Treatment response prediction of rehabilitation program in children with cerebral palsy using radiomics strategy: protocol for a multicenter prospective cohort study in west China
Introduction
Cerebral palsy (CP) is a motor disorder accompanied by disturbances of sensation, cognition, communication, perception, and behavior, by epilepsy, and by secondary musculoskeletal problems (1). There is a substantial public health cost with the economic burden estimated at USD$900,000 per person in the USA and Europe (2), highlighting the importance of effective intervention in CP. The effective treatment should be directed at stimulating the child’s development with the aim to achieve maximal independence in activities of daily life. Rehabilitation strategies are typically multidisciplinary. Conventional rehabilitation programme includes physical therapy (PT) and occupational therapy (OT). PT is an integral part of CP treatment. Although its effectiveness in promoting physical function is uncertain, PT aids in encouraging caretakers to learn how best to handle, toilet, wash, and feed their children and to promote posture, mobility, and transfer. OT seeks to improve function, but focus on maximizing a child’s ability to accomplish activities of daily life, education, and work (3-5). Although these are particularly helpful in maximizing available hand function, the efficiency of conventional treatment in children at individual level is still undetermined.
Advancement in the magnetic resonance imaging (MRI) techniques in recent decades provides an unique opportunity to search for functional biomarkers for CP. Equipped also with advanced radiomics, researchers have made unprecedented progress in discovering biomarkers that can increasingly enable identification of individual patients (6-9). However, it is still much more challenging to identify patients who will have effective treatment response (10,11). Using these biomarkers to make individual predictions of future treatment responses in the early phase of CP will be clinically important.
In the proposed study, we aim to predict the individual responses to the treatment. The results of this study may represent an important step towards the development of translational tools in personalized treatment approaches for treatment in newly diagnosed CP in the future.
Objectives
Primary objective
This study aims to develop and validate an individual-based model for prediction of treatment response based on MRI and clinical measures in children with CP.
Secondary objective
To establish a practical method for automatic detection and segmentation of periventricular white matter injury (PWMI). Cortical thickness (CT), fractional anisotropy (FA), and related network measures (structural covariance network based on CT, and DTI network based on FA) are being quantified.
Methods
Study design and rationale
Study design
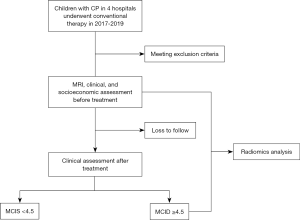
This study will be implemented as a multicenter prospective cohort study. The study has been registered with clinicaltrials.gov (NCT02979743). This study will be reported in accordance with the standard protocol items: recommendations for clinical trials (SPIRIT) (12). The flow chart of this study protocol is shown in Figure 1.
Study setting
The First Affiliated Hospital of Xi’an Jiaotong University (No. 277, Yanta West Road, Xi’an City, Shaanxi Province, China); Xi’an Brain Disease Hospital of Traditional Chinese Medicine (No. 368, Kuangshan Road, Xi’an City, Shaanxi Province, China); The Affiliated Hospital of Zunyi Medical University (No. 149, Dalian Road, Huichuan District, Zunyi City, Guizhou Province, China); The First Affiliated Hospital of Henan University of Traditional Chinese Medicine (No. 19, Renmin Road, Zhengzhou City, Henan Province, China).
Ethics and dissemination
All participating centers have approved the study protocol. This study has been approved by the Institutional Review Board of the First Affiliated Hospital of Xi’an Jiaotong University (KYLLSL-2015-179-01). An informed consent form will be obtained from the subjects’ parents or guardians. Participating families will receive detailed summaries of the results. The results of this study will be presented at national and international conferences and submitted to peer-reviewed journals. Subjects will have the freedom to withdraw from the study at any time. Since this study is supported by the national foundation, the access to the full protocol, participant-level data, and statistical code will be decided by the government.
Participants
The children will be recruited from inpatients at the four above-mentioned hospitals. The anticipated date for this study is from January 2017 to December 2019.
Inclusion criteria
Children presenting with all the following conditions will be considered for enrollment into this study: (I) diagnosis of spastic CP by pediatric neurologists; (II) aged 6 to 72 months; (III) GMFCS and MACS levels I–III; (IV) PWMI as described on the MRI imaging report; (V) following the conventional rehabilitation programme.
