
Functional endoscopy techniques for tracking stem cell fate
Introduction
Stem cell therapies (SCT) hold great potential for treating various human diseases and regenerating tissues; however, the heterogeneity and plasticity of stem cells render such SCT either beneficial (cell engraftment, differentiation, integration) or detrimental (e.g., the death of transplanted stem cells and subsequent inflammation and cancerous changes), depending upon the residual microenvironment (host inflammatory responses) at the recipient site (1). Such detrimental effects include the use of stem cell derivatives [e.g., conditioned medium (CM) and microvesicles (MVs)] to regenerate lung tissues on the release of TGF-β and IL-6 (2). To address these issues, we described a comprehensive biological Global Positioning System (bGPS) to track transplanted stem cells (3) with eight desired elements for tracking and monitoring the implanted stem cells to ensure successful SCT: these include (I) sensitivity for single cell detection, (II) real-time positioning, (III) an inducible system, (IV) retractable, (V) targeted and durable, (VI) monitoring cell fate, (VII) compliant with the FDA GMP guidelines for clinical applications, and (VIII) quantification capacity (refer to Table 1) (3). Thus far, none of the existing imaging modalities meets all of these criteria (5); however, all currently available platforms appear to be complementary, conjuring up hope for integration. The measurable fabric matrix of such integration has yet to be fully elucidated and developed.
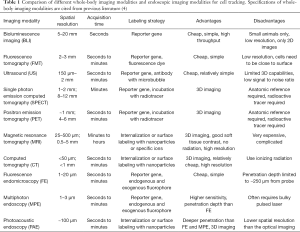
Full table
Tracking of implanted cells can be generally performed by (I) labeling cells using passive or active transport or (II) integrating a specific reporter gene to the targeted cells. The signal from the cells are then observed with magnetic resonance imaging (MRI), bioluminescence imaging (BLI), fluorescence imaging (FLI), positron emission tomography (PET), or single photon emission computed tomography (SPECT). The advantages and disadvantages of each imaging system are summarized in Table 1, as detailed in other reviews (4). However, none of the modalities can provide comprehensive and detailed visualization of the implanted stem cells to meet those eight criteria (3). As endoscopy can access internal organs (gastrointestinal tract, vaginal tract, and airway) and can treat and monitor the tissue simultaneously, we will discuss the clinical impact of endoscopic-based imaging techniques, focusing on the tools and methods that have been developed and tested in recent years. For each endoscopic imaging technique, we will summarize the currently used (I) cell labeling methods, (II) imaging probes, and (III) advantages and disadvantages.
Fluorescence endomicroscopy (FE)
FE or confocal laser endomicroscopy (CLE) is a new imaging tool that allows minimally-invasive, real-time in vivo imaging with sub-cellular spatial resolution (6). It combines the advantages of the confocal microscope to image biological tissue with high spatial resolution and the endoscope to reach tissues intravitally. FE has been applied in colon cancer detection (7) and the longitudinal study of live cells (8).
In stem cell research, FE has been used to study stem cell niches (9), homing and engraftment (10,11), and to investigate the safety of the implanted stem cells (12). Some studies showed the potential application of FE in monitoring stem cells during organ repair such as lung (13). Perez et al. labeled mesenchymal stem cells (MSCs) with fluorescence dye (Vybrant DiD Cell-Labeling Solution) which were injected into damaged lung tissue. Then, FE was used to monitor and quantify those cells over several days in live rats. This report was the first demonstration of stem cell tracking using FE in live animals (13).
Cell labeling
The cell labeling method for FE is similar to standard fluorescence imaging. Labeling can be performed through endogenous fluorophores, exogenous fluorescence dyes, and genetic modification. A cellular auto-fluorescence signal can be utilized to distinguish cellular morphological changes without exogenous contrast agents. Lin et al. demonstrated the FE system could detect a tissue auto-fluorescence signal from ex vivo human esophageal tissue by UV excitation (266 and 325 nm) (14). Membrane dyes, such as DiD and topical methylene blue, have been used to stain cells for endomicroscopy imaging (13,15,16). In other cases, MSCs are engineered to co-express the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and enhanced green fluorescent protein (EGFP) to track the integrity of the implanted stem cells in the tumor (17).
Endoscopic probe
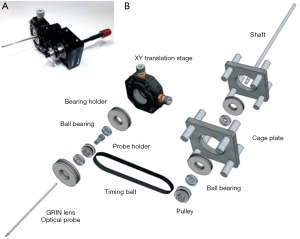
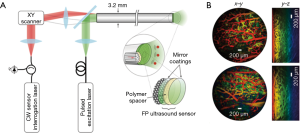
Most FE utilizes a fiber-bundle to image the tissue. Currently, there are two products on the market: FIVE1 from Optician and Cellvizio from Mauna Kea Technologies. Cellvizio uses two lasers (488 and 660 nm) and two channels to detect fluorescence signals from two spectral regions. The probe diameter is 2.6 mm, and the field of view is around 240 µm. The image acquisition speed is 12 frames/sec. This system is much faster than MRI and CT. Recently, a side-view endomicroscope has also been developed and tested, as shown in Figure 1A and B (8).
