Prenatal ultrasound heating impacts on fluctuations in haematological analysis of Oryctolagus cuniculus
Introduction
After the invention and development of an ultrasound (US) machine in late 1950s, it has been used for diagnostic purposes throughout the developed world in various specialties and most obviously in obstetrics and gynecology field (1,2). Diagnostic US was utilized in screening for pregnant women and it was extensively used for decades (1-3). However, the increase of power outputs and extended time of scans along with weaker regulations for the use of US machine has caused it to become increasingly difficult to ignore the contribution of increasing fetal US exposure during recent decades (3-5). Thus, the potential thermal and non-thermal insults or injury are also increased (1,3,4,6). In the official statements of the American Institute of Ultrasound in Medicine (AIUM) advocates the prudent use of prenatal US is strongly restricted for nonmedical usage as such for entertainment purposes including fetal viewing, obtaining fetal images or even fetal gender screening without clinical indications (7).
There are several sources that might contribute into knowledge or information regarding US bioeffects, including the mechanisms of action of US, epidemiological studies in human, experimental studies in animal (in vivo) and cell studies (in vitro) (8). Two components in the mechanisms of action of US which produce bioeffects are heating and cavitation. The parameter that determines the estimation of temperature tissue rise is thermal index (TI) and it should be kept at less than 0.5 as proposed by Barnett et al. (9) for nonmedical use of US as such for entertainment purposes without clinical indications. While the parameter that determines the likelihood of cavitation occurring is mechanical index (MI), which should be at less than 0.4 in medical use (10) and as low as 0.3 in nonmedical use (9). TI and MI are not sole indicators responsible for thermal and non-thermal bioeffects that occur following to US insonation, but these indexes are currently accepted as potential risk’s estimators (11).
The primary concern of diagnostic US in obstetrical examination is tissue heating (1,12). The heating effects are the results of the absorption of US energy from an US beam (13). The heating effect is highly dependent on absorption coefficient of insonated tissue and because bones have high absorption coefficient values (12-14), it tends to absorb 60 percents or more of the incident US energy. During 3rd trimester of pregnancy, fetal bones are almost completed since it has reached the final stage of organogenesis. The surrounded soft tissues are the responsible area for an inevitable rise of temperature during insonation (13) which can go up to a factor of five (4). It is considered hazardous when embryo and fetus have been exposed to US for five minutes or more, as this will elevate both embryonic and fetal temperature to 4 °C above the normal body temperature (12). Large body of evidence has also proven that heat stimulates the abnormalities in studied animal and the sensitivity varies throughout gestational stages. For example, neuronal tube closure and neurogenesis of central nervous system have been noted to become hypersensitive to thermal injury (4,15,16) and even 2 °C elevation of temperature may develop anomaly or defect (17).
The non-thermal effect (mechanical effect) of US insonation causal to acoustic cavitation is defined as the production of bubbles in liquid that may exhibit behavioral of collapse and contribute to sudden release of energy (8,12). Mechanical effect is also regarded as any biological effect from US when accompanied by elevation of temperature less than 1 °C above normal physiologic levels (18). It might then disrupt the molecular bonds and release free radicals that consequently interfere with DNA (deoxyribonucleic acid) of the cells and lead to chromosomal damage (12,19). As there are thermal and non-thermal effects of US, hence this study aimed to assess the association between US exposure time and blood constituents such as red blood cell (RBC) count, white blood cell (WBC) count, haemoglobin (Hb) concentration and platelet (PLT) count in newborns of Oryctolagus cuniculus.
Materials and methods
This in vivo experimental study involved the use of eight female New Zealand White rabbits (Oryctolagus cuniculus). Two does (female rabbit) served as controlled group while the rest were categorized as treated group. The research was carried out in Animal Handling Research Laboratory, Universiti Teknologi MARA (UiTM), Puncak Alam Campus. The ethical approval was obtained from Committee on Animal Research and Ethics (UiTM CARE).
All pregnant does were treated equally with all external factors were kept constant (surrounding temperature: 28 °C, humidity: 60-65%). Good ventilation was provided by using BioGS air purifier, from RabbitAir, USA to evacuate harmful gases released by rabbits such as CO2 (carbon dioxide) and NH3 (ammonia) (20,21). This was critical to prevent incidental confounding factors that might affect the validity of subjects since the goal was to access the heating effect of US to a fetus in a womb.
