
Preliminary evaluation of dynamic glucose enhanced MRI of the human placenta during glucose tolerance test
Introduction
Placental glucose transfer is essential to sustain fetal development (1). Insufficient glucose transfer may result in intrauterine growth restriction (IUGR) while too much glucose is associated with fetal macrosomia. Such conditions can further lead to complications at birth and an increased burden of chronic diseases in adulthood (1). The fetal glucose level varies with the gestational age and the maternal glucose level. In addition, diabetes and gestational diabetes have been associated with elevated maternal glucose levels and risk of birth complications. Whereas the glucose tolerance test screens for gestational diabetes (2), it does not provide information about placental glucose transfer. Routine ultrasound exams are not are not able to characterize maternal-fetal glucose relationships. Although PET can map tracer distribution across the placenta and in fetal organs in experimental animals, it is not feasible in humans due to the ionizing radiation (3). Hence, it is urgent to develop a safe non-invasive means to assess placental glucose transport.
Advanced MRI techniques are being investigated for placental imaging. For example, placental perfusion can be measured by dynamic contrast-enhanced (DCE) MRI (4,5), arterial spin labeling (ASL) (6) and intravoxel-incoherent motion (IVIM) MRI (7-9). In addition, placental oxygenation can be assessed using T2 and T2* mapping (10,11) and BOLD MRI (12-14). In addition, MR spectroscopy (MRS) has be used to study the placenta (15-17) but its spatial resolution is limited. Recently, chemical exchange saturation transfer (CEST) MRI has been shown to be glucose sensitive via the exchange between bulk water and the glucose hydroxyl protons, dubbed GlucoCEST MRI (18,19). Dynamic glucose enhanced (DGE) MRI provides time-resolved glucose measurement in brain tumors at high field (20,21), which has been extended for head and neck cancer imaging at 3.0 Tesla (22). Recently, Wu et al. demonstrated DGE MRI at 11.7 Tesla in a mouse model of intrauterine inflammation (IUI), which is known to result in acute placental injury (23). It is necessary to note that most of these DGE experiments were performed with intravenous (IV) glucose administration.
Although it is advantageous to perform CEST MRI at high field (24), it is important to develop GlucoCEST MRI at 3.0 Tesla, particularly for the pregnant population. For patient acceptance, getting MRI scan after an oral glucose drink is far preferable to an IV load. Oral glucose tolerance test is commonly used during pregnancy to screen for gestational diabetes. To address these two issues, our study here optimized continuous wave (CW) GlucoCEST echo planar imaging (EPI) at 3.0 Tesla (25). We tested GlucoCEST in ex vivo placentas and characterized the relationship between GlucoCEST and placental vascular density. We also demonstrated GlucoCEST MRI following the glucose tolerance test and established the feasibility of placental DGE as an ancillary exam.
Methods
The study has been approved by the Institutional Review Board (IRB) at the Boston Children’s Hospital and Massachusetts General Hospital.
Glucose phantom study
D-glucose was dissolved in PBS at concentrations of 4, 8, 12 and 20 mM with pH titrated to 7.4. The solution was transferred into separate 50 mL Falcon tubes, and positioned in a cylindrical phantom holder.
Ex vivo placental tissue study
Fresh placentas were obtained from uncomplicated term deliveries within 6 h. Cannulation was performed on the surface fetal chorionic arteries of the placenta specimen. The lobules were flushed with normal saline and 5,000 IU/L heparin (NSH) until the venous return was clear. Subsequently, NSH supplemented with D-glucose (5, 10, 20 mM) and 1 mL/L yellow India ink (Cancer Diagnostics, Inc.) were perfused in separate placenta lobules of a single placenta. After the perfusion, the area around the perfused lobule was clamped, sutured, and fit into a cylindrical plastic container of 10 cm diameter. Four placenta sections were prepared and stacked in the container, which was then filled with agar gel (1%) (Figure 1). The sample was maintained at room temperature before imaging. Hematoxylin and Eosin histology was obtained after MR imaging and examined by an experienced placenta pathologist.
