Imaging the aged brain: pertinence and methods
Biological aged brain
Aging has become the next global public health challenge (1). For the first time in history, the number of people aged 65 years and older in the world outnumbers children aged younger than 5 years (2-4). A total of 10–20% of people aged 60–80 years are estimated to have one of three neurological diseases, including stroke (5,6), Alzheimer’s disease (7), and Parkinson’s disease (8,9). The notion that the aged brain differs from the young brain is not a new one. Intuition alone emphasizes notable differences in maturation, processing speed, and baseline function between the preadolescent and the elderly brain. In order to advise on healthy ageing, it is critical to first understand the fundamental biological differences between the young and old brain. The anatomical aged brain is marked by distinct changes in brain volume, general cognition, vasculature, and neurovascular coupling (10) (Figure 1). For the purposes of this concise review paper, the young brain is considered to be a preadolescent brain before neural pruning; the elderly brain is considered to be in the middle stages of degradation around the US senior citizen average age of 62. Further, imaging discussed in this paper considers both healthy and degenerative disease affected aging.
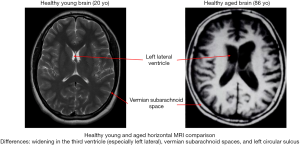
The volume and size of the brain is one of the most obvious targets of age. Over time, the aged brain loses 5% of its volume with each decade past the age of 40, with this rate of mass loss often escalating after the age of 70 (10-18). Additionally, there is specific volume loss in white matter tracts and myelin degeneration (19-26). Loss of white matter tracts behaves in a “last-in-first-out” manner beginning in posterior, frontal, and parietal sections of the brain and first degrading tracts that typically form towards the end of puberty (19). Of the subdivisions of the brain, the prefrontal cortex and striatum seem to be most affected by this age-induced deterioration while the occipital lobe remains the least affected (10,27). The hippocampus also suffers degradation, and this can be linked to a vast number of age-related memory disorders (10,28-31). Substantial loss in brain volume over time heavily impacts imaging and statistical significance when a healthy aged brain is compared to a structurally different, healthy adolescent brain.
The aged brain also presents differences in cognition. Incorrectly attributed to decreased volume, cognitive decline is better explained by changes in dendritic shape and connection (32-36). Deterioration of dendritic spine length or shape heavily impacts the functionality of neural networks and can be linked to age related impairments in processing and integrating information (32,36). Even discounting aged related disease, the aged brain has stark differences in cognition and processing that impact elderly citizens in society. The biggest changes in cognition occur in reasoning, episodic and semantic memory tasks, and integration speed (37-42). Most notably, younger brains perform better in tasks that require problem-solving or adaptable application of skills, whereas older brains perform better at tasks that require accessing stored information or applying an often practiced skill (37).
Change in vasculature and blood pressure in the brain is the third most notable effect of age (43-50). Blood vessels become more fragile with time and use and become vulnerable to white matter lesions, clots, and tears (51). Atrophy and grey matter loss can additionally result in higher blood pressure (10,52-55). Changes in blood vessel size and durability are associated with increased risk of dementia, Alzheimer’s disease, and stroke (10). These changes should be paid special attention in imaging because they are present even in the normal ageing process and can be indicators of predisposition for age related degenerative diseases.
Finally, even in healthy ageing, the brain has reduced increases in blood flow at moments of key firing and in response to various stimulations. This phenomenon, named neurovascular coupling or functional hyperemia, is the brain’s method of supplying sufficient amounts of oxygen and vital nutrients via blood flow during elevated brain activity and activation (56). The study of the relationship between blood micro-vessels and astrocytes is paramount in the analysis of healthy ageing. Healthy ageing also impacts both elements of this mechanism. It has been suggested that dysfunction in both the astrocyte end of calcium signaling and the blood vessel mechanism through cellular oxidative stress or IGF-1 deficiency could affect the efficiency of neurovascular coupling in the brain (56). This mechanism has particular importance in brain imaging because functional magnetic resonance imaging (fMRI) fundamentally relies on measuring this speed of reactance. The blood oxygenation level dependent (BOLD) signal in fMRI is heavily related to the magnitude of oxygenation in the brain and, in aged brains, the decreased neurovascular coupling can be used as an indicator of age-related neurodegenerative diseases (57). Indeed, neurovascular coupling has already been considered in a large number of aged brain imaging and research (58-61).
Challenges of imaging the aged brain
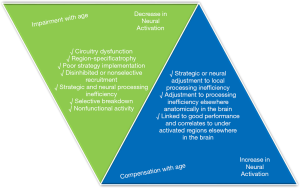
As discussed above, the rate, type, and general presentation of ageing presents drastically differently in each person, in health or in disease. This makes standardizing imaging and designing experiments that are widely applicable a very challenging task (Figure 2). For example, MRI is arguably one of the most powerful forms of imaging in anatomical and functionality studies that relies heavily on BOLD signals (63-69). However, changes in blood vasculature and neuronal structure of the brain can be misinterpreted in MRI as activity or lack thereof in functionality studies (70,71). The standard hemodynamic response function (HRF) is fit to data for younger brains and applying this standard to a different vasculature system can result in a less accurate fit and misleading result (70). This issue also manifests itself in structural standards to identify aging-induced anomaly. Spatial normalization is applied in imaging to account for small differences in structure between individuals. However, the standard will continue to bend a participant’s images to match the HRF which can lead to overestimation errors in structural analysis (70). In fact, all statistical analysis using young brain data must fundamentally warp the data of older brains because of the identified structural and functional differences. This presents the big challenge of lacking a proper standard against which to compare healthy aged brains.
Beyond the functional challenges, there are also many participant and involvement based challenges when imaging elderly volunteers. Health becomes a larger factor in activity of elderly adults, so running self-selecting studies could result in bias due to which adults feel healthy enough to participate (37,72-74). Participants with decreased mobility or limited independence will by nature of their living circumstances be underrepresented in such studies. Since the participant pool is limited by health and mobility among other factors, the problem of getting a statistically relevant sample size then arises (73).
Similarly, longitudinally assessing healthy ageing through imaging tools becomes increasingly difficult when studying elderly adults. Longitudinal studies are the most helpful tool in quantifying change over time, but they require multiple visits or attempts at a task with a healthy participant, and this is not always the easiest parameter to achieve (37,75-82). Participants are always at risk of developing a cognitive or physical impairment during the course of a study even if they began the trial neurologically and physically fit. Such consistency is hard to achieve due to the changing nature of physical health and the long symptomatic manifestation time of cognitive disorders.
Impact (meeting the need)
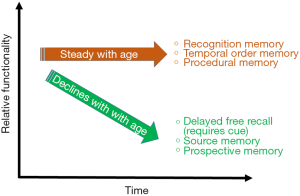
Increasingly, our society is becoming more and more flushed with elderly citizens. With better health care and longer lifespans, the number of Americans over the age of 65 is projected to reach 88.5 million in the year 2050 (37). Healthy ageing is taking center stage as society must learn how to cater to this new generation of older adults who are experiencing cognitive decline despite being free of degenerative disorders. These changes to their cognition, for example memories as shown in Figure 3, however small, are impacting the daily lives of an increasing number of people and efforts must be made to further understand healthy ageing in order to cater to the day to day lives of the elderly.
Imaging the aged brain would not only give us better standards against which to create the benchmark of healthy cognitive ageing, but it would also reveal more about the connectivity and functional methods of the brain. If certain functions are discovered to be lost over time due to degeneration, more could be illuminated about the functionality of both older and younger brains. Especially on the technological brink of imaging, more information on the structure, function, and working principles of the brain can only help in establishing both the standard of and deviations from healthy brain function.