Exclusion criteria
Children presenting with any one or more of the following will be excluded from this study: (I) receiving other treatments (i.e., orthopedic surgery, or BoNT-A injections within 6 weeks) may affect the efficacy; (II) accompanied by other diseases (i.e., congenital muscular disease, hereditary disease, progressive central nervous system diseases, cancer, severe heart disease, or severe infectious disease); (III) insufficient cooperation or cognitive understanding to participate in the assessment; (IV) contraindication to MRI or obvious MRI image artifacts.
Withdrawal criteria
Subjects will be withdrawn from the study at the discretion of the investigator or the sponsor if judged non-compliant with study procedures or if there are safety concerns.
Data collection
Upon screening, the following data will be collected: demographic data (sex, age, body weight and side of injury); medical history; concomitant medications (e.g., epilepsy, intellectual disability); findings from a complete physical examination and neurological examination; neuropsychological assessment; and family socioeconomic status. Inclusion and exclusion criteria will be assessed. Structural brain MRI will be carried out if the patient meets the inclusion and exclusion criteria. These details will be collected for identification of prediction factors and to help establish predictive variables.
A follow-up assessment will be scheduled after 3 months of treatment. Data from the following examinations will be collected: physical and neurological examinations; neuropsychological assessment.
Data monitoring
All participants will be evaluated by the trained therapists and undergo brain MRI scan. Imaging data will be checked for quality and protocol conformity after each scanning session. Clinical symptoms and MRI findings will be input into the electronic data capture system. All documents collected in this study will be stored safely and confidentially. On all study-specific documents, other than the signed consent, the subject will be referred to by number/code, not by name. Study documentation will be archived for a period of 6 years after the study. Data will be statistically analyzed by a professional statistician. Only study members will have access to the data.
Blinding
The radiologists responsible for reporting the MRI results will be blinded to all other imaging results, clinical information, and neurobehavioral scores. The neonatologists carrying out the clinical assessments will be blinded to the MRI findings. Follow-up assessments will be scored by well-trained physical and occupational therapists who are not involved in the treatment and data collection. These therapists will be blinded to the perinatal history, primary assessment, and MRI findings.
Conventional therapy
The children will follow the regular routine of the conventional rehabilitation programme. This includes hand splinting, muscle strengthening and stretching, using neurodevelopmental facilitation techniques, and so on, for 2 hours per day, 5 days per week, for 3 months. The conventional treatments can include PT and OT. PT is a crucial part of CP treatment. PT helps caretakers to learn how best to handle, toilet, wash, and feed their children and to promote posture, mobility, and transfer. OT focuses on improving functions, and seeks to maximize a child’s ability to accomplish activities in daily life, education, and work (3-5). These are particularly useful in maximizing available hand function. The children undergo rehabilitation according to their doctors’ clinical care, but they do not receive any intentional rehabilitation program. During the period, they can freely start, change or cease any conventional treatments.
Outcome measures
Primary outcome measures
The primary outcome is the change in value of motor scales at the third month from a baseline measured by the Peabody Developmental Motor Scales, Second Edition (PDMS-2). PDMS-2 is a standardized and norm-referenced test used for the assessment of upper limb functions. It includes gross motor (reflexes, stationary, locomotion, and object manipulation) and fine motor (grasping and visual-motor integration) functions. The PDMS-2 scores have good test-retest reliability (intraclass correlation coefficients =0.88–1.00), with sensitivity-to-change coefficients ranging from 1.6 to 2.1, and responsiveness coefficients ranging from 1.7 to 2.3 (13). The patients will be divided into two groups according to minimal clinically important difference (MCID) (group A ≥4.5, group B <4.5) (14,15). The MCID focuses on the change scores from pretreatment to post-treatment, and provides the smallest change that is important by a respondent.
Secondary outcome measures
The secondary outcome measures include neurobehavioral development scores: Gross Motor Function Measure (GMFM) score, Gross Motor Function Classification System (GMFCS), Manual Ability Classification System (MACS), Hand Assessment for Infants (HAI), Assisting Hand Assessment (AHA), Pediatric Evaluation of Disability Inventory (PEDI), and neuroplasticity in vivo MRI.
- GMFM score: the GMFM is a widely accepted scale used to evaluate gross motor function that can quantitatively assess the dysfunction and developmental delay of patients with CP. The scale comprises five dimensions divided by 88 items, with a total score of 264 (16). This tool has been validated in the Chinese population (17,18).
- GMFCS: the GMFCS is a five-level classification system, with level I indicating the best voluntary movement and level V representing the worst voluntary movement (19,20). This tool has been validated in the Chinese population (17).