US exposures of 30, 60 and 90 minutes were used as a teratogen, given prenatally to pregnant does at the middle of 3rd trimester pregnancy, gestational day (GD) 28-29. Gestational period was varied between 31-33 days (22). For control group, no US exposure was given. This study used Philips HD3 US machine from Koninklijke Philips N.V, Netherlands 2D operating at B-mode pulsed-US.
With an exception of the lengths of exposure time (e.g., 30, 60, 90 minutes), US parameters such as transducer frequency, focal distance, TI and MI were kept constant. Transducer of linear array, L 5-9 with the ability to transmit frequency between 5 until 9 MHz was used. Focal distance was kept constant at 4.5 cm throughout the experiments. The displayed TI and MI values were 0.2 and 1.0, respectively. The output power and intensities, spatial peak temporal average intensity (ISPTA) of US were varied from 0.4 to 0.7 W and 0.13 to 0.19 W/cm2 respectively when measured in water for durations of 30, 60 and 90 minutes of US exposures. The parameter of interest were usually determined in free-field experimental conditions where there were without reflectors or other disturbances to an ultrasonic field (23). When soft tissue replaced water along the ultrasonic pathways in researcher’s real experiment involving animal subject as a model, a decrease in intensity was expected, mainly because of soft tissues had much higher rate of attenuation as compared to water (24,25) and also with the probability of fetal bones presented in the studied area.
Forty-eight newborns were analyzed for full blood count (FBC) using haematology analyzer Coulter LH 500 from Beckman Coulter Inc. United States. Blood were drained by method of cardiac puncture using 23G sterile needle and 3 mL sterile syringe to subjects and collected in 5 mL purple cap EDTA (Ethylene Diamine Tetraacetic Acid) tubes. The tubes were gently mixed by inverting the specimens 5-10 times and were placed on automatic rotator for 15 minutes for the blood to properly mix with the anticoagulant content of EDTA tube wall. Then, they were transferred to the haematological laboratory in 2-8 °C to stabilize the blood for complete blood count analysis. The analysis must be performed within 24 hours (4 °C) after the blood withdrawal for the purpose of obtaining reliable and accurate results (26). The parameters that had been taken into account were RBC count, WBC count, Hb concentration and PLT count.
Statistical Package for the Social Sciences, SPSS version 21.0 was used to analyze the data, to make inference and draw robust conclusions.
Results
The descriptive statistic was initially done to evaluate the distribution, normality and homogeneity of the data. Table 1 summarized the results of descriptive statistic. For the test of normality, The Shapiro-Wilk statistic showed that most of P-values (marked as * in Table 1) were more than 0.05 (P>0.05), which indicated the insignificant value, and hence the normality had been assumed. Gaussian distribution in histograms showed normal distribution in all groups of US exposure time except for groups in WBC count. Skewness and kurtosis values were within range of –2 to 2 in all groups of US exposure time except for groups in WBC count. Lastly, test of homogeneity of variances was also carried out to make sure that the population variances were not differed among groups of US exposure time. Levene statistic showed that P-value (marked as * in Table 1) was not significant (P>0.05), and hence equal variance had been assumed. Therefore, it can be concluded that the variances were homogeneous among groups of US exposure time in WBC count. Researcher decided to use parametric tests since all the assumptions were not violated.
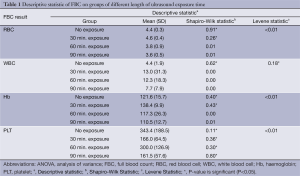
Full Table
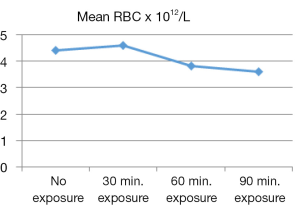
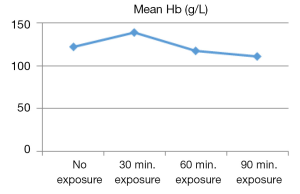
From the descriptive statistics the calculated mean was displayed in graphical view. According to Figures 1-3, the mean RBC count, WBC count and Hb concentration reached its peaked at 30 minutes of US exposure group, and later gradually decreased along the length with increasing US exposure time. Figure 4 showed that the mean PLT count was the highest at no US exposure group, and then it fluctuated before dropped to its lowest value at 90 minutes of US exposure group.