Technologies for imaging aged brains
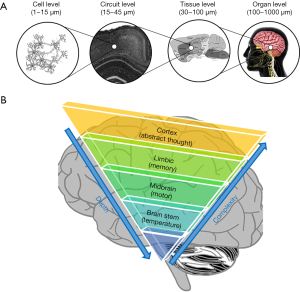
The human brain is the most complex, powerful and mysterious organ of the human body (83,84). Although scientists have been avidly discovering the secrets of the brain, the knowledge accumulated so far still falls far short of a comprehensive understanding. Thanks to modern biomedical imaging technologies, our understanding of the brain has advanced over the last few decades at an accelerated speed (85,86). Looking back, the history of neuroscience is also a history of applying new imaging technologies to look at the brain in a more informative way. However, imaging the human brain is also the most challenging application for many imaging technologies because the brain functions as a highly-coordinated system with functional connections at various spatial scales ranging from the single cell level (e.g., within a cortical circuit) to the tissue level (e.g., between cortex and hippocampus) (Figure 4). Large efforts are currently supported by the NIH BRAIN Initiative to image brain functions at different scales and to understand the relevance of its dynamics during development, aging, and in disease (87,88). Human brain mapping has become one of the most exciting contemporary research areas with major breakthroughs expected in the following decades. So far, many imaging technologies have been applied for imaging aged brains in preclinical studies and clinical practice (89-95). These technologies can be grouped into three major categories based on their spatial resolutions and corresponding maximum imaging depths: microscopic imaging, mesoscopic imaging, and macroscopic imaging. Here, we will briefly introduce the representative imaging technologies in each group, together with their strengths and limitations in brain imaging.
Microscopic brain imaging
At the microscopic level (<10 µm), optical imaging has been the dominating player, providing cellular and subcellular images of brain structures and functions, especially at the neuronal level (96-102). Taking advantage of the short wavelengths of photons, optical imaging, including confocal and multiphoton microscopy (103-107), can achieve a spatial resolution on the level of ~1 µm, which is sufficient to resolve single neurons and even dendrites, the basic communication units of the brain. Moreover, optical imaging can provide rich image contrast by using a large library of exogenous optical labels such as fluorescent dyes, quantum dots, and genetically encoded fluorescent proteins. These optical labelling tools have been widely applied to small animal brain imaging providing direct or indirect measurements of the brain’s morphological and functional status, including neuronal connection, hemodynamics, action potential firing, and signal transmission (Figure 5A). One important example is non-invasive real-time optical reading of the brain’s neuronal activities based on calcium or voltage-sensitive indicators (112-117). The advantage of optical imaging over electrode recording is the high throughput that supports simultaneous interrogation of thousands of neurons, allowing the study of neural circuits and networks. However, the drawback of optical imaging is also clear: the penetration depth is limited to the superficial brain tissue, typically less than 1 mm into the brain tissue, mainly because of the strong optical scattering of the tissue. Multi-photon microscopy takes advantage of the longer excitation wavelengths and has achieved a penetration depth of 1.5 mm (118-121). Nevertheless, optical imaging is mainly used for small animal brain imaging, such as on fruit flies, zebra fish, and mice. Invasive methods have also been developed to circumvent the imaging depth by inserting miniaturized optics into the brain tissue (122,123), which, however, may induce undesired damage to the brain functions.
Mesoscopic imaging
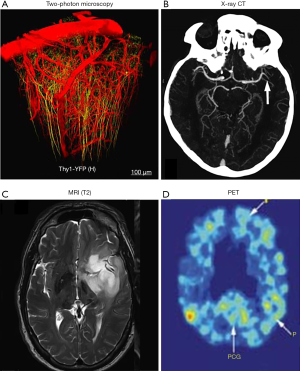
On the mesoscopic scale (10 µm–1 mm), several imaging modalities have been used for brain imaging, including X-ray, CT, MRI, and ultrasound imaging. Mesoscopic imaging can provide structural and functional information on the neural circuit level, and more importantly, deep penetration into the brain. In particular, X-ray CT and MRI are routinely used for human brain imaging in clinical practice (124,125), allowing simultaneous mapping of the whole brain including structures such as the gray and white matter volumes as well as tissue density (Figure 5B,C). More advanced technologies such as contrast-enhanced X-ray CT and MRI have been used in imaging brain vasculature in neurological diseases such as stroke, AD, and brain tumor (126,127). The advent of in vivo diffusion tensor imaging (DTI) allows direct measurement of the bulk tissue microstructure ordering by virtue of mapping water proton motions within the tissue microenvironment (128). DTI has been playing an important role in studying the aged brain, especially in studies on neurodegenerative processes that cause changes at the microstructural level through the rate of myelination or demyelination, degradation of microtubules, or loss of axonal structure (129). Ultrasound imaging is not typically used for the brain, with the skull as a physical barrier to the acoustic waves (130,131). However, using low frequency ultrasound around 2 MHz, transcranial Doppler ultrasound imaging is able to measure the cerebral blood flow through the skull’s acoustic windows, including temporal, orbital, suboccipital, and submandibular windows (132). Transcranial Doppler ultrasound has been applied to study blood flow velocity, arterial pulsatility, and resistance with aging. The results have collectively shown that cerebrovascular hemodynamics may carry important implications in vascular diseases associated with advanced age, increased risk of cerebrovascular disease, cognitive decline, and dementia.
Macroscopic imaging
At the macroscopic level (1 mm to 1 cm), fMRI (133-135), PET (136-139), and diffuse optical tomography (DOT) (140-143) are the major imaging techniques being used to study brain function and metabolism. Based on different contrast mechanisms, all these imaging techniques can provide the macroscopic functional status of the brain in the resting state and under stress. fMRI is sensitive to the blood oxygenation dependent signals, which are closely correlated with neuronal activities through neurovascular coupling. As a totally noninvasive imaging modality, fMRI has been the most popular tool in studying the cognitive decline in both diseased and aged brains and has shown that healthy aging reduces the cerebral hemodynamic responses to visual challenges (144). It has also shown that the brain’s resting state activities are significantly different in normal aging, mild cognitive impairment, and Alzheimer’s disease (145). PET relies on the accumulation of radiolabeled tracers to map the brain’s metabolism status and other important pathophysiological indicators. Despite the low resolution and the ionizing radiation, PET has been a powerful tool in studying the brain’s normal aging process and neurodegenerative diseases, with its high sensitivity and specificity. For example, PET has been increasingly used in studying the rate of accumulation of pathological tau in normal aging and Alzheimer’s disease and has shown different tau deposition rates over the whole brain in the early Alzheimer’s disease onsets (111,138) (Figure 5D). DOT has been a relatively new player in functional brain imaging, compared with fMRI and PET. DOT shares the same contrast principle as fMRI, and optically detects the neuronal activities through the brain’s hemodynamic responses. Increased blood volume and oxygenation are two important physiological parameters measured in DOT. DOT typically can provide brain functions only in the neocortical layer, limited by the penetration depth of near-infrared photons through the intact scalp and skull. However, compared with fMRI and PET, DOT is more portable, much faster, and can provide real-time monitoring of brain function. Moreover, DOT is a much less expensive technology. DOT has recently gained more popularity in mapping distributed brain functions and networks (146), such as in patients with Parkinson’s disease and implanted deep brain stimulators that preclude fMRI.
Conclusions and prospects
As the brain ages in health and in disease, there are numerous structural, functional, molecular, and cognitive changes at a wide range of scales from cellular to whole organ levels. These changes are intrinsically interconnected through the extremely complex signal pathways and neural networks in the brain. Alterations of the aging brain can result from multifactorial processes and be reflected by many functional and molecular biomarkers. However, the knowledge accumulated so far still falls short of a comprehensive understanding. The knowledge gap about the functional disruption and remodeling of the aging brain is largely due to a lack of imaging technologies that can provide longitudinal imaging with the required spatial-temporal resolutions and imaging depth (147). Due to relatively low spatial-temporal resolution, MRI and PET are not suited for microscopic studies (148,149). Optical imaging methods lack the penetration depth for accessing the brain regions beyond the cortex, and thus cannot study the spatial heterogeneity of brain damage and restoration (150). Ultrasound imaging still lacks the sensitivity to most brain functions outside of blood flow. Histological examination of brain slices cannot provide functional information. Therefore, to better understand aging brains, we still need an imaging technology that can provide high spatial-temporal resolution, deep penetration, and functional information.
