CT features of metanephric adenoma: a case report and review of the literature
Introduction
Metanephric adenoma (MA) is a rare neoplasm, accounting for 0.2% of adult renal epithelial neoplasms (1). MA can present at any age, ranging from 15 months to 83 years, and the majority of cases occurs in patients in fifth and sixth decades and are seen preponderantly in females by a 2:1 ratio (2,3). Only a few case reports deal with MA in the literatures and imaging manifestations has not been well documented. Here, we presented a case of MA to describe its ultrasound and computed tomography (CT) findings. Pathological appearances were correlated and literatures were reviewed.
Case report
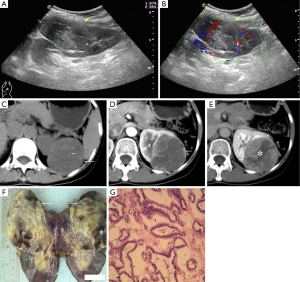
A 49-year-old woman visited our hospital due to an intermittently vague pain in left flank for a duration of 3 weeks. Her physical examination and laboratory tests were unremarkable. A protruding hypoechoic mass measured 80 mm × 55 mm was detected in the superior pole of her left kidney by ultrasound (Figure 1A). No capsule was detected, and the inner echo was heterogeneous. The renal sinus was pushed to the other side of the mass while no tumor thrombus had been found in the renal veins and inferior vena. Color Doppler flow imaging showed that there were a little blood flow signal in the surrounding and the inner of the mass (Figure 1B). Dynamic contrast-enhanced CT examination was performed with unenhanced scanning, the corticomedullary phase and the nephrographic phase. In unenhanced scanning, the mass was well-demarcated with a few small calcification spots and protruded out of renal outline (Figure 1C). It was of iso-attenuation to renal parenchyma. After the injection of contrast materials, peripheral mild nodular enhancement occurred in the corticomedullary phase (Figure 1D). Enhancement degree of the peripheral enhanced nodular was lower than that of renal cortex, but slight higher than medullar with CT value of 96, 180 and 80 HU, respectively. Centripetal and more pronounced enhancement with an irregular unenhanced area was noticed in the nephrographic phase, but enhancement of the mass was lower than that of renal cortex and medullar with CT value of 103, 166 and 232 HU, respectively (Figure 1E). Although the classical renal cell carcinoma was excluded, other malignant entities, such as papillary renal cell carcinoma and sarcoma, were still suspected. The patient received a laparoscopic total nephrectomy. At gross pathological examination, the mass was in oval shape and showed a well-defined pushing border with no capsule. The cut-surface was shown as a mixture color of yellowish-grey and tan pink (Figure 1F). Multiple foci of hemorrhage, one focus of necrosis and calcification were also noted. Microscopy revealed small, tightly packed acini and tubules in organized arrays (Figure 1G). The tumor cells were small and uniform with round nuclei and scant cytoplasm. No karyokinesis was observed. A final diagnosis of MA was made pathologically.
Discussion
MA is often found incidentally without any related clinical manifestations or with several unspecific presentations, such as flank pain, abdominal mass, hematuria, dysuria, or hypertension (3). Due to its benign characteristics, partial nephrectomy is highly recommended and some successful cases have been reported (4-6). Therefore, it is important to differentiate MA from other clinically aggressive renal tumors preoperatively. But unfortunately, even at pathological examination, MA still has some overlapping features with papillary renal cell carcinoma (7).
In present case, the MA appeared as a well-demarcated soft tissual mass protruding out of renal outline with a few spot-like calcification inside it. Dynamic CT enhancement revealed slight peripheral enhancement in the corticomedullary phase and the enhancement became centripetal and more pronounced with an irregular unenhanced area in the nephrographic phase. The enhancement degree was lower than renal cortex in both the corticomedullary and nephrographic phases. Well-defined margin, calcification and irregular necrosis area were also noted at gross pathological examination. Other characteristical CT finding of this case is its heterogenous enhancement, which is consistenced with of irregular necrosis at gross specimen. Masuda et al. reported a case of MA as a hypovascular renal mass that showed gradual and prolonged enhancement on multiphase enhanced CT (8), but the peripheral to centripetal and more pronounced enhancement was not mentioned by them. Zhu and colleagues reported CT findings of eight cases of MAs recently (9). Unlike the present case, all their cases were poorly-defined and seven of eight were centered in the renal medulla. In a study of Mantoan Padilha and colleagues, which included 21 MAs, all the MAs were well demarcated at pathological examinations (7). Other characteristics of MAs were hypovascular in relation to adjacent renal parenchyma. In present case, we also found that the enhancement of the solid components was lower than that of the renal cortex in both the corticomedullary and the nepherographic phases. Another feature seen in this case was peripheral enhancement with centripetal filling-in.