- MACS score: the MACS measures five levels of the abilities of children with CP to use their hands to handle objects in daily activities, with level I indicating that objects are handled easily and successfully and level V indicating that the child is not able to handle objects or to complete even simple actions with his/her hands (21-23).
- HAI: HAI is the first assessment tool that can quantify hand function in infants. It is valid for measuring bilateral hand use and quantifying side difference between hands in infants between 3 and 12 months corrected age (24,25).
- AHA: AHA is a hand function evaluation instrument, which measures and describes how children with an upper limb disability in one hand use his/her affected hand (assisting hand) collaboratively with the non-affected hand in bimanual play. The AHA measures how effectively the affected hand and arm is used in bimanual performance. The assessment is performed by observing the child's spontaneous handling of toys in a relaxed and playful session. This makes the AHA a measure of usual performance. AHA has been frequently used to assess motor function in children with bilateral and unilateral SCP because of good inter-rater reliability (26-28).
- PEDI score: the PEDI assesses key functional capabilities and performance in children of 6 months to seven years of age (29). This tool has been validated in the Chinese population (30).
The secondary outcome measure will also include neuroplasticity changes, as measured by MRI. MRI is a powerful translational imaging technique able to extract functional, structural, and biochemical information from the entire brain (31). Specifically, the brain structural change, including CT, surface area, and volume, will be measured. White matter microstructure, measured by diffusion tensor imaging, will be assessed.
Acquisition of MRI images
Brain MRI will be performed using 3.0-T scanners with 8-channel head coils at baseline. All children will be required to sleep soundly and to wear sponge earplugs for hearing protection. Each subject’s sleeping routine will be adjusted in order to reduce motion artifacts and ensure a complete MRI examination. The subject’s head will be immobilised using moulded foam. The children who can not remain still will be sedated with 10% chloral hydral (25–50 mg/kg) to reduce motion artifacts during the MRI examination. The potential risks of the chloral hydrate will be fully explained to the parents of the children. The patient selection, monitoring, and management will be performed in strict compliance with the guidelines (32). Heart rate, transcutaneous oxygen saturation, and respiration rate will be monitored throughout the procedure.
The brain MRI will be performed using an optimized protocol with 3-T MRI scanners across the study site. Three-dimensional fast spoiled gradient-recalled echo (3D-FSPGR) T1WI and T2-FLAIR (fluid attenuated inversion recovery) will be performed with an eight-channel head coil (Table 1). A single-shot echo planar imaging sequence will be performed for the acquisition of DTI (Table 2). The total scan time will be approximately 20 minutes. Providing that children are well prepared for the examination, the completion rate for all three sequences is close to 90% at The First Affiliated Hospital of Xi’an Jiaotong University, so this protocol should be suitable for implementation at the other study sites. Each subject’s vital signs will be monitored during the MRI examination; if an adverse event occurs, the scan will be stopped immediately with intervention depending on the circumstances. The details of the protocol used at each participating site are shown in Tables 1,2.
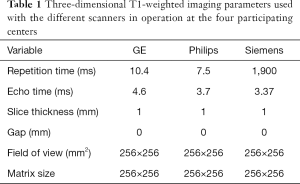
Full table
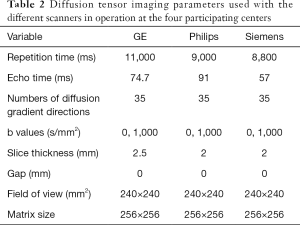
Full table
Structural MRI image analysis
T1-weighted structural images will be preprocessed using SPM12 and computational anatomy toolbox (CAT) based on MATLAB (33,34). CT will be calculated. The main steps are summarized as follows. The original T1 high-resolution anatomical images will be visually checked to ensure that there is no obvious artifact, incomplete scan, or poor contrast, then these images will be format-converted and manually reoriented. The format-converted and reoriented T1 images will be entered in preprocessing pipeline in CAT12 with the default parameters, except for the brain template using a pediatric template for 6- to 12-year-old children from the Imaging Research Center at Cincinnati Children’s Hospital Medical Center (CCHMC), USA. Briefly, this automated method allows for central surface reconstruction and CT measurement in one step, then the topological defects of cortical surface mesh will be repaired by using a spherical harmonic method. Prior to the statistical analyses, the individual CT maps will be smoothed by using a Gaussian filter with full-width at half-maximum of 15 mm (35). We will compute structural covariance network based on interregional CT correlation and graph-theoretical analysis toolbox.