New imaging technologies that harness novel contrast mechanisms may provide new opportunities in studying aged brains. One example is photoacoustic tomography that physically combines light and ultrasound to probe the tissue’s functional and molecular information with balanced spatial resolution, penetration depth, and imaging speed (151-158). Although the skull still acts as a significant barrier for the ultrasound waves, photoacoustic tomography shows great promise in human brain imaging, with advances in light delivery, ultrasound detection, and image reconstruction. Another promising technology is magnetic resonance fingerprinting that permits the non-invasive quantification of multiple important properties of the brain simultaneously with improved sensitivity, specificity, and speed when compared to conventional MRI (159,160). More importantly, when combined with appropriate pattern recognition and data mining methods, magnetic resonance fingerprinting can potentially reduce the cost of the MRI by using lower magnetic field and shorter scanning times (161-163).
Further, to understand the aged brain, one also needs to understand how one region of the brain influences another. The ability to image these changes at different scales will help not only to understand normal brain functional architectures but also how complex diseases disrupt normal brain functions (164,165). For example, we could better understand how normal aging changes the connection between the hippocampus and cerebral cortex to translate the transient cortical neuronal activities (microscopic connection) to long-term memory (macroscopic connection). We may also better understand how Alzheimer’s disease progresses from memory loss in the hippocampus to impaired judgment and reasoning in the cortex. However, there exist substantial barriers in scale and contrast mechanism among the traditional brain imaging modalities, which can study the aged brain only at the microscopic scale (e.g., two-photon microscopy) or the macroscopic scale (e.g., fMRI) with dramatically different signal origins. Correlation between different imaging tools is truly an engineering challenge due to their dramatically different imaging scales and contrast mechanisms. To study the complex brain systems, we need imaging technologies that can simultaneously image the aged brain at both microscopic and macroscopic scales. In other words, to better understand the brain, in vivo brain imaging at different scales needs to be joined to best extract the information. On one hand, imaging systems that can operate at multiple scales have been reported (166), such as ultrasound and optical imaging with multiple detection frequencies or wavelengths, or incorporate multiple modalities, such as the integrated PET and X-ray CT (167). On the other hand, with the fast advances in machine learning technologies (168-170), data fusion among different brain imaging modalities becomes more practical, not only to match the anatomical structures at various scales, but also to correlate the functions at different hierarchy levels.
Acknowledgments
Funding: This work was supported by the Duke MEDx fund, Duke GCB faculty award, AHA collaborative sciences grant 18CSA34080277, and NIH grant R01EB028143 (all to J Yao).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ezeh AC, Bongaarts J, Mberu B. Global population trends and policy options. Lancet 2012;380:142-8. [Crossref] [PubMed]
- Wasay M, Grisold W, Carroll W, Shakir R. World Brain Day 2016: celebrating brain health in an ageing population. Lancet Neurol 2016;15:1008. [Crossref] [PubMed]
- Wen YM. Health in an Aging World: What Should We Do? Engineering 2016;2:40-3. [Crossref]
- Suzman R, Beard JR, Boerma T, Chatterji S. Health in an ageing world-what do we know? Lancet 2015;385:484. [Crossref] [PubMed]
- Ferri CP, Schoenborn C, Kalra L, Acosta D, Guerra M, Huang YQ, Jacob KS, Rodriguez JJL, Salas A, Sosa AL, Williams JD, Liu ZR, Moriyama T, Valhuerdi A, Prince MJ. Prevalence of stroke and related burden among older people living in Latin America, India and China. J Neurol Neurosurg Psychiatry 2011;82:1074-82. [Crossref] [PubMed]
- Sousa RM, Ferri CP, Acosta D, Albanese E, Guerra M, Huang Y, Jacob KS, Jotheeswaran AT, Rodriguez JJ, Pichardo GR, Rodriguez MC, Salas A, Sosa AL, Williams J, Zuniga T, Prince M. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet 2009;374:1821-30. [Crossref] [PubMed]
- Alzheimer's A. 2016 Alzheimer's disease facts and figures. Alzheimers Dement 2016;12:459-509. [Crossref] [PubMed]
- Tison F, Dartigues JF, Dubes L, Zuber M, Alperovitch A, Henry P. Prevalence of Parkinson's disease in the elderly: a population study in Gironde, France. Acta Neurol Scand 1994;90:111-5. [Crossref] [PubMed]
- de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, Fratiglioni L, Lobo A, Martinez-Lage J, Trenkwalder C, Hofman A. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000;54:S21-3. [PubMed]
- Peters R. Ageing and the Brain. Postgrad Med J 2006;82:84-8. [Crossref] [PubMed]
- Bonte S, Vandemaele P, Verleden S, Audenaert K, Deblaere K, Goethals I, Van Holen R. Healthy brain ageing assessed with 18F-FDG PET and age-dependent recovery factors after partial volume effect correction. Eur J Nucl Med Mol Imaging 2017;44:838-49. [Crossref] [PubMed]
- Braskie MN, Boyle CP, Rajagopalan P, Gutman BA, Toga AW, Raji CA, Tracy RP, Kuller LH, Becker JT, Lopez OL, Thompson PM. Physical activity, inflammation, and volume of the aging brain. Neuroscience 2014;273:199-209. [Crossref] [PubMed]
- Vuong P, Drucker D, Schwarz C, Fletcher E, Decarli C, Carmichael O. Effects of T2-Weighted MRI Based Cranial Volume Measurements on Studies of the Aging Brain. Proc SPIE Int Soc Opt Eng 2013;8669.