For renal cell carcinoma (RCC) is the most common renal renal tumor and needs total nephrectomy at most situations, it is necessary to include RCC in differential diagnosis when a solid mass without visible fat is confronted in the kidney at cross-section imaging. Clear cell RCC often shows marked and heterogeneous enhancement at the corticomedullary phase and decreasing at the nephrographic phase (10). These hypervascular characteristics can be helpful to distinguish MA from clear cell RCC, but MA is difficult to differentiate from other hypovascular renal masses, especially solid papillary RCC. Therefore, many patients received total nephrectomy. Except for hypovascular, the present case also showed a characteristic of peripheral enhancement with centripetal filling-in. However, 74% of papillary RCC (10) showed predominantly peripheral enhancement, neither can this feature be used as a sign to differentiate the two entities.
In summary, we presented the CT features of a case of MA with pathological correlated. It manifested as a well-circumscribed, heterogenerous renal mass protruding out of renal outline. The dynamic enhancement pattern was of progressive and centripetal enhancement, and hypovascular related to renal parenchyma. Although it could be differentiated from conventional RCC, it is difficult to be distinguished from other hypovascular renal masses, especially solid papillary RCC. Familiarity with its imaging features can help radiologists to include MA into differential diagnostic array and to guide further evaluation and management.
Disclosure: The authors declare no conflict of interest.
References
- Amin MB, Amin MB, Tamboli P, Javidan J, Stricker H, de-Peralta Venturina M, Deshpande A, Menon M. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol 2002;26:281-91. [PubMed]
- Chaudhary H, Raghvendran M, Dubey D, Srivastava A, Mandhani A, Kapoor R, Kumar A. Correlation of radiological and clinical features of metanephric neoplasms in adults. Indian J Cancer 2004;41:37-40. [PubMed]
- Davis CJ Jr, Barton JH, Sesterhenn IA, Mostofi FK. Metanephric adenoma. Clinicopathological study of fifty patients. Am J Surg Pathol 1995;19:1101-14. [PubMed]
- Xu B, Zhang Q, Jin J. A case of central metanephric adenoma successfully treated with laparoscopic nephron-sparing surgery. Urology 2012;80:e19-20. [PubMed]
- Kumar S, Mandal AK, Acharya NR, Kakkad N, Singh SK. Laparoscopic nephron-sparing surgery for metanephric adenoma. Surg Laparosc Endosc Percutan Tech 2007;17:573-5. [PubMed]
- Kosugi M, Nagata H, Nakashima J, Murai M, Hata J. A case of metanephric adenoma treated with partial nephrectomy. Nihon Hinyokika Gakkai Zasshi 2000;91:489-92. [PubMed]
- Mantoan Padilha M, Billis A, Allende D, Zhou M, Magi-Galluzzi C. Metanephric adenoma and solid variant of papillary renal cell carcinoma: common and distinctive features. Histopathology 2013;62:941-53. [PubMed]
- Masuda A, Kamai T, Mizuno T, Kambara T, Abe H, Tomita S, Fukabori Y, Yamanishi T, Kaji Y, Yoshida K. Renal metanephric adenoma mimicking papillary renal cell carcinoma on computed tomography: a case report. Urol Int 2013;90:369-72. [PubMed]
- Zhu Q, Zhu W, Wu J, Chen W, Wang S. The clinical and CT imaging features of metanephric adenoma. Acta Radiol 2014;55:231-8. [PubMed]
- Kim JK, Kim TK, Ahn HJ, Kim CS, Kim KR, Cho KS. Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am J Roentgenol 2002;178:1499-506. [PubMed]