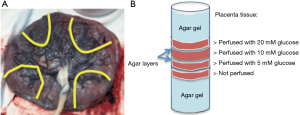
In vivostudy
The GlucoCEST scans were performed in pregnant mothers (Table 1). After a glucose drink (TrutolTM) in a sitting position with a dose of 50 gm, equivalent to glucose tolerance test, subjects re-entered the scanner while keeping the same maternal position for the dynamic GlucoCEST scan. T2*-weighted scans were performed simultaneously.
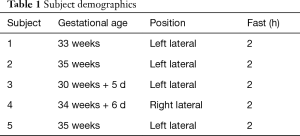
Full table
Data acquisition
All studies were performed on a 3.0 Tesla Skyra (Siemens Healthcare, Erlangen, Germany). While phantom and ex vivo tissue was scanned using a 32-channel array head coil, in vivo subjects were scanned using a combined 18-channel body and 12-channel spine receive arrays. CEST imaging was performed with single-shot gradient echo EPI (26) with a CW RF irradiation of 1 second. For the phantom study, we repeated CEST MRI measurements under B1 fields of 1, 1.5, 2 and 3 µT, and flip angle (FA) of 20, 45, 70 and 90 degrees. Z-spectra were obtained with RF saturation frequency from –5 to 5 ppm with intervals of 0.25 ppm. Imaging matrix 48 × 48; in-plane resolution 3 × 3 mm2, slice thickness 10 mm; TR/TE =4 s/17 ms, FA =90° , BW =2.3 kHz/px, averages =2. WASSR scan was performed with the same FOV and resolution as CEST scan at B1 =0.5 µT. For the ex vivo experiment, double spin echo MRI was performed for T2 imaging (TR =1 s, FA=90 degree, TE1/TE2 =8.5/50 ms). In addition, T1 was calculated based on images acquired with a double FA gradient echo sequence (TR/TE =15/1.7 ms, FA1/FA2 =5/26 degrees). Phantom and ex vivo experiments were performed at room temperature (25 °C).
For in vivo study, all scans were performed free-breathing. The baseline (before glucose drink) CEST image was collected with a CW CEST MRI (RF saturation time =1 s, B1 =1.5 µT). Upon mother re-entering the scanner, the dynamic CEST scan was performed with saturation frequency sweep densely between 0.75 and 4 ppm with intervals of 0.25 ppm, and sparsely between –4 to 0 with intervals of 2.0 ppm. Each dynamic scan has a total of 17 frequency offsets plus two reference scans without on resonance saturation, which adds up to 1 min 16 s for each dynamic scan. At the end of the scan, a full CEST z-spectrum was acquired. A field map was collected with a double gradient echo MRI.
Data processing
B0 map was obtained by Lorentzian fitting of z-spectrum, followed by Water saturation shift referencing (WASSR) correction of z-spectrum (27). ROIs of placental perfusion regions (and phantom tubes) were manually outlined and magnetization transfer asymmetry (MTRasym) [defined as Msat (–) – Msat(+)/Mctrl, where Mctrl is the control scan without saturation, Msat(–) and Msat(+) are saturated scans with RF irradiation applied on negative and positive ppms, respectively] was plotted for each ROI. The GlucoCEST enhancement (GCE) is defined as the change in the integral of MTRasym between 0.75 and 4 ppm. The DGE is defined as the change in integral of Z spectra between 0.75 and 4 ppm with reference to the Z spectrum of the first time point. The reference scans of each dynamic CEST acquisition, which are T2*-weighted EPI images, were used to obtain the dynamic change of R2*, as ∆R2* = log(S0/S0 baseline)/TE. Therefore R2* changes were recorded concurrently as GCE changes.
Results
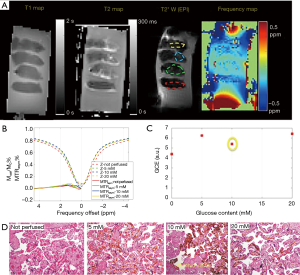
Figure 2 shows the phantom GlucoCEST MRI. As shown in Figure 2B, the GCE effect increases linearly with glucose concentration. Moreover, the GCE peaked when B1 =2 uT, consistent with previous simulation results (25). The FA affects the magnitude of GlucoCEST MRI effect and its signal-to-noise ratio (SNR) in opposite ways, and this finding was used as a reference in optimization placental tissue GlucoCEST MRI.