- Knoops AJ, Gerritsen L, van der Graaf Y, Mali WP, Geerlings MI. Loss of entorhinal cortex and hippocampal volumes compared to whole brain volume in normal aging: the SMART-Medea study. Psychiatry Res 2012;203:31-7. [Crossref] [PubMed]
- Takao H, Hayashi N, Ohtomo K. A longitudinal study of brain volume changes in normal aging. Eur J Radiol 2012;81:2801-4. [Crossref] [PubMed]
- Nagai M, Kario K. Blood pressure, aging, vascular disease, and their effects on brain volume. Am J Hypertens 2009;22:1135. [Crossref] [PubMed]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology 2009;72:1906-13. [Crossref] [PubMed]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol 2008;65:113-20. [Crossref] [PubMed]
- Simon W. Davis NAD, Norbou G. Buchler, Leonard E. White, David J. Madden, Roberto Cabeza. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. NeuroImage 2009;46:530-41. [Crossref]
- Madden DJ, Parks EL, Tallman CW, Boylan MA, Hoagey DA, Cocjin SB, Packard LE, Johnson MA, Chou YH, Potter GG, Chen NK, Siciliano RE, Monge ZA, Honig JA, Diaz MT. Sources of disconnection in neurocognitive aging: cerebral white-matter integrity, resting-state functional connectivity, and white-matter hyperintensity volume. Neurobiol Aging 2017;54:199-213. [Crossref] [PubMed]
- Marstaller L, Williams M, Rich A, Savage G, Burianova H. Aging and large-scale functional networks: white matter integrity, gray matter volume, and functional connectivity in the resting state. Neuroscience 2015;290:369-78. [Crossref] [PubMed]
- King KS, Peshock RM, Rossetti HC, McColl RW, Ayers CR, Hulsey KM, Das SR. Effect of normal aging versus hypertension, abnormal body mass index, and diabetes mellitus on white matter hyperintensity volume. Stroke 2014;45:255-7. [Crossref] [PubMed]
- Papp KV, Kaplan RF, Springate B, Moscufo N, Wakefield DB, Guttmann CR, Wolfson L. Processing speed in normal aging: effects of white matter hyperintensities and hippocampal volume loss. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2014;21:197-213. [Crossref] [PubMed]
- Leritz EC, Shepel J, Williams VJ, Lipsitz LA, McGlinchey RE, Milberg WP, Salat DH. Associations between T1 white matter lesion volume and regional white matter microstructure in aging. Hum Brain Mapp 2014;35:1085-100. [Crossref] [PubMed]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer's disease. Neuroimage 2009;44:1247-58. [Crossref] [PubMed]
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging 2008;29:102-16. [Crossref] [PubMed]
- Chung HK, Tymula A, Glimcher P. The Reduction of Ventrolateral Prefrontal Cortex Gray Matter Volume Correlates with Loss of Economic Rationality in Aging. J Neurosci 2017;37:12068-77. [Crossref] [PubMed]
- Hamezah HS, Durani LW, Yanagisawa D, Ibrahim NF, Aizat WM, Bellier JP, Makpol S, Ngah WZW, Damanhuri HA, Tooyama I. Proteome profiling in the hippocampus, medial prefrontal cortex, and striatum of aging rat. Exp Gerontol 2018;111:53-64. [Crossref] [PubMed]
- Lanke V, Moolamalla STR, Roy D, Vinod PK. Integrative Analysis of Hippocampus Gene Expression Profiles Identifies Network Alterations in Aging and Alzheimer's Disease. Front Aging Neurosci 2018;10:153. [Crossref] [PubMed]
- Adler DH, Wisse LEM, Ittyerah R, Pluta JB, Ding SL, Xie L, Wang J, Kadivar S, Robinson JL, Schuck T, Trojanowski JQ, Grossman M, Detre JA, Elliott MA, Toledo JB, Liu W, Pickup S, Miller MI, Das SR, Wolk DA, Yushkevich PA. Characterizing the human hippocampus in aging and Alzheimer's disease using a computational atlas derived from ex vivo MRI and histology. Proc Natl Acad Sci U S A 2018;115:4252-7. [Crossref] [PubMed]
- Thomsen K, Yokota T, Hasan-Olive MM, Sherazi N, Fakouri NB, Desler C, Regnell CE, Larsen S, Rasmussen LJ, Dela F, Bergersen LH, Lauritzen M. Initial brain aging: heterogeneity of mitochondrial size is associated with decline in complex I-linked respiration in cortex and hippocampus. Neurobiol Aging 2018;61:215-24. [Crossref] [PubMed]
- Dara L., Dickstein DK, Anne B. Rocher, Jennifer I. Luebke, Susan L. Wearne, Patrick R. Hof. Changes in the structural complexity of the aged brain. Aging Cell 2007;6:275-84. [Crossref]
- Boros BD, Greathouse KM, Gearing M, Herskowitz JH. Dendritic spine remodeling accompanies Alzheimer's disease pathology and genetic susceptibility in cognitively normal aging. Neurobiol Aging 2019;73:92-103. [Crossref] [PubMed]
- Le Y, Liu S, Peng M, Tan C, Liao Q, Duan K, Ouyang W, Tong J. Aging differentially affects the loss of neuronal dendritic spine, neuroinflammation and memory impairment at rats after surgery. PLoS One 2014;9:e106837. [Crossref] [PubMed]
- Dickstein DL, Weaver CM, Luebke JI, Hof PR. Dendritic spine changes associated with normal aging. Neuroscience 2013;251:21-32. [Crossref] [PubMed]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci 2011;31:7831-9. [Crossref] [PubMed]
- Harada CN, Natelson Love MC, Triebel KL. Normal Cognitive Aging. Clin Geriatr Med 2013;29:737-52. [Crossref] [PubMed]
- Rizio AA, Diaz MT. Language, aging, and cognition: frontal aslant tract and superior longitudinal fasciculus contribute toward working memory performance in older adults. Neuroreport 2016;27:689-93. [Crossref] [PubMed]
- Negash S, Bennett DA, Wilson RS, Schneider JA, Arnold SE. Cognition and neuropathology in aging: multidimensional perspectives from the Rush Religious Orders Study and Rush Memory And Aging Project. Curr Alzheimer Res 2011;8:336-40. [Crossref] [PubMed]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn Sci 2005;9:496-502. [Crossref] [PubMed]
- Zelinski EM, Kennison RF, Long Beach Longitudinal S. The Long Beach Longitudinal Study: evaluation of longitudinal effects of aging on memory and cognition. Home Health Care Serv Q 2001;19:45-55. [Crossref] [PubMed]
- Sandin M, Jasmin S, Levere TE. Aging and cognition: facilitation of recent memory in aged nonhuman primates by nimodipine. Neurobiol Aging 1990;11:573-5. [Crossref] [PubMed]
- Lee J, Yanckello LM, Ma D, Hoffman JD, Parikh I, Thalman S, Bauer B, Hartz AMS, Hyder F, Lin AL. Neuroimaging Biomarkers of mTOR Inhibition on Vascular and Metabolic Functions in Aging Brain and Alzheimer's Disease. Front Aging Neurosci 2018;10:225. [Crossref] [PubMed]
- Lin CH, Cheng HM, Chuang SY, Chen CH. Vascular Aging and Cognitive Dysfunction: Silent Midlife Crisis in the Brain. Pulse (Basel) 2018;5:127-32. [Crossref] [PubMed]
- Lo RY, Lo YC, Chen SC, Li YY, Yang YL, Chang YL, Sung HC, Chiu THT, Goh JOS. Vascular burden and brain aging in a senior volunteer cohort: A pilot study. Ci Ji Yi Xue Za Zhi 2017;29:91-7. [PubMed]
- Deak F, Freeman WM, Ungvari Z, Csiszar A, Sonntag WE. Recent Developments in Understanding Brain Aging: Implications for Alzheimer's Disease and Vascular Cognitive Impairment. J Gerontol A Biol Sci Med Sci 2016;71:13-20. [Crossref] [PubMed]
- Villeneuve S, Jagust WJ. Imaging Vascular Disease and Amyloid in the Aging Brain: Implications for Treatment. J Prev Alzheimers Dis 2015;2:64-70. [PubMed]
