01January/Pages/government-review-advises-on-french-pip-breast-implants.aspx
- Scientific committee on emerging and newly identified health risks (SCENIHR). The safety of PIP silicone breast implants. Scintific committee of the European commission. Available online: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr
- O’Neill JK, Rigby H, Kenealy JM. Leakage and osmotic shifts in PIP Hydrogel implants. A case demonstrating increase and decrease of implant volume in the same patient. J Plast Reconstr Aesthet Surg 2008;61:1122-3. [PubMed]
- Khan UD. Poly Implant Prothèse (PIP) incidence of device failure and capsular contracture: a retrospective comparative analysis. Aesthetic Plast Surg 2013;37:906-13. [PubMed]
- Quaba O, Quaba A. PIP silicone breast implants: rupture rates based on the explantation of 676 implants in a single surgeon series. J Plast Reconstr Aesthet Surg 2013;66:1182-7. [PubMed]
- Maijers MC, Niessen FB. Prevalence of rupture in poly implant Prothèse silicone breast implants, recalled from the European market in 2010. Plast Reconstr Surg 2012;129:1372-8. [PubMed]
- Berry MG, Stanek JJ. PIP implant biodurability: a post-publicity update. J Plast Reconstr Aesthet Surg 2013;66:1174-81. [PubMed]
- Vassiou K, Kanavou T, Vlychou M, Poultsidi A, Athanasiou E, Arvanitis DL, Fezoulidis IV. Characterization of breast lesions with CE-MR multimodal morphological and kinetic analysis: comparison with conventional mammography and high-resolution ultrasound. Eur J Radiol 2009;70:69-76. [PubMed]
- Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, Manoliu RA, Kok T, Peterse H, Tilanus-Linthorst MM, Muller SH, Meijer S, Oosterwijk JC, Beex LV, Tollenaar RA, de Koning HJ, Rutgers EJ, Klijn JG; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004;351:427-37. [PubMed]
- Heywang SH, Hilbertz T, Beck R, Bauer WM, Eiermann W, Permanetter W. Gd-DTPA enhanced MR imaging of the breast in patients with postoperative scarring and silicon implants. J Comput Assist Tomogr 1990;14:348-56. [PubMed]
- Kuhl CK. MR imaging for surveillance of women at high familial risk for breast cancer. Magn Reson Imaging Clin N Am 2006;14:391-402. [PubMed]
- Yang N, Muradali D. The augmented breast: a pictorial review of the abnormal and unusual. AJR Am J Roentgenol 2011;196:W451-60. [PubMed]
- Huch RA, Künzi W, Debatin JF, Wiesner W, Krestin GP. MR imaging of the augmented breast. Eur Radiol 1998;8:371-6. [PubMed]
- Di Benedetto G, Cecchini S, Grassetti L, Baldassarre S, Valeri G, Leva L, Giuseppetti GM, Bertani A. Comparative study of breast implant rupture using mammography, sonography, and magnetic resonance imaging: correlation with surgical findings. Breast J 2008;14:532-7. [PubMed]
- Juanpere S, Perez E, Huc O, Motos N, Pont J, Pedraza S. Imaging of breast implants-a pictorial review. Insights Imaging 2011;2:653-70. [PubMed]
- Goodman CM, Cohen V, Thornby J, Netscher D. The life span of silicone gel breast implants and a comparison of mammography, ultrasonography, and magnetic resonance imaging in detecting implant rupture: a meta-analysis. Ann Plast Surg 1998;41:577-85; discussion 585-6. [PubMed]
- Hedén P, Nava MB, van Tetering JP, Magalon G, Fourie le R, Brenner RJ, Lindsey LE, Murphy DK, Walker PS. Prevalence of rupture in inamed silicone breast implants. Plast Reconstr Surg 2006;118:303-8; discussion 309-12. [PubMed]
- Helyar V, Burke C, McWilliams S. The ruptured PIP breast implant. Clin Radiol 2013;68:845-50. [PubMed]
- Netscher DT, Weizer G, Malone RS, Walker LE, Thornby J, Patten BM. Diagnostic value of clinical examination and various imaging techniques for breast implant rupture as determined in 81 patients having implant removal. South Med J 1996;89:397-404. [PubMed]
- Hölmich LR, Friis S, Fryzek JP, Vejborg IM, Conrad C, Sletting S, Kjøller K, McLaughlin JK, Olsen JH. Incidence of silicone breast implant rupture. Arch Surg 2003;138:801-6. [PubMed]
- Cunningham B, McCue J. Safety and effectiveness of Mentor's MemoryGel implants at 6 years. Aesthetic Plast Surg 2009;33:440-4. [PubMed]
- Hedén P, Bronz G, Elberg JJ, Deraemaecker R, Murphy DK, Slicton A, Brenner RJ, Svarvar C, van Tetering J, van der Weij LP. Long-term safety and effectiveness of style 410 highly cohesive silicone breast implants. Aesthetic Plast Surg 2009;33:430-6; discussion 437-8. [PubMed]
- Collis N, Litherland J, Enion D, Sharpe DT. Magnetic resonance imaging and explantation investigation of long-term silicone gel implant integrity. Plast Reconstr Surg 2007;120:1401-6. [PubMed]
- Oulharj S, Pauchot J, Tropet Y. PIP breast implant removal: a study of 828 cases. J Plast Reconstr Aesthet Surg 2014;67:302-7. [PubMed]
- Department of Health, NHS Medical Directorate. Poly Implant Prothese (PIP) breast implants: final report of the working Group. 18 June 2012.
- Taylor KO, Webster HR, Prince HM. Anaplastic large cell lymphoma and breast implants: five Australian cases. Plast Reconstr Surg 2012;129:610e-7e. [PubMed]
- Aladily TN, Medeiros LJ, Amin MB, Haideri N, Ye D, Azevedo SJ, Jorgensen JL, de Peralta-Venturina M, Mustafa EB, Young KH, You MJ, Fayad LE, Blenc AM, Miranda RN. Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Surg Pathol 2012;36:1000-8. [PubMed]
- AFSSAPS medical devices evaluation direction. Silicone based filling gel breast implants from Poly Implant Prothèse company: update of test results, issued 14th April 2011.