In the ex vivo placenta, the sub-regions of perfused tissue were identified based on the T2* weighted image (Figure 3A). CEST effect in the non-perfused tissue may be due to blood sugar in the fetal vasculature (10% v/v) (28). The normal serum glucose level in the placenta is reported to be around 4 mM in vivo (29). Glucose-perfused placental tissue showed higher GlucoCEST effect than the non-perfused control placental tissue (Figure 3B). This observation is also consistent with previous work that compared GCE effect in “glucose perfused”, “non-perfused”, and “saline perfused” placenta tissue (30). However, the GlucoCEST MRI effect did not scale linearly with the glucose perfusion concentration (Figure 3C). Notably, the section of placenta tissue perfused with 10 mM glucose was found to have chronic villitis (Figure 3D), which may explain its lower GCE. Nevertheless, the tissue perfused by 20 mM glucose showed only slightly higher GCE than that of 5 mM glucose may be due to normal variations in packing density of villi and the number of vessels/capillaries per villi, which could be a major confounding factor when inferring glucose content from the GCE intensity.
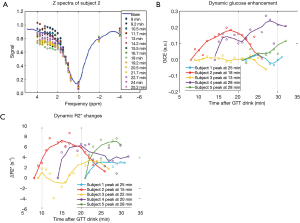
The in vivo experiments were performed in five volunteers whose gestational age ranged from 30 to 35 weeks. The duration of the dynamic scans varied for each subject, from 10 to 18 min, due to the different time needed to re-position the imaging field of view to match the baseline scan, while the maximum stay in the scanner for each subject was fixed. Nonetheless, the dynamic scans were kept within 30 min of the glucose drink. Example of the z-spectra of one subject is shown in Figure 4A. The baseline scans that were taken before glucose drink resulted in sizable variations of the GCE (–0.8 to 1.8) among individuals. Given the subject position changes between the baseline and the dynamic scans, the DGE of glucose was reported in reference to the first dynamic scan (Figure 4B). And the tendency of DGE increase during the first 30 min within glucose drink is consistent for all five subjects. Further, the ∆R2* was also assessed by dynamic T2* weighted images in reference to the first time point after the glucose drink (Figure 4C). The time series of ∆R2* and the time series DGE exhibit similarities in most subjects, however, they do not have significant correlations.
Discussion
Our study investigated GlucoCEST MRI at 3.0 Tesla and preliminarily tested DGE MRI in human placenta following oral glucose tolerance test. The DGE signal increase was observed for each individual within 30 min after the glucose drink. We have demonstrated the feasibility of GlucoCEST as a novel imaging test for characterizing placental glucose transport.
The ex vivo study demonstrated that GCE is promising for detection of placental pathologies. Glucose perfused tissue exhibited higher GCE than the non-perfused tissue. Although we did not show a simple linear relationship between GCE and glucose content, the results could be partially explained by the histological findings of variable tissue perfusion possibly affected by localized placental pathology. The vessel perfusion and vascular density in the placenta tissue directly affect the volume fraction of fetal blood in a single imaging voxel and thus affect the magnitude of GCE effect. Because the maternal/fetal glucose gradient is only 1.22 mM (fetal 3.48–3.87 and maternal 4.4–4.7 mM) (29), the glucose concentration is largely homogenous across the placenta, and therefore GCE might be sensitive to regional pathologies such as infarction and chronic villitis.