- Marchant NL, Reed BR, Sanossian N, Madison CM, Kriger S, Dhada R, Mack WJ, DeCarli C, Weiner MW, Mungas DM, Chui HC, Jagust WJ. The aging brain and cognition: contribution of vascular injury and abeta to mild cognitive dysfunction. JAMA Neurol 2013;70:488-95. [Crossref] [PubMed]
- Wardlaw JM, Bastin ME, Valdes Hernandez MC, Maniega SM, Royle NA, Morris Z, Clayden JD, Sandeman EM, Eadie E, Murray C, Starr JM, Deary IJ. Brain aging, cognition in youth and old age and vascular disease in the Lothian Birth Cohort 1936: rationale, design and methodology of the imaging protocol. Int J Stroke 2011;6:547-59. [Crossref] [PubMed]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011;77:461-8. [Crossref] [PubMed]
- Rabin JS, Schultz AP, Hedden T, Viswanathan A, Marshall GA, Kilpatrick E, Klein H, Buckley RF, Yang HS, Properzi M, Rao V, Kirn DR, Papp KV, Rentz DM, Johnson KA, Sperling RA, Chhatwal JP. Interactive Associations of Vascular Risk and beta-Amyloid Burden With Cognitive Decline in Clinically Normal Elderly Individuals: Findings From the Harvard Aging Brain Study. JAMA Neurol 2018;75:1124-31. [Crossref] [PubMed]
- Elias MF, Dore GA. Brain indices predict blood pressure control: aging brains and new predictions. Hypertension 2008;52:1014-5. [Crossref] [PubMed]
- Kasper SO, Carter CS, Ferrario CM, Ganten D, Ferder LF, Sonntag WE, Gallagher PE, Diz DI. Growth, metabolism, and blood pressure disturbances during aging in transgenic rats with altered brain renin-angiotensin systems. Physiol Genomics 2005;23:311-7. [Crossref] [PubMed]
- Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol Aging 2000;21:57-62. [PubMed]
- Parnetti L, Mecocci P, Ciuffetti G, Bellomo G, Senin U. Blood pressure and functional aspects of the aging brain. Arch Gerontol Geriatr 1989;9:155-61. [Crossref] [PubMed]
- Tarantini S, Tran C, Gordon G, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer's disease: Contribution of astrocycte dysfunction and endothelilal impairment to cognitive decline. Exp Gerontol 2017;94:52-8. [Crossref] [PubMed]
- Fabiani M, Gordon B, Maclin E, Pearson M, Brumback-Peltz C, Low K, McAuley E, Sutton BP, Kramer AF, Gratton G. Neurovascular coupling in normal aging: A combined optial, ERP, and fMRI study. Neuroimage 2014;85:592-607. [Crossref] [PubMed]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 2006;100:328-35. [Crossref] [PubMed]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci 2005;28:202-8. [Crossref] [PubMed]
- Toth P, Tarantini S, Tucsek Z, Ashpole N, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Reversal treatment resources neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol 2014;306:H299-308. [Crossref] [PubMed]
- Latimer CS, Searcy J, Bridges M, Brewer L, Popović J, Blalock EM, Landfield PW, Thibault O, Porter NM. Reversal of glial and neurovascular markers of unhealthy brain agin by exercise in middle-aged female mice. PLoS One 2011;6:e26812. [Crossref] [PubMed]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol 2005;15:245-51. [Crossref] [PubMed]
- Farina E, Baglio F, Pomati S, D'Amico A, Campini IC, Di Tella S, Belloni G, Pozzo T. The Mirror Neurons Network in Aging, Mild Cognitive Impairment, and Alzheimer Disease: A functional MRI Study. Front Aging Neurosci 2017;9:371. [Crossref] [PubMed]
- Guo H, Siu W, D'Arcy RC, Black SE, Grajauskas LA, Singh S, Zhang Y, Rockwood K, Song X. MRI assessment of whole-brain structural changes in aging. Clin Interv Aging 2017;12:1251-70. [Crossref] [PubMed]
- Coupé P, Catheline G, Lanuza E, Manjon JV. Alzheimer's Disease Neuroimaging I. Towards a unified analysis of brain maturation and aging across the entire lifespan: A MRI analysis. Hum Brain Mapp 2017;38:5501-18. [Crossref] [PubMed]
- Werden E, Cumming T, Li Q, Bird L, Veldsman M, Pardoe HR, Jackson G, Donnan GA, Brodtmann A. Structural MRI markers of brain aging early after ischemic stroke. Neurology 2017;89:116-24. [Crossref] [PubMed]
- Keuken MC, Bazin PL, Backhouse K, Beekhuizen S, Himmer L, Kandola A, Lafeber JJ, Prochazkova L, Trutti A, Schafer A, Turner R, Forstmann BU. Effects of aging on T(1), T(2)*, and QSM MRI values in the subcortex. Brain Struct Funct 2017;222:2487-505. [Crossref] [PubMed]
- Zhang N, Gordon ML, Goldberg TE. Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer's disease. Neurosci Biobehav Rev 2017;72:168-75. [Crossref] [PubMed]
- Hakkers CS, Arends JE, Barth RE, Du Plessis S, Hoepelman AI, Vink M. Review of functional MRI in HIV: effects of aging and medication. J Neurovirol 2017;23:20-32. [Crossref] [PubMed]
- Samanez-Larkin GR, D'Esposito M. Group Comparisons: imaging the aging brain. Soc Cogn Affect Neurosci 2008;3:290-7. [Crossref] [PubMed]
- West KL, Zuppichini MD, Turner MP, Sivakolundu DK, Zhao Y, Abdelkarim D, Spence JS, Rypma B. BOLD hemodynamic response function changes significantly with healthy aging. Neuroimage 2019;188:198-207. [Crossref] [PubMed]
- Rodríguez Laso Á, Urdaneta Artola E, de la Fuente Sánchez M, Galindo Moreno E, Yanguas Lezáun JJ, Rodríguez Rodríguez V. Analysis of selection bias in the pilot study of a longitudinal study on aging in Spain. Gac Sanit 2013;27:425-32. [PubMed]
- Nummela O, Sulander T, Helakorpi S, Haapola I, Uutela A, Heinonen H, Valve R, Fogelholm M. Register-based data indicated nonparticipation bias in a health study among aging people. J Clin Epidemiol 2011;64:1418-25. [Crossref] [PubMed]
- Adam J. Statistical bias in cross-sequential studies of aging. Exp Aging Res 1977;3:325-33. [Crossref] [PubMed]
- Shao Y, Chen ZH, Ming S, Ye Q, Shu ZY, Gong C, Pang PP, Gong XY. Predicting the Development of Normal-Appearing White Matter With Radiomics in the Aging Brain: A Longitudinal Clinical Study. Front Aging Neurosci 2018;10:393. [Crossref] [PubMed]
- Gracien RM, Nurnberger L, Hok P, Hof SM, Reitz SC, Rub U, Steinmetz H, Hilker-Roggendorf R, Klein JC, Deichmann R, Baudrexel S. Evaluation of brain ageing: a quantitative longitudinal MRI study over 7 years. Eur Radiol 2017;27:1568-76. [Crossref] [PubMed]
- Paulsen DJ, Hallquist MN, Geier CF, Luna B. Effects of incentives, age, and behavior on brain activation during inhibitory control: A longitudinal fMRI study. Dev Cogn Neurosci 2015;11:105-15. [Crossref] [PubMed]
- Wu K, Taki Y, Sato K, Qi HC, Kawashima R, Fukuda H. A longitudinal study of structural brain network changes with normal aging. Front Hum Neurosci 2013;7:113. [Crossref] [PubMed]
- Callisaya ML, Beare R, Phan TG, Blizzard L, Thrift AG, Chen J, Srikanth VK. Brain ageing and gait decline in older people - A longitudinal study. Australas J Ageing 2012;31:28.
- van Haren N, Pol HEH, Cahn W, Schnack HG, Brans R, Laponder AJ, Kahn RS. Brain volume changes in patients with schizophrenia: a 5-year longitudinal MRI study across the age range. Eur Neuropsychopharm 2004;14:S65-6. [Crossref]
- van Haren NEM, Pol HEH, Cahn W, Schnack HG, Brans R, Laponder AJ, Kahn RS. Brain volume changes in 109 patients with schizophrenia compared to 130 control subjects: A 5-year longitudinal MRI study across the age range. Schizophr Res 2004;67:96.