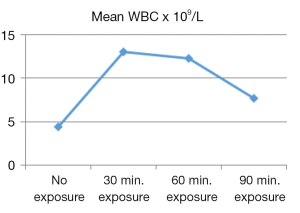

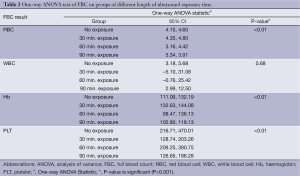
Further statistical analysis was carried out to compare the mean between groups. One way analysis of variance (ANOVA) test was carried out (refer to Table 2). In the readings of RBC, Hb and PLT between groups of US exposure time, P-values were <0.01 (P<0.001). These results suggested that there are significant differences in RBC count, Hb concentration and PLT count in groups of different US exposure time. However, P-value for WBC count between groups of US exposure time was 0.68 (P>0.05) which demonstrated no significant difference in WBC count in groups of different US exposure time.
Full Table
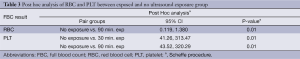
The significant values from ANOVA test suggested that at least one pair among the groups were significantly different. Since the reference values for the statistical test are the group without US exposure, it needs to be confirmed that which group is differed from the predetermined reference range in no US exposure group. Subsequent post hoc analysis using Scheffe procedure was carried out (refer to Table 3). Scheffe test suggested that the mean RBC count was significantly differed between group of “no exposure and 90 min. exposure” with P-value of 0.01 (P<0.05). While, the mean PLT count was significantly differed between group of “no exposure and 30 min. exposure” and “no exposure and 90 min. exposure” with P-value of 0.01 each (P<0.05).
Full Table
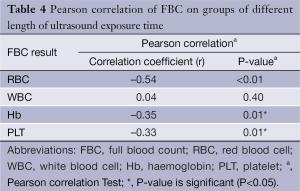
To correlate both dependent and independent variables, correlation statistics were carried out (refer to Table 4). Correlation between RBC count and the groups of different length of US exposure time revealed Pearson’s correlation coefficient; r, was –0.54 and P-value was <0.01 (P<0.05). There is significant good negative relationship (correlation) between groups and RBC count. Therefore, longer US exposure time is associated with lower RBC count. However the correlation between WBC and the groups of different length of US exposure time revealed Pearson’s correlation coefficient; r, was 0.04 and P-value was 0.40 (P>0.05). There is no significant relationship (correlation) between the groups and WBC count. Therefore, length of US exposure time is not associated with WBC count. For Hb concentration and the groups of different length of US exposure time, result of Pearson’s correlation coefficient; r, was –0.35 and P-value was 0.01 (P<0.05). There is fair significant negative relationship (correlation) between the groups and Hb concentration. Therefore, longer US exposure time is associated with lower Hb concentration. Lastly, correlation between PLT count and the groups of different length of US exposure time revealed Pearson’s correlation coefficient; r, was –0.33 and P-value was 0.01 (P<0.05). This result indicates fair significant negative relationship (correlation) between the groups and PLT count, therefore longer US exposure time is associated with lower PLT count.
Full Table
Discussion
This study showed that the RBC count, Hb concentration and PLT count were significantly differed among exposed and controlled group. The mean RBC count and Hb concentration were documented as the lowest in 90 minutes US exposure group when compared to 60 minutes US exposure, 30 minutes US exposure and no US exposure groups. RBC count and Hb concentration reported to have a considerable variation throughout period of life (27,28). They might be higher immediately after birth with the probability of cessation of umbilical artery and uterine contractions of a mother resulting blood in placenta reentering the infant’s circulation (26). This condition was seen in the group without US exposure since the mean value for RBC count and Hb concentration were still in the reference range, while they were peaked in 30 minutes US exposure group before gradually fell to lowest value in 90 minutes US exposure group. This might suggest cells destruction due to US heating that creates blood cells haemolysis in newborn of Oryctolagus cuniculus. Poikilocytosis is a damage of RBCs after its formation and may be a result from extrinsic factor by drugs, chemicals, toxins, heat or abnormal mechanical forces (26). In this case US exposure is a reliable source of delivering heat and also creating mechanical forces, as has been discussed earlier.