Diffusion MRI image analysis
DTI-derived FA and mean diffusivity maps will be obtained after the brain extraction and the eddy current correction by using FMRIB’s Diffusion Toolbox (FDT) in the FMRIB’s Software Library (FSL) (36). FA will be estimated from pre-processed diffusion data using a diffusion tensor model. The diffusion metrics of bilateral corticospinal tract (CST), posterior thalamic radiation (PTR), genu of corpus callosum (GCC) and splenium of corpus callosum (SCC) will be quantified. Whole-brain voxel-based analysis of diffusion parameters will be performed using optimised tract-based spatial statistics (37). Deterministic tractography will be performed using automated fiber quantification (38). The structural network of the brain will be constructed based on the diffusion data, including cortical parcellation, tractography, and generation of a connectivity matrix (39).
Harmonization
Because of multi-site neuroimaging studies, there is a need for handling non-biological variance introduced by differences in MRI scanners and acquisition protocols. Such unwanted sources of variation, which we refer to as "scanner effects", can hinder the detection of imaging features associated with clinical covariates of interest and cause spurious findings. In this study, we will use ComBat (40,41), a technique adopted from the genomics literature and recently applied to diffusion tensor imaging data, to combine and harmonize CT values across scanners and the unwanted inter-site variability in FA and MD maps.
Sample size
The statistical power measures are calculated using the G*Power 3.1.9.2 Version (Christian-Albrechts-University, Kiel, Germany) (42,43). Power calculations has been performed using the observed mean (0.592, 0.584) and standard deviation of FA (0.02, 0.01) of affected corticospinal tract between patients who respond vs. don't respond to intervention (44). Significant improvement (responders) on FA is defined as greater than 5% as described pre-intervention. Based on these data, sample sizes of 104 are required to obtain a statistical power of 95% with a significance level of 5% and a maximum dropout rate of 20%. A total of 130 children will be enrolled.
Statistical analysis
Statistical analysis will be conducted using SPSS 20.0 (IBM, Armonk, NY, USA). Continuous variables will be expressed as the mean ± SD if normally distributed and median (range) if not. Categorical variables will be presented as number and percentage. Mann-Whitney U test with the Bonferroni correction will be used to assess the structural and diffused imaging differences between treatment responders and non-responders. The spearman correlation coefficient will be used to associate the selected features and clinical indices (e.g., perinatal history, clinical information, and socioeconomic status) with the response/nonresponse outcome. Missing data will be handled using the full information maximum likelihood in the estimation of path models, which is robust against biases introduced by data missing at random. Models will be constructed to identify predictors of the treatment response. Variables that are statistically significant (P<0.05) in univariable analyses will be entered in multivariable models. To control scanner effects, which is a potential source of variation, our multivariable models will contain confounder variable represents different scanners along with patient’s age and gender. The results will be corrected for multiple comparisons using a Monte Carlo simulation, with 10,000 iterations (family-wise error).
Radiomics pipeline (Figure 2), (II) first, the regions of interest (ROIs) will be drawn to segment periventricular white matter lesions, which is defined as T2 hyperintense areas on T2-FLAIR images, using ITK-SNAP Version 3.6.0 (45). The ROIs will be drawn by a neuroradiologist (5 years’ experience in radiology), and confirmed by another neuroradiologist (10 years’ experience in radiology). (II) Second, radiomics features of ROIs will be extracted using the Artificial Intelligence Kit Version 3.0.1A (Life sciences), which is a software of GE Healthcare (46). Radiomics features will include histograms, form factor parameters, grey-level co-occurrence matrix (GLCM), and grey-level run-length matrix (GLRLM). The extracted features will be selected by the analysis of variance and Mann–Whitney U-test, correlation analysis, Spearman’s correlation, and the LASSO in sequence. (III) Third, the whole dataset will be divided into a “Training Cohort” and a “Test Cohort” with a proportion of 7:3. A model will be built by a multivariable logistic regression analysis, using the optimal characteristic parameters of dimension reduction by LASSO. (IV) Fourth, the selected combination of features will be used to define models with 10-fold stratified cross-validation on the training cohort. Several classification algorithms will be evaluated using: random forests (RF), support vector machine (SVM), K-nearest neighbors (KNN), and logistic regression (LR). The parameters of those estimators will be optimized by cross-validated grid-search. The selected classifiers will be trained with the whole dataset and applied on the test set using the same preprocessing method. The cross-validation step will be repeated 100 times with shuffled folds composition. Receiver operating characteristic (ROC) curves will be used to assess the classification validity of the models for differentiation responders from non-responders. The significance levels of all ROC analyses will be tested (47). The thresholds for sensitivity, specificity, positive and negative predictive values will be determined by Youden J statistics.