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 2003;60:989-94. [Crossref] [PubMed]
- Raichle ME. Functional brain Imaging and human brain function. J Neurosci 2003;23:3959-62. [Crossref] [PubMed]
- Raichle ME. A Paradigm Shift in Functional Brain Imaging. J Neurosci 2009;29:12729-34. [Crossref] [PubMed]
- Insel TR, Landis SC, Collins FS. Research priorities. The NIH BRAIN Initiative. Science 2013;340:687-8. [Crossref] [PubMed]
- Devor A, Bandettini PA, Boas DA, Bower JM, Buxton RB, Cohen LB, Dale AM, Einevoll GT, Fox PT, Franceschini MA, Friston KJ, Fujimoto JG, Geyer MA, Greenberg JH, Halgren E, Hamalainen MS, Helmchen F, Hyman BT, Jasanoff A, Jernigan TL, Judd LL, Kim SG, Kleinfeld D, Kopell NJ, Kutas M, Kwong KK, Larkum ME, Lo EH, Magistretti PJ, Mandeville JB, Masliah E, Mitra PP, Mobley WC, Moskowitz MA, Nimmerjahn A, Reynolds JH, Rosen BR, Salzberg BM, Schaffer CB, Silva GA, So PTC, Spitzer NC, Toote RB, Van Essen DC, Vanduffe W, Vinogradov SA, Wald LL, Wang LV, Weber B, Yodh AG. The Challenge of Connecting the Dots in the BRAIN. Neuron 2013;80:270-4. [Crossref] [PubMed]
- Yuste R. The Origins of the BRAIN Initiative: A Personal Journey. Cell 2017;171:726-35. [Crossref] [PubMed]
- Yuste R, Bargmann C. Toward a Global BRAIN Initiative. Cell 2017;168:956-9. [Crossref] [PubMed]
- Sakai T, Kondo M, Kawana Y, Nakagawa T, Tomimoto H. Clinical Features of Very Elderly Patients Aged 90 Years or Above with Acute Ischemic Stroke: A Study by Using Diffusion Weighted Brain Magnetic Resonance Imaging. Brain Nerve 2017;69:1337-45. [PubMed]
- Lin CC, Barker JW, Sparto PJ, Furman JM, Huppert TJ. Functional near-infrared spectroscopy (fNIRS) brain imaging of multi-sensory integration during computerized dynamic posturography in middle-aged and older adults. Exp Brain Res 2017;235:1247-56. [Crossref] [PubMed]
- Lwin TT, Yoneyama A, Hara A, Ohbu M, Maruyama H, Taguchi M, Esashi S, Matsushima T, Terazaki K, Hyodo K, Takeda T. Spontaneous brain tumor imaging of aged rat by crystal X-ray interferometer-based phase-contrast X-ray CT. Acta Radiol Open 2016;5:2058460115626958. [Crossref] [PubMed]
- Ito K. Functional brain imaging in the aged. Nihon Ronen Igakkai Zasshi 2003;40:586-9. [Crossref] [PubMed]
- Holtkamp M, Buchheim K, Siegmund H, Meierkord H. Optical imaging reveals reduced seizure spread and propagation velocities in aged rat brain in vitro. Neurobiol Aging 2003;24:345-53. [Crossref] [PubMed]
- Wahlund LO, Agartz I, Almqvist O, Basun H, Forssell L, Saaf J, Wetterberg L. The brain in healthy aged individuals: MR imaging. Radiology 1990;174:675-9. [Crossref] [PubMed]
- Imai Y. CT imaging of the aged brain and senile dementia. Nihon Rinsho 1985;43:1428-32. [PubMed]
- Nguyen D, Marchand PJ, Planchette AL, Nilsson J, Sison M, Extermann J, Lopez A, Sylwestrzak M, Sordet-Dessimoz J, Schmidt-Christensen A, Holmberg D, Van De Ville D, Lasser T. Optical projection tomography for rapid whole mouse brain imaging. Biomed Opt Express 2017;8:5637-50. [Crossref] [PubMed]
- Lefebvre J, Castonguay A, Pouliot P, Descoteaux M, Lesage F. Whole mouse brain imaging using optical coherence tomography: reconstruction, normalization, segmentation, and comparison with diffusion MRI. Neurophotonics 2017;4:041501. [Crossref] [PubMed]
- Dai X, Zhang T, Yang H, Tang J, Carney PR, Jiang H. Fast noninvasive functional diffuse optical tomography for brain imaging. J Biophotonics 2018;11. [Crossref] [PubMed]
- Morone KA, Neimat JS, Roe AW, Friedman RM. Review of functional and clinical relevance of intrinsic signal optical imaging in human brain mapping. Neurophotonics 2017;4:031220. [Crossref] [PubMed]
- Gratton G, Chiarelli AM, Fabiani M. From brain to blood vessels and back: a noninvasive optical imaging approach. Neurophotonics 2017;4:031208. [Crossref] [PubMed]
- Afrashteh N, Inayat S, Mohsenvand M, Mohajerani MH. Optical-flow analysis toolbox for characterization of spatiotemporal dynamics in mesoscale optical imaging of brain activity. Neuroimage 2017;153:58-74. [Crossref] [PubMed]
- Zhu X, Xia Y, Wang X, Si K, Gong W. Optical Brain Imaging: A Powerful Tool for Neuroscience. Neurosci Bull 2017;33:95-102. [Crossref] [PubMed]
- Silva AJ. Miniaturized two-photon microscope: seeing clearer and deeper into the brain. Light Sci Appl 2017;6:e17104. [Crossref] [PubMed]
- Sato M, Motegi Y, Yagi S, Gengyo-Ando K, Ohkura M, Nakai J. Fast varifocal two-photon microendoscope for imaging neuronal activity in the deep brain. Biomed Opt Express 2017;8:4049-60. [Crossref] [PubMed]
- Ma Y, Tang Y, Zhao Y, Gao S, Lin W. Two-Photon and Deep-Red Emission Ratiometric Fluorescent Probe with a Large Emission Shift and Signal Ratios for Sulfur Dioxide: Ultrafast Response and Applications in Living Cells, Brain Tissues, and Zebrafishes. Anal Chem 2017;89:9388-93. [Crossref] [PubMed]
- Le VH, Yoo SW, Yoon Y, Wang T, Kim B, Lee S, Lee KH, Kim KH, Chung E. Brain tumor delineation enhanced by moxifloxacin-based two-photon/CARS combined microscopy. Biomed Opt Express 2017;8:2148-61. [Crossref] [PubMed]
- Zong W, Wu R, Li M, Hu Y, Li Y, Li J, Rong H, Wu H, Xu Y, Lu Y, Jia H, Fan M, Zhou Z, Zhang Y, Wang A, Chen L, Cheng H. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nat Methods 2017;14:713-9. [Crossref] [PubMed]
- Kislin M, Sword J, Fomitcheva IV, Croom D, Pryazhnikov E, Lihavainen E, Toptunov D, Rauvala H, Ribeiro AS, Khiroug L, Kirov SA. Reversible Disruption of Neuronal Mitochondria by Ischemic and Traumatic Injury Revealed by Quantitative Two-Photon Imaging in the Neocortex of Anesthetized Mice. J Neurosci 2017;37:333-48. [Crossref] [PubMed]
- Smit EJ, Vonken EJ, Meijer FJ, Dankbaar JW, Horsch AD, van Ginneken B, Velthuis B, van der Schaaf I, Prokop M, Timing-Invariant CT. Angiography Derived from CT Perfusion Imaging in Acute Stroke: A Diagnostic Performance Study. AJNR Am J Neuroradiol 2015;36:1834-8. [Crossref] [PubMed]
- Jha B, Kothari M. Pearls & oy-sters: hyperdense or pseudohyperdense MCA sign: a Damocles sword? Neurology 2009;72:e116-7. [Crossref] [PubMed]
- Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang SC, Satyamurthy N, Phelps ME, Barrio JR. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med 2006;355:2652-63. [Crossref] [PubMed]
- Oscar BG, Liu W, Zhao Y, Tang L, Wang Y, Campbell RE, Fang C. Excited-state structural dynamics of a dual-emission calmodulin-green fluorescent protein sensor for calcium ion imaging. Proc Natl Acad Sci U S A 2014;111:10191-6. [Crossref] [PubMed]
- Qian Y, Piatkevich KD, Mc Larney B, Abdelfattah AS, Mehta S, Murdock MH, Gottschalk S, Molina RS, Zhang W, Chen Y, Wu J, Drobizhev M, Hughes TE, Zhang J, Schreiter ER, Shoham S, Razansky D, Boyden ES, Campbell RE. A genetically encoded near-infrared fluorescent calcium ion indicator. Nat Methods 2019;16:171-4. [Crossref] [PubMed]
- Barykina NV, Subach OM, Piatkevich KD, Jung EE, Malyshev AY, Smirnov IV, Bogorodskiy AO, Borshchevskiy VI, Varizhuk AM, Pozmogova GE, Boyden ES, Anokhin KV, Enikolopov GN, Subach FV. Green fluorescent genetically encoded calcium indicator based on calmodulin/M13-peptide from fungi. PLoS One 2017;12:e0183757. [Crossref] [PubMed]
- Bethge P, Carta S, Lorenzo DA, Egolf L, Goniotaki D, Madisen L, Voigt FF, Chen JL, Schneider B, Ohkura M, Nakai J, Zeng H, Aguzzi A, Helmchen F. An R-CaMP1.07 reporter mouse for cell-type-specific expression of a sensitive red fluorescent calcium indicator. PLoS One 2017;12:e0179460. [Crossref] [PubMed]
- Tada M, Takeuchi A, Hashizume M, Kitamura K, Kano M. A highly sensitive fluorescent indicator dye for calcium imaging of neural activity in vitro and in vivo. Eur J Neurosci 2014;39:1720-8. [Crossref] [PubMed]
- Muschol M, Dasgupta BR, Salzberg BM. Caffeine interaction with fluorescent calcium indicator dyes. Biophys J 1999;77:577-86. [Crossref] [PubMed]
- Kantelhardt SR, Kalasauskas D, Konig K, Kim E, Weinigel M, Uchugonova A, Giese A. In vivo multiphoton tomography and fluorescence lifetime imaging of human brain tumor tissue. J Neurooncol 2016;127:473-82. [Crossref] [PubMed]
- Stutzmann G. Seeing the brain in action: how multiphoton imaging has advanced our understanding of neuronal function. Microsc Microanal 2008;14:482-91. [Crossref] [PubMed]
- Levene MJ, Dombeck DA, Kasischke KA, Molloy RP, Webb WW. In vivo multiphoton microscopy of deep brain tissue. J Neurophysiol 2004;91:1908-12. [Crossref] [PubMed]
- Yoder EJ. In Vivo Microscopy of the Mouse Brain Using Multiphoton Laser Scanning Techniques. Proc SPIE Int Soc Opt Eng 2002;4620:14-29.