As supported by S. B Barnett, slight increase in temperature of embryonic and fetal above normal body temperature even at 4 °C elevation for five minutes of US exposure or more was considered hazardous (12). Damage to erythrocytes, haemolysis were even reported upon microwave heating in blood cells (29). In haemolysis, blood cell will become microcytic and together can develop iron deficiency (26). Hb is an iron containing compound that transports oxygen in blood. This explains why Hb concentration is also affected when RBCs undergo haemolysis. Furthermore the results of these findings were also supported by earlier research done by S.M. Dom. It was found that RBC mean value was significantly differed when US exposure was given at 3rd trimester pregnant Oryctolagus cuniculus as compared to control group with no US exposure (30). More US energy was absorbed and converted to heat by bones that had high absorption coefficient (12-14). As the insonation was given during 3rd trimester of pregnancy, the amount of fetal bones were at the highest at this time as they were highly developed in which complete organogenesis in fetus had occurred.
PLT count was at highest at no US exposure group, and then it fluctuated before decreased to its lowest value at 90 minutes US exposure group. A dramatic increased in PLT count at 60 minutes US exposure group suggested that bodily mechanism of PLT had taken its action in defending the inflammatory stress or bleeding occurrence denoted by a decreased in RBC count and Hb concentration at certain heat stress delivered by an US exposure.
Conclusions
The findings of this in vivo experimental study suggest that there are effects on newborn of Oryctolagus cuniculus’s full blood count constituents, such as RBC count, Hb concentration and PLT count when prenatal US exposure have been given on 3rd trimester pregnant female Oryctolagus cuniculus. This scientific information can be applied in the field of US application for its safety and prudent use. This result can also be utilised to inspire other researchers to further investigate diseases that are anticipated to be associated later in life among those who had been exposed to prenatal US during fetal age.
Acknowledgements
The authors would like to acknowledge the Research Management Institute (RMI) of Universiti Teknologi MARA, Shah Alam, Malaysia for the Research Intensive Faculty (RIF) grant awarded (600-RMI/DANA 5/3/RIF(229/2012).
Disclosure: The authors declare no conflict of interest.
References
- Chau MT. Safety of diagnostic ultrasound. Medical Section. Queen Mary Hospital, Hong Kong 2002;7:3-4.
- Kieler H, Cnattingius S, Haglund B, et al. Sinistrality--a side-effect of prenatal sonography: a comparative study of young men. Epidemiology 2001;12:618-23. [PubMed]
- Miller MW, Brayman AA, Abramowicz JS. Obstetric ultrasonography: a biophysical consideration of patient safety--the “rules” have changed. Am J Obstet Gynecol 1998;179:241-54. [PubMed]
- Church CC, Miller MW. Quantification of risk from fetal exposure to diagnostic ultrasound. Prog Biophys Mol Biol 2007;93:331-53. [PubMed]
- Duck FA. Safety aspects of the use of ultrasound in pregnancy. Fetal and Maternal Medicine Review 2003;14:1-21.
- Deane C. Safety of diagnostic ultrasound in fetal imaging. In: Nicolaides K, Rizzo G, Hecker K, et al. eds. Doppler in Obstetrics, 2002.
- Official Statements: Prudent Use in Pregnancy. Available online: http://www.aium.org/officialStatements/33, 2012
- Kremkau FW. Performance and safety. In: Diagnostic Ultrasound Principles and Instruments, 6th ed. Philadelphia, USA: Saunders, 2002:335-51.
- Barnett SB, Abramowicz JS, Ziskin MC, et al. WFUMB Symposium on Safety of Nonmedical Use of Ultrasound. Ultrasound Med Biol 2010;36:1209-12. [PubMed]
- Barnett SB, Duck F, Ziskin M. Recommendations on the safe use of ultrasound contrast agents. Ultrasound Med Biol 2007;33:173-4. [PubMed]
- Abramowicz JS, Kossoff G, Marsal K, et al. Safety Statement 2000 (reconfirmed 2003). Ultrasound Obstet Gynecol 2003;21:100. [PubMed]
- Barnett SB. Key issues in the analysis of safety of diagnostic ultrasound. ASUM Ultrasound Bull 2003;6:41-3.