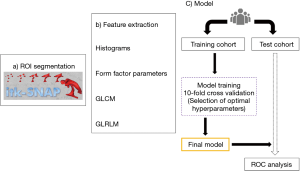
Inter and intra-observer ICC of the two neuroradiologists will be assessed. Finally, we will increase the number of features that are included in the model in a forward stepwise fashion according to their P-value at univariate analysis and we will calculate the corresponding classifiers test metrics. A nomogram will be developed for visualisation of an individual-level prediction model. Significance levels will be set to 0.05.
Confidentiality
The original data will be preserved by The First Affiliated Hospital of Xi’an Jiaotong University, China. Unless required by law, patient information will not be publicly disclosed divulged. Anonymized study data will be published for scientific purpose.
Trial status
This trial is currently recruiting participants.
Discussion
This protocol will combine brain MRI and clinical information to establish an individual-based model for prediction of treatment response in children with CP.
This study will have some important strengths. It will use an advanced radiomics method to extract significant imaging and clinical features for development of a model that can predict the treatment response in CP children at an individual level. Most of the previous studies of CP have been retrospective and included relatively small sample sizes. Furthermore, researchers have focused mainly on MRI changes in microstructure caused by the treatment. Previous studies have suggested that the location and volume of lesions and certain structural parameters can predict adverse motor or cognitive outcomes in these children (48-50). However, all those studies compared group-level data, and the predictive values reported need further validation. This study will provide an individual-based model for prediction of treatment response via a prospective cohort design, which can be expected to contribute to decision-making for intervention. Moreover, the combination of multiple modalities in our study, rather than relying on a single modality, will improve our ability to predict treatment response in children with CP.
An individualized MRI examination procedure for CP children has been developed and implemented in the four hospitals to ensure safety and feasibility. In addition, the neurological assessments are widely used in routine clinical practice. With the multi-site neuroimaging studies, there is a need for methods that can overcome the non-biological variance introduced by differences in MRI scanners and acquisition protocols between centers. These sources of bias can hinder the detection of features associated with the clinical covariates of interest and lead to spurious findings (51-54). The ComBat method has been found to be an effective harmonization technique that both removes unwanted variation introduced by different sites in multi-center studies and preserves biological variation in the data (51,52). In addition, the brain pathogenic patterns of CP differs significantly (55), which makes lesion segmentation more challenging. Furthermore, post-processing of MRI images of CP brain is difficult (56). Thus we will attempt to apply a deep learning algorithm to deal better with this critical issue. Finally, this study is expected to develop an automatic detection and segmentation pipeline for CP by multiple training and testing of manually applied labels on FLAIR. This method will not only help in rapid identification of the responsible lesions, but also provide a new reference to post-processing of images for other pediatric brain diseases.
The study has several limitations. Although CP can be associated with motor, cognitive, and visual impairments, in this study we mainly focus on upper limb function for improving the sensitivity, specificity, and accuracy of our prediction model. We plan to investigate other deficits in the future based on the readouts developed in this protocol. Functional MRI has great potential for characterizing typical development and abnormality (57). However, given the poor ability of children to tolerate MRI scanning, functional MRI is not included in our present study.
Acknowledgments
The authors would like to thank Dr. Guanceng Qu, Associate Professor Jingwei Ma (Shaanxi Provincial Rehabilitation Hospital), Dr. Dan Wen, Dr. Junwei Liu, and Professor Tijiang Zhang (Affiliated Hospital of Zunyi Medical University) for their contributions to this protocol and kind support to this study.
Funding: This work was supported by grants from the National Key Research and Development Program of China (2016YFC0100300), the National Natural Science Foundation of China (81771810, 81171317, and 81471631), the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438), and the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University (XJTU1AF-CRF-2015-004).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of the First Affiliated Hospital of Xi’an Jiaotong University (No. KYLLSL-2015-179-01) and written informed consent was obtained from all patients.