- Ohayon S, Caravaca-Aguirre A, Piestun R, DiCarlo JJ. Minimally invasive multimode optical fiber microendoscope for deep brain fluorescence imaging. Biomed Opt Express 2018;9:1492-509. [Crossref] [PubMed]
- Xie Y, Bonin T, Loffler S, Huttmann G, Tronnier V, Hofmann UG. Coronal in vivo forward-imaging of rat brain morphology with an ultra-small optical coherence tomography fiber probe. Phys Med Biol 2013;58:555-68. [Crossref] [PubMed]
- Schmainda KM, Prah MA, Rand SD, Liu Y, Logan B, Muzi M, Rane SD, Da X, Yen YF, Kalpathy-Cramer J, Chenevert TL, Hoff B, Ross B, Cao Y, Aryal MP, Erickson B, Korfiatis P, Dondlinger T, Bell L, Hu L, Kinahan PE, Quarles CC. Multisite Concordance of DSC-MRI Analysis for Brain Tumors: Results of a National Cancer Institute Quantitative Imaging Network Collaborative Project. AJNR Am J Neuroradiol 2018;39:1008-16. [Crossref] [PubMed]
- Messaris GAT, Georgakopoulos DN, Zampakis P, Kalogeropoulou CP, Petsas TG, Panayiotakis GS. Patient dose in brain perfusion imaging using an 80-slice CT system. J Neuroradiol 2018. [Crossref] [PubMed]
- Hatzoglou V, Yang TJ, Omuro A, Gavrilovic I, Ulaner G, Rubel J, Schneider T, Woo KM, Zhang Z, Peck KK, Beal K, Young RJ. A prospective trial of dynamic contrast-enhanced MRI perfusion and fluorine-18 FDG PET-CT in differentiating brain tumor progression from radiation injury after cranial irradiation. Neuro Oncol 2016;18:873-80. [Crossref] [PubMed]
- Merali Z, Wong T, Leung J, Gao MM, Mikulis D, Kassner A. Dynamic contrast-enhanced MRI and CT provide comparable measurement of blood-brain barrier permeability in a rodent stroke model. Magn Reson Imaging 2015;33:1007-12. [Crossref] [PubMed]
- Walker L, Chang LC, Nayak A, Irfanoglu MO, Botteron KN, McCracken J, McKinstry RC, Rivkin MJ, Wang DJ, Rumsey J, Pierpaoli C. Brain Development Cooperative G. The diffusion tensor imaging (DTI) component of the NIH MRI study of normal brain development (PedsDTI). Neuroimage 2016;124:1125-30. [Crossref] [PubMed]
- Zhang S, Ye X, Bai G, Fu Y, Mao C, Wu A, Liu X, Yan Z. Alterations in Cortical Thickness and White Matter Integrity in Mild-to-Moderate Communicating Hydrocephalic School-Aged Children Measured by Whole-Brain Cortical Thickness Mapping and DTI. Neural Plast 2017;2017:5167973. [Crossref] [PubMed]
- Desmidt T, Hachemi ME, Remenieras JP, Lecomte P, Ferreira-Maldent N, Patat F, Camus V. Ultrasound Brain Tissue Pulsatility is decreased in middle aged and elderly type 2 diabetic patients with depression. Psychiatry Res 2011;193:63-4. [Crossref] [PubMed]
- Kamihira S, Honda T, Tonomoto N, Suzuki Y, Ishiguro S, Kuroda H, Sasaki S, Mori T. The influence of aging on cerebral blood flow and oxygen metabolism during moderate hypothermic cardiopulmonary bypass--a clinical study by means of transcranial Doppler ultrasound. Nihon Kyobu Geka Gakkai Zasshi 1994;42:1163-70. [PubMed]
- Topcuoglu MA, Aydin H, Saka E. Occipital cortex activation studied with simultaneous recordings of functional transcranial Doppler ultrasound (fTCD) and visual evoked potential (VEP) in cognitively normal human subjects: effect of healthy aging. Neurosci Lett 2009;452:17-22. [Crossref] [PubMed]
- Chen JJ. Functional MRI of brain physiology in aging and neurodegenerative diseases. Neuroimage 2019;187:209-25. [Crossref] [PubMed]
- Griffanti L, Stratmann P, Rolinski M, Filippini N, Zsoldos E, Mahmood A, Zamboni G, Douaud G, Klein JC, Kivimaki M, Singh-Manoux A, Hu MT, Ebmeier KP, Mackay CE. Exploring variability in basal ganglia connectivity with functional MRI in healthy aging. Brain Imaging Behav 2018;12:1822-7. [Crossref] [PubMed]
- Baciu M, Boudiaf N, Cousin E, Perrone-Bertolotti M, Pichat C, Fournet N, Chainay H, Lamalle L, Krainik A. Functional MRI evidence for the decline of word retrieval and generation during normal aging. Age (Dordr) 2016;38:3. [Crossref] [PubMed]
- Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, Rabinovici GD, Jagust WJ. Alzheimer's Disease Neuroimaging I. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer's disease. Neuroimage 2017;157:448-63. [Crossref] [PubMed]
- Lagarde J, Sarazin M, Chauvire V, Stankoff B, Kas A, Lacomblez L, Peyronneau MA, Bottlaender M. Cholinergic Changes in Aging and Alzheimer Disease: An [18F]-F-A-85380 Exploratory PET Study. Alzheimer Dis Assoc Disord 2017;31:8-12. [Crossref] [PubMed]
- Schöll M, Lockhart SN, Schonhaut DR, O'Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, Rabinovici GD, Jagust WJ. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron 2016;89:971-82. [Crossref] [PubMed]
- Krell-Roesch J, Ruider H, Lowe VJ, Stokin GB, Pink A, Roberts RO, Mielke MM, Knopman DS, Christianson TJ, Machulda MM, Jack CR, Petersen RC, Geda YE. FDG-PET and Neuropsychiatric Symptoms among Cognitively Normal Elderly Persons: The Mayo Clinic Study of Aging. J Alzheimers Dis 2016;53:1609-16. [Crossref] [PubMed]
- Chitnis D, Cooper RJ, Dempsey L, Powell S, Quaggia S, Highton D, Elwell C, Hebden JC, Everdell NL. Functional imaging of the human brain using a modular, fibre-less, high-density diffuse optical tomography system. Biomed Opt Express 2016;7:4275-88. [Crossref] [PubMed]
- Huppert TJ, Franceschini MA, Boas DA. Noninvasive Imaging of Cerebral Activation with Diffuse Optical Tomography. In: Frostig RD. editor. In Vivo Optical Imaging of Brain Function. Frontiers in Neuroscience. Boca Raton (FL): 2009.