The assessment of placental glucose is somewhat confounded by several known factors that may affect the GlucoCEST MRI effect, such as field inhomogeneity and motion (31,32) and factors that are specific to placenta structure, such as volume fractions of fetal and maternal blood. For example, the absolute GCE effect is sensitive to the B1 field. In the human placenta in vivo (~20 cm in diameter), the B1 filed may vary up to 40%, which could translate to 20% difference in GCE according to previous analysis of CEST effect (25). In addition, the blood sugar level varies throughout the day, whereas the normal range of fasting blood sugar level (3.9–5.5 mM) is close to that of prediabetes (5.5–7 mM) and diabetes (more than 7 mM) (33). In light of the technical challenges, further development of GCE is needed before it can reliably characterize placental glucose transport. Although the time to peak generally occurs within 2–4 min after IV glucose infusion (21), the time to peak is highly variable following oral glucose drink (34). Despite these challenges, the in vivo DGE showed a consistent trend in all subjects during glucose challenge, which is very encouraging.
It has been shown that apart from the CEST effect, glucose also reduces the water T2 relaxation due to chemical exchange, which would broaden the water saturation curve (35,36). T2 change of the glucose perfused ex vivo tissue is consistent with the GCE effect, though may be confounded by blood wash out. The in vivo experiments show the ∆R2* increases that are largely similar to the DGE signal, which is confirmative of the glucose enhancement effect.
There are several limitations of the current study, for example, the protocol of glucose drink required the subject to sit up and drink the glucose in order to prevent reflux, thus causing inconsistency between baseline measurements and the dynamic measurements. It was also challenging to get a blood sugar level validation, since even a simple serum glucose measure with a finger prick requires the patient to be out of the scan room and sitting down comfortably, which takes about 5–10 min. Thus, the values would not be matched in time. Finally, fetal organs such as fetal livers would be of interest to observe, but accurate assessment of glucose change was challenging due to the fetal motion. In addition, motion caused by maternal free breathing, fetal motion, Braxton-hicks contractions, as well as blood perfusion change induced by increased blood sugar level, needs to be considered in future studies. Quantitative validation of the dynamic GCE signal may also require iv administration for a more rapid bolus, so as to enable correlation between serum glucose and each dynamic scan. Here, we chose to do a simple noninvasive feasibly study as the more invasive study design would need to be justified by a successful feasibility study.
Conclusions
Our study demonstrated the feasibility of DGE MRI in the human placenta following an oral glucose load at 3.0 Tesla. We found that in vivo DGE showed a consistent trend in all subjects during glucose challenge.
Acknowledgments
Funding: The study was supported in part by NIH human placenta project U01HD087211 (to Grant) and Shanghai Sailing Program 18YF1410900 (to Luo).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board at the Boston Children’s Hospital and Massachusetts General Hospital.
References
- Desoye G, Gauster M, Wadsack C. Placental transport in pregnancy pathologies. Am J Clin Nutr 2011;94:1896S-902S. [Crossref] [PubMed]
- Close K. Oral Glucose Tolerance Test Uses, Procedure and Results. 2018. Available online: https://www.verywellhealth.com/the-oral-glucose-tolerance-test-1087684