References
- Oskoui M, Coutinho F, Dykeman J, Jette N, Pringsheim T. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol 2013;55:509-19. [Crossref] [PubMed]
- Eunson P. The long-term health, social, and financial burden of hypoxic-ischaemic encephalopathy. Dev Med Child Neurol 2015;57 Suppl 3:48-50. [Crossref] [PubMed]
- Gulati S, Sondhi V. Cerebral Palsy: An Overview. Indian J Pediatr 2018;85:1006-16. [Crossref] [PubMed]
- Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, Stumbles E, Wilson SA, Goldsmith S. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol 2013;55:885-910. [Crossref] [PubMed]
- Plasschaert VFP, Vriezekolk JE, Aarts PBM, Geurts ACH, Van den Ende CHM. Interventions to improve upper limb function for children with bilateral cerebral palsy: a systematic review. Dev Med Child Neurol 2019;61:899-907. [Crossref] [PubMed]
- Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue R, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017;14:749-62. [Crossref] [PubMed]
- Hou Z, Yang Y, Li S, Yan J, Ren W, Liu J, Wang K, Liu B, Wan S. Radiomic analysis using contrast-enhanced CT: predict treatment response to pulsed low dose rate radiotherapy in gastric carcinoma with abdominal cavity metastasis. Quant Imaging Med Surg 2018;8:410-20. [Crossref] [PubMed]
- Mao L, Chen H, Liang M, Li K, Gao J, Qin P, Ding X, Li X, Liu X. Quantitative radiomic model for predicting malignancy of small solid pulmonary nodules detected by low-dose CT screening. Quant Imaging Med Surg 2019;9:263-72. [Crossref] [PubMed]
- Kim S, Shin J, Kim DY, Choi GH, Kim MJ, Choi JY. Radiomics on gadoxetic acid-enhanced magnetic resonance imaging for prediction of postoperative early and late recurrence of single hepatocellular carcinoma. Clin Cancer Res 2019;25:3847-55. [Crossref] [PubMed]
- Schertz M, Shiran SI, Myers V, Weinstein M, Fattal-Valevski A, Artzi M, Ben Bashat D, Gordon AM, Green D. Imaging Predictors of Improvement From a Motor Learning-Based Intervention for Children With Unilateral Cerebral Palsy. Neurorehabil Neural Repair 2016;30:647-60. [Crossref] [PubMed]
- Laporta-Hoyos O, Fiori S, Pannek K, Ballester-Plane J, Leiva D, Reid LB, Pagnozzi AM, Vazquez E, Delgado I, Macaya A, Pueyo R, Boyd RN. Brain lesion scores obtained using a simple semi-quantitative scale from MR imaging are associated with motor function, communication and cognition in dyskinetic cerebral palsy. Neuroimage Clin 2018;19:892-900. [Crossref] [PubMed]
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krležajerić K, Hróbjartsson A, Mann H, Dickersin K, Berlin JA. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann Intern Med 2013;158:200-7. [Crossref] [PubMed]
- Wang HH, Liao HF, Hsieh CL. Reliability, sensitivity to change, and responsiveness of the peabody developmental motor scales-second edition for children with cerebral palsy. Phys Ther 2006;86:1351-9. [Crossref] [PubMed]
- Iyer LV, Haley SM, Watkins MP, Dumas HM. Establishing minimal clinically important differences for scores on the pediatric evaluation of disability inventory for inpatient rehabilitation. Phys Ther 2003;83:888-98. [PubMed]
- Lin KC, Chen HF, Chen CL, Wang TN, Wu CY, Hsieh YW, Wu LL. Validity, responsiveness, minimal detectable change, and minimal clinically important change of the Pediatric Motor Activity Log in children with cerebral palsy. Res Dev Disabil 2012;33:570-7. [Crossref] [PubMed]
- Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S. The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol 1989;31:341-52. [Crossref] [PubMed]
- Shi W, Yang H, Li CY, Zhou MQ, Zhu M, Wang Y, Qian X. Expanded and revised gross motor function classification system: study for Chinese school children with cerebral palsy. Disabil Rehabil 2014;36:403-8. [Crossref] [PubMed]
- Han T, Gray N, Vasquez MM, Zou LP, Shen K, Duncan B. Comparison of the GMFM-66 and the PEDI Functional Skills Mobility domain in a group of Chinese children with cerebral palsy. Child Care Health Dev 2011;37:398-403. [Crossref] [PubMed]
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214-23. [Crossref] [PubMed]