- Gent R. Biological effects and safety of diagnostic ultrasound. In: Applied Physics and Technology of Diagnostic Ultrasound, 1st ed. Australia: Milner, 1997:301-16.
- Rapid Response Group, Executive Board of the International Society of Ultrasound in Obstetrics and Gynecology. Safety statement, 2000. International Society of Ultrasound in Obstetrics and Gynecology (ISUOG). Ultrasound Obstet Gynecol 2000;16:594-6. [PubMed]
- Edwards MJ, Saunders RD, Shiota K. Effects of heat on embryos and foetuses. Int J Hyperthermia 2003;19:295-324. [PubMed]
- Miller MW, Nyborg WL, Dewey WC, et al. Hyperthermic teratogenicity, thermal dose and diagnostic ultrasound during pregnancy: implications of new standards on tissue heating. Int J Hyperthermia 2002;18:361-84. [PubMed]
- Health Protection Agency-Radiation, Chemical and Environmental Hazards, “Health effect of exposure to ultrasound and infrasound: Report of the independent advisory group on non-ionising radiation”, 2010 (Online). Available online: http://www.hpa.org.uk. (Accessed Jan. 14, 2012).
- Stratmeyer ME, Greenleaf JF, Dalecki D, et al. Fetal ultrasound: mechanical effects. J Ultrasound Med 2008;27:597-605. [PubMed]
- National Council on Radiation Protection and Measurements (NCRP), Diagnostic Ultrasound Safety: A summary of NCRP technical report no. 140: Exposure Criteria for Medical Diagnostic Ultrasound: II. Criteria Based on all Known Mechanisms, 2002 (Online). Available online: http://www.ncrponline.org/Publications/Reports/Misc_PDFs/UltrasoundSummary--NCRP.pdf. (Accessed Jan. 8, 2012).
- Lebas F, Coudert P, De Rochambeau H, et al. The rabbit husbandry, health and production, 1997.
- Lebas F, Coudert P, Rouvier R, et al. The rabbit: husbandry, health and production,” in Food and Agriculture Organization (FAO) Animal Product and Health Series, (Online). Available online: http://www.fao.org/docrep/X5082E/X5082E00.HTM (Accessed Dec. 29, 2010).
- Palmer AK. Spontaneous malformations of the New Zealand White rabbit: the background to safety evaluation tests. Laboratory Animal 1968;2:195-206.
- Hedrick WR, Hykes DL, Starchman DE. Intensity and power. In: Ultrasound Physics and Instrumentation, 4th ed. Philadelphia, USA: Elsevier, 2005:294-300.
- Hedrick WR, Hykes DL, Starchman DE. Clinical safety. In: Ultrasound Physics and Instrumentation, 4th ed. United States of America: Elsevier Mosby, 2005:317-32.
- Miller DL. Safety assurance in obstetrical ultrasound. Semin Ultrasound CT MR 2008;29:156-64. [PubMed]
- Lewis SM, Bain BJ, Bates I. Basic Haematological Techniques. In: Practical Haematology, 10th ed. Philadelphia: Elsevier, 2006:25-57.
- Lilleyman JS, Hann IM, SBlanchette V. Pediatric Hematology, 2nd ed. London: Churchill Livingstone, 1999.
- Ozbek N, Gürakan B, Kayiran SM. Complete blood cell counts in capillary and venous blood of healthy term newborns. Acta Haematol 2000;103:226-8. [PubMed]
- Hirsch J, Menzebach A, Welters ID, et al. Indicators of erythrocyte damage after microwave warming of packed red blood cells. Clin Chem 2003;49:792-9. [PubMed]
- Dom SM. Teratogenic effect of diagnostic ultrasound exposure on rabbit foetal physiological development related to bone. Ph.D dissertation, Medical Imaging Department, Universiti Teknologi MARA, Kuala Lumpur, 2011.