- Ferradal SL, Liao SM, Eggebrecht AT, Shimony JS, Inder TE, Culver JP, Smyser CD. Functional Imaging of the Developing Brain at the Bedside Using Diffuse Optical Tomography. Cereb Cortex 2016;26:1558-68. [Crossref] [PubMed]
- Habermehl C, Schmitz CH, Steinbrink J. Contrast enhanced high-resolution diffuse optical tomography of the human brain using ICG. Opt Express 2011;19:18636-44. [Crossref] [PubMed]
- Cliff M, Joyce DW, Lamar M, Dannhauser T, Tracy DK, Shergill SS. Aging effects on functional auditory and visual processing using fMRI with variable sensory loading. Cortex 2013;49:1304-13. [Crossref] [PubMed]
- Liu CY, Krishnan AP, Yan L, Smith RX, Kilroy E, Alger JR, Ringman JM, Wang DJ. Complexity and synchronicity of resting state blood oxygenation level-dependent (BOLD) functional MRI in normal aging and cognitive decline. J Magn Reson Imaging 2013;38:36-45. [Crossref] [PubMed]
- Eggebrecht AT, Ferradal SL, Robichaux-Viehoever A, Hassanpour MS, Dehghani H, Snyder AZ, Hershey T, Culver JP. Mapping distributed brain function and networks with diffuse optical tomography. Nature Photonics 2014;8:448. [Crossref] [PubMed]
- Magistretti PJ, Allaman I. A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron 2015;86:883-901. [Crossref] [PubMed]
- Jain V, Langham MC, Wehrli FW. MRI estimation of global brain oxygen consumption rate. J Cereb Blood Flow Metab 2010;30:1598-607. [Crossref] [PubMed]
- Hu S, Wang LV. Photoacoustic imaging and characterization of the microvasculature. Journal of Biomedical Optics 2010;15:011101. [Crossref] [PubMed]
- Kerr JND, Denk W. Imaging in vivo: watching the brain in action. Nat Rev Neurosci 2008;9:195-205. [Crossref] [PubMed]
- Wang LV, Yao J. A practical guide to photoacoustic tomography in the life sciences. Nat Methods 2016;13:627-38. [Crossref] [PubMed]
- Yao J, Wang L, Yang JM, Maslov KI, Wong TT, Li L, Huang CH, Zou J, Wang LV. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat Methods 2015;12:407-10. [Crossref] [PubMed]
- Yao J, Wang LV. Photoacoustic tomography: fundamentals, advances and prospects. Contrast Media Mol Imaging 2011;6:332-45. [Crossref] [PubMed]
- Yao J, Xia J, Maslov KI, Nasiriavanaki M, Tsytsarev V, Demchenko AV, Wang LV. Noninvasive photoacoustic computed tomography of mouse brain metabolism in vivo. Neuroimage 2013;64:257-66. [Crossref] [PubMed]
- Estrada H, Huang X, Rebling J, Zwack M, Gottschalk S, Razansky D. Virtual craniotomy for high-resolution optoacoustic brain microscopy. Sci Rep 2018;8:1459. [Crossref] [PubMed]
- Deán-Ben XL, Gottschalk S, Sela G, Shoham S, Razansky D. Functional optoacoustic neuro-tomography of calcium fluxes in adult zebrafish brain in vivo. Opt Lett 2017;42:959-62. [Crossref] [PubMed]
- Deán-Ben XL, Sela G, Lauri A, Kneipp M, Ntziachristos V, Westmeyer GG, Shoham S, Razansky D. Functional optoacoustic neuro-tomography for scalable whole-brain monitoring of calcium indicators. Light Sci Appl 2016;5:e16201. [Crossref] [PubMed]
- Olefir I, Mercep E, Burton NC, Ovsepian SV, Ntziachristos V. Hybrid multispectral optoacoustic and ultrasound tomography for morphological and physiological brain imaging. J Biomed Opt 2016;21:86005. [Crossref] [PubMed]
- Badve C, Yu A, Rogers M, Ma D, Liu Y, Schluchter M, Sunshine J, Griswold M, Gulani V. Simultaneous T1 and T2 Brain Relaxometry in Asymptomatic Volunteers using Magnetic Resonance Fingerprinting. Tomography 2015;1:136-44. [Crossref] [PubMed]
- Ma D, Jiang Y, Chen Y, McGivney D, Mehta B, Gulani V, Griswold M. Fast 3D magnetic resonance fingerprinting for a whole-brain coverage. Magn Reson Med 2018;79:2190-7. [Crossref] [PubMed]
- Ma D, Pierre EY, Jiang Y, Schluchter MD, Setsompop K, Gulani V, Griswold MA. Music-based magnetic resonance fingerprinting to improve patient comfort during MRI examinations. Magn Reson Med 2016;75:2303-14. [Crossref] [PubMed]
- Ye H, Ma D, Jiang Y, Cauley SF, Du Y, Wald LL, Griswold MA, Setsompop K. Accelerating magnetic resonance fingerprinting (MRF) using t-blipped simultaneous multislice (SMS) acquisition. Magn Reson Med 2016;75:2078-85. [Crossref] [PubMed]
- European Society of Radiology (ESR). Magnetic Resonance Fingerprinting - a promising new approach to obtain standardized imaging biomarkers from MRI. Insights Imaging 2015;6:163-5. [Crossref] [PubMed]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SARB, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A 2010;107:4734-9. [Crossref] [PubMed]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron 2008;57:634-60. [Crossref] [PubMed]
- Hogstrom LJ, Guo SM, Murugadoss K, Bathe M. Advancing multiscale structural mapping of the brain through fluorescence imaging and analysis across length scales. Interface Focus 2016;6:20150081. [Crossref] [PubMed]
- Salmon E, Bernard Ir C, Hustinx R. Pitfalls and Limitations of PET/CT in Brain Imaging. Semin Nucl Med 2015;45:541-51. [Crossref] [PubMed]
- Chen T, Mu J, Xue Q, Yang L, Dun W, Zhang M, Liu J. Whole-brain structural magnetic resonance imaging-based classification of primary dysmenorrhea in pain-free phase: a machine learning study. Pain 2019;160:734-41. [Crossref] [PubMed]
- Sartori JM, Reckziegel R, Passos IC, Czepielewski LS, Fijtman A, Sodre LA, Massuda R, Goi PD, Vianna-Sulzbach M, Cardoso TA, Kapczinski F, Mwangi B, Gama CS. Volumetric brain magnetic resonance imaging predicts functioning in bipolar disorder: A machine learning approach. J Psychiatr Res 2018;103:237-43. [Crossref] [PubMed]
- Lemm S, Blankertz B, Dickhaus T, Muller KR. Introduction to machine learning for brain imaging. Neuroimage 2011;56:387-99. [Crossref] [PubMed]