- Salavati M, Krijnen WP, Rameckers EA, Looijestijn PL, Maathuis CG, van der Schans CP, Steenbergen B. Reliability of the modified Gross Motor Function Measure-88 (GMFM-88) for children with both Spastic Cerebral Palsy and Cerebral Visual Impairment: A preliminary study. Res Dev Disabil 2015;45-46:32-48. [Crossref] [PubMed]
- Öhrvall AM, Krumlinde-Sundholm L, Eliasson AC. The stability of the Manual Ability Classification System over time. Dev Med Child Neurol 2014;56:185-9. [Crossref] [PubMed]
- Eliasson AC, Krumlinde-Sundholm L, Rosblad B, Beckung E, Arner M, Ohrvall AM, Rosenbaum P. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol 2006;48:549-54. [Crossref] [PubMed]
- Eliasson AC, Ullenhag A, Wahlström U, Krumlinde-Sundholm L. Mini-MACS: development of the Manual Ability Classification System for children younger than 4 years of age with signs of cerebral palsy. Dev Med Child Neurol 2017;59:72-8. [Crossref] [PubMed]
- Krumlinde-Sundholm L, Ek L, Eliasson AC. What assessments evaluate use of hands in infants? A literature review. Dev Med Child Neurol 2015;57 Suppl 2:37-41. [Crossref] [PubMed]
- Krumlinde-Sundholm L, Ek L, Sicola E, Sjostrand L, Guzzetta A, Sgandurra G, Cioni G, Eliasson AC. Development of the Hand Assessment for Infants: evidence of internal scale validity. Dev Med Child Neurol 2017;59:1276-83. [Crossref] [PubMed]
- Pannek K, Boyd RN, Fiori S, Guzzetta A, Rose SE. Assessment of the structural brain network reveals altered connectivity in children with unilateral cerebral palsy due to periventricular white matter lesions. Neuroimage Clin 2014;5:84-92. [Crossref] [PubMed]
- Kuczynski AM, Carlson HL, Lebel C, Hodge JA, Dukelow SP, Semrau JA, Kirton A. Sensory tractography and robot-quantified proprioception in hemiparetic children with perinatal stroke. Hum Brain Mapp 2017;38:2424-40. [Crossref] [PubMed]
- Hodge J, Goodyear B, Carlson H, Wei XC, Kirton A. Segmental Diffusion Properties of the Corticospinal Tract and Motor Outcome in Hemiparetic Children With Perinatal Stroke. J Child Neurol 2017;32:550-9. [Crossref] [PubMed]
- Feldman AB, Haley SM, Coryell J. Concurrent and construct validity of the Pediatric Evaluation of Disability Inventory. Phys Ther 1990;70:602-10. [Crossref] [PubMed]
- Chen KL, Hsieh CL, Sheu CF, Hu FC, Tseng MH. Reliability and validity of a Chinese version of the Pediatric Evaluation of Disability Inventory in children with cerebral palsy. J Rehabil Med 2009;41:273-8. [Crossref] [PubMed]
- Hamaide J, De Groof G, Van der Linden A. Neuroplasticity and MRI: A perfect match. Neuroimage 2016;131:13-28. [Crossref] [PubMed]
- Coté CJ, Wilson S. Guidelines for Monitoring and Management of Pediatric Patients Before, During, and After Sedation for Diagnostic and Therapeutic Procedures: Update 2016. Pediatr Dent 2016;38:13-39. [PubMed]
- Malone IB, Leung KK, Clegg S, Barnes J, Whitwell JL, Ashburner J, Fox NC, Ridgway GR. Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage 2015;104:366-72. [Crossref] [PubMed]
- Adduru VR, Michael AM, Helguera M, Baum SA, Moore GJ. Leveraging Clinical Imaging Archives for Radiomics: Reliability of Automated Methods for Brain Volume Measurement. Radiology 2017;284:862-9. [Crossref] [PubMed]
- Fischl B, Dale AM. Measuring the Thickness of the Human Cerebral Cortex from Magnetic Resonance Images. Proc Natl Acad Sci U S A 2000;97:11050-5. [Crossref] [PubMed]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23 Suppl 1:S208-19. [Crossref] [PubMed]
- Li X, Gao J, Wang M, Wan M, Yang J. Rapid and reliable tract-based spatial statistics pipeline for diffusion tensor imaging in the neonatal brain: Applications to the white matter development and lesions. Magn Reson Imaging 2016;34:1314-21. [Crossref] [PubMed]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One 2012;7:e49790. [Crossref] [PubMed]
- Huang H, Shu N, Mishra V, Jeon T, Chalak L, Wang ZJ, Rollins N, Gong G, Cheng H, Peng Y, Dong Q, He Y. Development of human brain structural networks through infancy and childhood. Cereb Cortex 2015;25:1389-404. [Crossref] [PubMed]
- Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, Adams P, Cooper C, Fava M, Mcgrath PJ. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 2018;167:104-20. [Crossref] [PubMed]
- Fortin JP, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, Roalf DR, Satterthwaite TD, Gur RC, Gur RE. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 2017;161:149. [Crossref] [PubMed]
- Faul F, Erdfelder E, Lang AG, Buchner A. G. *Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175-91. [Crossref] [PubMed]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149-60. [Crossref] [PubMed]
- Weinstein M, Myers V, Green D, Schertz M, Shiran SI, Geva R, Artzi M, Gordon AM, Fattal-Valevski A, Ben Bashat D. Brain Plasticity following Intensive Bimanual Therapy in Children with Hemiparesis: Preliminary Evidence. Neural Plast 2015;2015:798481. [Crossref] [PubMed]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006;31:1116-28. [Crossref] [PubMed]
- Xue X, Yang Y, Huang Q, Cui F, Lian Y, Zhang S, Yao L, Peng W, Li X, Pang P, Yan J, Chen F. Use of a Radiomics Model to Predict Tumor Invasiveness of Pulmonary Adenocarcinomas Appearing as Pulmonary Ground-Glass Nodules. Biomed Res Int 2018;2018:6803971. [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. [Crossref] [PubMed]
- Fiori S, Guzzetta A, Pannek K, Ware RS, Rossi G, Klingels K, Feys H, Coulthard A, Cioni G, Rose S, Boyd RN. Validity of semi-quantitative scale for brain MRI in unilateral cerebral palsy due to periventricular white matter lesions: Relationship with hand sensorimotor function and structural connectivity. Neuroimage Clin 2015;8:104-9. [Crossref] [PubMed]
- Reid SM, Ditchfield MR, Bracken J, Reddihough DS. Relationship between characteristics on magnetic resonance imaging and motor outcomes in children with cerebral palsy and white matter injury. Res Dev Disabil 2015;45-46:178-87. [Crossref] [PubMed]
- Pagnozzi AM, Dowson N, Fiori S, Doecke J, Bradley AP, Boyd RN, Rose S. Alterations in regional shape on ipsilateral and contralateral cortex contrast in children with unilateral cerebral palsy and are predictive of multiple outcomes. Hum Brain Mapp 2016;37:3588-603. [Crossref] [PubMed]
- Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, Adams P, Cooper C, Fava M, McGrath PJ, McInnis M, Phillips ML, Trivedi MH, Weissman MM, Shinohara RT. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 2018;167:104-20. [Crossref] [PubMed]
- Fortin JP, Parker D, Tunc B, Watanabe T, Elliott MA, Ruparel K, Roalf DR, Satterthwaite TD, Gur RC, Gur RE, Schultz RT, Verma R, Shinohara RT. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 2017;161:149-70. [Crossref] [PubMed]
- Mirzaalian H, Ning L, Savadjiev P, Pasternak O, Bouix S, Michailovich O, Grant G, Marx CE, Morey RA, Flashman LA, George MS, McAllister TW, Andaluz N, Shutter L, Coimbra R, Zafonte RD, Coleman MJ, Kubicki M, Westin CF, Stein MB, Shenton ME, Rathi Y. Inter-site and inter-scanner diffusion MRI data harmonization. Neuroimage 2016;135:311-23. [Crossref] [PubMed]
- Mirzaalian H, Ning L, Savadjiev P, Pasternak O, Bouix S, Michailovich O, Karmacharya S, Grant G, Marx CE, Morey RA, Flashman LA, George MS, McAllister TW, Andaluz N, Shutter L, Coimbra R, Zafonte RD, Coleman MJ, Kubicki M, Westin CF, Stein MB, Shenton ME, Rathi Y. Multi-site harmonization of diffusion MRI data in a registration framework. Brain Imaging Behav 2018;12:284-95. [Crossref] [PubMed]
- Himmelmann K, Horber V, De La Cruz J, Horridge K, Mejaski-Bosnjak V, Hollody K, Krageloh-Mann I. MRI classification system (MRICS) for children with cerebral palsy: development, reliability, and recommendations. Dev Med Child Neurol 2017;59:57-64. [Crossref] [PubMed]
- Pagnozzi AM, Gal Y, Boyd RN, Fiori S, Fripp J, Rose S, Dowson N. The need for improved brain lesion segmentation techniques for children with cerebral palsy: A review. Int J Dev Neurosci 2015;47:229-46. [Crossref] [PubMed]
- Cusack R, Wild C, Linke AC, Arichi T, Lee DS, Han VK. Optimizing stimulation and analysis protocols for neonatal fMRI. PLoS One 2015;10:e0120202. [Crossref] [PubMed]