- Benveniste H, Fowler JS, Rooney WD, Moller DH, Backus WW, Warner DA, Carter P, King P, Scharf B, Alexoff DA, Ma Y, Vaska P, Schlyer D, Volkow ND. study PET, study MRI. Maternal-fetal in vivo imaging: a combined PET and MRI study. J Nucl Med 2003;44:1522-30. [PubMed]
- Brunelli R, Masselli G, Parasassi T, De Spirito M, Papi M, Perrone G, Pittaluga E, Gualdi G, Pollettini E, Pittalis A, Anceschi MM. Intervillous circulation in intra-uterine growth restriction. Correlation to fetal well being. Placenta 2010;31:1051-6. [Crossref] [PubMed]
- Frias AE, Schabel MC, Roberts VH, Tudorica A, Grigsby PL, Oh KY, Kroenke CD. Using dynamic contrast-enhanced MRI to quantitatively characterize maternal vascular organization in the primate placenta. Magn Reson Med 2015;73:1570-8. [Crossref] [PubMed]
- Zun Z, Zaharchuk G, Andescavage NN, Donofrio MT, Limperopoulos C. Non-Invasive Placental Perfusion Imaging in Pregnancies Complicated by Fetal Heart Disease Using Velocity-Selective Arterial Spin Labeled MRI. Sci Rep 2017;7:16126. [Crossref] [PubMed]
- Moore RJ, Issa B, Tokarczuk P, Duncan KR, Boulby P, Baker PN, Bowtell RW, Worthington BS, Johnson IR, Gowland PA. In vivo intravoxel incoherent motion measurements in the human placenta using echo-planar imaging at 0.5 T. Magn Reson Med 2000;43:295-302. [Crossref] [PubMed]
- Moore RJ, Ong SS, Tyler DJ, Duckett R, Baker PN, Dunn WR, Johnson IR, Gowland PA. Spiral artery blood volume in normal pregnancies and those compromised by pre-eclampsia. NMR Biomed 2008;21:376-80. [Crossref] [PubMed]
- Moore RJ, Strachan BK, Tyler DJ, Duncan KR, Baker PN, Worthington BS, Johnson IR, Gowland PA. In utero perfusing fraction maps in normal and growth restricted pregnancy measured using IVIM echo-planar MRI. Placenta 2000;21:726-32. [Crossref] [PubMed]
- Huen I, Morris DM, Wright C, Parker GJ, Sibley CP, Johnstone ED, Naish JH. R1 and R2 * changes in the human placenta in response to maternal oxygen challenge. Magn Reson Med 2013;70:1427-33. [Crossref] [PubMed]
- Huen I, Morris DM, Wright C, Sibley CP, Naish JH, Johnstone ED. Absence of PO2 change in fetal brain despite PO2 increase in placenta in response to maternal oxygen challenge. BJOG 2014;121:1588-94. [Crossref] [PubMed]
- Luo J, Abaci Turk E, Bibbo C, Gagoski B, Roberts DJ, Vangel M, Tempany-Afdhal CM, Barnewolt C, Estroff J, Palanisamy A, Barth WH, Zera C, Malpica N, Golland P, Adalsteinsson E, Robinson JN, Grant PE. In Vivo Quantification of Placental Insufficiency by BOLD MRI: A Human Study. Sci Rep 2017;7:3713. [Crossref] [PubMed]
- Sørensen A, Peters D, Fründ E, Lingman G, Christiansen O, Uldbjerg N. Changes in human placental oxygenation during maternal hyperoxia estimated by blood oxygen level-dependent magnetic resonance imaging (BOLD MRI). Ultrasound Obstet Gynecol 2013;42:310-4. [Crossref] [PubMed]
- Sørensen A, Sinding M, Peters DA, Petersen A, Frøkjær JB, Christiansen OB, Uldbjerg N. Placental oxygen transport estimated by the hyperoxic placental BOLD MRI response. Physiol Rep 2015. [Crossref] [PubMed]
- Denison FC, Semple SI, Stock SJ, Walker J, Marshall I, Norman JE. Novel use of proton magnetic resonance spectroscopy (1HMRS) to non-invasively assess placental metabolism. PLoS One 2012;7:e42926. [Crossref] [PubMed]
- Kay HH, Gordon JD, Ribeiro AA, Spicer LD. Phosphorus 31 magnetic resonance spectroscopy of human placenta and quantitation with perchloric acid extracts. Am J Obstet Gynecol 1991;164:80-7. [Crossref] [PubMed]
- Macnaught G, Semple SI, Gray C, Simpson M, Norman JE, Walker J, Denison FC. 1H MRS as a biomarker for placental insufficiency in the growth restricted fetus. 2014. doi:. [Crossref]
- Chan KW, McMahon MT, Kato Y, Liu G, Bulte JW, Bhujwalla ZM, Artemov D, van Zijl PC. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med 2012;68:1764-73. [Crossref] [PubMed]
- Walker-Samuel S, Ramasawmy R, Torrealdea F, Rega M, Rajkumar V, Johnson SP, Richardson S, Goncalves M, Parkes HG, Arstad E, Thomas DL, Pedley RB, Lythgoe MF, Golay X. In vivo imaging of glucose uptake and metabolism in tumors. Nat Med 2013;19:1067-72. [Crossref] [PubMed]
- Xu X, Chan KW, Knutsson L, Artemov D, Xu J, Liu G, Kato Y, Lal B, Laterra J, McMahon MT, van Zijl PC. Dynamic glucose enhanced (DGE) MRI for combined imaging of blood-brain barrier break down and increased blood volume in brain cancer. Magn Reson Med 2015;74:1556-63. [Crossref] [PubMed]
- Xu X, Yadav NN, Knutsson L, Hua J, Kalyani R, Hall E, Laterra J, Blakeley J, Strowd R, Pomper M, Barker P, Chan K, Liu G, McMahon MT, Stevens RD, van Zijl PC. Dynamic Glucose-Enhanced (DGE) MRI: Translation to Human Scanning and First Results in Glioma Patients. Tomography 2015;1:105-14. [Crossref] [PubMed]
- Wang J, Weygand J, Hwang KP, Mohamed AS, Ding Y, Fuller CD, Lai SY, Frank SJ, Zhou J. Magnetic Resonance Imaging of Glucose Uptake and Metabolism in Patients with Head and Neck Cancer. Sci Rep 2016;6:30618. [Crossref] [PubMed]
- Wu D, Xu J, Lei J, McLane M, van Zijl PC, Burd I. Dynamic glucose enhanced MRI of the placenta in a mouse model of intrauterine inflammation. Placenta 2018;69:86-91. [Crossref] [PubMed]
- van Zijl PCM, Yadav NN. Chemical exchange saturation transfer (CEST): What is in a name and what isn't? Magn Reson Med 2011;65:927-48. [Crossref] [PubMed]
- Jiang W, Zhou IY, Wen L, Zhou X, Sun PZ. A theoretical analysis of chemical exchange saturation transfer echo planar imaging (CEST-EPI) steady state solution and the CEST sensitivity efficiency-based optimization approach. Contrast Media Mol Imaging 2016;11:415-23. [Crossref] [PubMed]
- Sun PZ, Lu J, Wu Y, Xiao G, Wu R. Evaluation of the dependence of CEST-EPI measurement on repetition time, RF irradiation duty cycle and imaging flip angle for enhanced pH sensitivity. Phys Med Biol 2013;58:N229-40.
- Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med 2009;61:1441-50. [Crossref] [PubMed]
- Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG 2006;113:580-9. [Crossref] [PubMed]
- Holme AM, Roland MC, Lorentzen B, Michelsen TM, Henriksen T. Placental glucose transfer: a human in vivo study. PLoS One 2015;10:e0117084. [Crossref] [PubMed]
- Luo J, Ji Y, Abaci Turk E, Zhou IY, Roberts DJ, Grant PE, Sun PZ. Feasibility of glucose CEST in the human placenta. Proc Intl Soc Mag Reson Med 2017. Available online: https://cds.ismrm.org/protected/17MPresentations/abstracts/0104.html
- Simegn GL, Van der Kouwe AJW, Robertson FC, Meintjes EM, Alhamud A. Real-time simultaneous shim and motion measurement and correction in glycoCEST MRI using double volumetric navigators (DvNavs). Magn Reson Med 2019;81:2600-13. [Crossref] [PubMed]
- Zaiss M, Herz K, Deshmane A, Kim M, Golay X, Lindig T, Bender B, Ernemann U, Scheffler K. Possible artifacts in dynamic CEST MRI due to motion and field alterations. J Magn Reson 2019;298:16-22. [Crossref] [PubMed]
- Yasinska IM, Sumbayev VV. S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett 2003;549:105-9. [Crossref] [PubMed]
- National Center for Health Statistics. The One-hour oral glucose tolerance test. DHEW Publication, 1973.
- Gore JC, Brown MS, Mizumoto CT, Armitage IM. Influence of glycogen on water proton relaxation times. Magn Reson Med 1986;3:463-6. [Crossref] [PubMed]
- Yadav NN, Xu J, Bar-Shir A, Qin Q, Chan KW, Grgac K, Li W, McMahon MT, van Zijl PC. Natural D-glucose as a biodegradable MRI relaxation agent. Magn Reson Med 2014;72:823-8. [Crossref] [PubMed]