Advantages and limitations
Fiber-probe FE has a higher spatial resolution, sensitivity, and is cheaper compared to MRI, ultrasound, and CT imaging techniques. The lateral resolution of FE depends on the type of fiber bundle and the lens which is typically 2.5–5 µm (18). The sensitivity of FE to detect the reporter is high (10−9–10−11 M) compared to MRI (10−3–10−5 M) (19,20). While MRI and CT require bulky and costly equipment, FE can be performed through the working channel of a conventional endoscope and serve as a point-of-care imaging tool. The manufacturing cost is also much less than MRI, CT, and PET. Additionally, FE can access the targeted tissue intravitally through flexible optical fibers. Therefore, the imaging depth of FE is not limited to the surface of the tissue, which is the case of the whole-body fluorescence imaging system (21). However, if the size of the fiber-bundle is equal to or bigger than the anatomical cavities, such as terminal bronchioles and the internal tracts of small animals, noninvasive imaging through FE may be challenging. For example, one of the smallest commercially available fiber-based FE probe is Cellvizio, and the probe size is about 0.6 mm (6). However, the airway structure of the rat can be smaller than 0.4 mm. It is possible to intentionally puncture the biological tissue through the FE probe to image the targeted region, but this procedure is invasive and can be very hazardous in small animals such as a rat.
Multiphoton endoscopy
Multiphoton microscopy (MPM) is a powerful imaging technique that provides functional histopathological information of biological tissue with sub-cellular resolution, minimum photothermal damage, and less tissue scattering (22,23). Due to the nonlinear two-photon effect of near-infrared light, two-photon microscopy can image deeper tissue with less photo-damage compared to the fluorescence microscope, which makes it ideal for in vivo applications (24). Similar to the previously introduced FE technologies, multiphoton endoscopic imaging allows in vivo and in situ visualization of the histopathological process and can be used to monitor stem cell behavior. In stem cell research, MPM has been used to investigate in vivo MSC homing and evaluate cellular response (25,26). Rompolas et al. tracked the hair-follicle stem cells and progeny using a transgenic mouse. The fluorescence signal from epithelial nuclei was visualized using an MPM by inducing expression of a fusion protein of histone H2B with a green fluorescent protein (GFP) by the keratin 14 promoter (K14H2BGFP). Then the stem cells and their progeny were identified based on their unique morphological features (26). However, few studies of stem cell tracking using multiphoton endoscopy have been reported as of February 2019.
Cell labeling
Similar to fluorescence microscopy, the targeted cells can be visualized through staining using exogenous contrast agents, such as Au nanoparticles (AuNPs) (27-30), EGFP (31), and Quantum dots (QDs) (32). Also, taking advantage of two-photon effects, endogenous fluorophores, such as reduced nicotinamide adenine dinucleotide (NADH) and oxidized flavin adenine (FAD), can be targeted for label-free cell imaging (33-35). Second harmonic generation (SHG) is another label-free imaging technique based on the non-linear effect of light which is often used to visualize the collagen architecture within the tissue (36,37). Also, a genetic marker, such as GFP, can be used to identify the transplanted stem cells (38).
Endoscopic probe
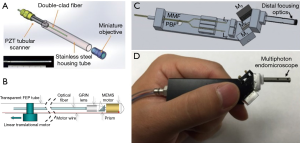
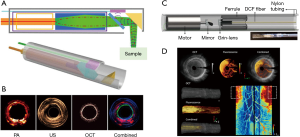
Combining the custom double-clad fiber (DCF) and achromatic miniature objective, Liang et al. developed a miniature flexible fiber-based endoscopic probe for two-photon imaging (39). The custom DCF is made of a silica single-mode core, and it suppresses in-fiber nonlinear luminescence. The outer diameter of the probe is around 2.1 mm (Figure 2A). Alternatively, the microelectromechanical system (MEMS) scanner-based multiphoton endoscopy has been developed by several groups (36,37,40). The advantage of MEMS is a large scanning angle compared to PZT-based fiber scanning with less off-axis aberration. Liu et al. demonstrated SHG endoscopy using a rotational MEMS motor and a custom-built 1-micrometer ultrashort-pulse fiber laser (Figure 2B) (37). A 360-degree wide-field view of an SHG signal was acquired using a rotational scanning probe. Duan et al. achieved a lateral resolution of 2 µm, large FOV of 300×300 µm2 and fast image acquisition speed of 5 frames/sec with a 3.4 mm outer diameter (OD) probe (Figure 2C,D) (40).
Advantages and limitations
Similar to FE, multiphoton endoscopic imaging can visualize in vivo tissue with subcellular resolution, high-sensitivity, and high acquisition speed close to real time. Also, two-photon excitation of near-infrared lowers the risk of photo-damage making it ideal for in vivo inspection and tracking of stem cells without damaging them. The limitation of two-photon microscopy is that it requires a bulky and expensive high-speed pulsed laser.
Photoacoustic endoscope (PAE)
PA imaging is a new imaging modality based on the photoacoustic effect in which the absorbed energy from the laser is transformed into kinetic energy through thermal expansion of the sample and detected by an ultrasound transducer. It combines the advantages of optical imaging to obtain high resolution, molecular sensitivity, and spectroscopic information and ultrasound imaging to acquire tomographic information of deep tissue.
Stem cells typically do not have optical contrast by themselves. However, numerous studies have used various contrast agents to monitor and track stem cells in vivo. Metal nanoparticles, such as gold, seem to be the most popular choice of contrast agents for PA imaging due to their high absorption coefficient and tunable optical properties (41). Recently, Kim et al. implanted stem cells labeled with Prussian blue nanoparticles (PBNPs) into a nude mouse. The labeled stem cells show a strong photoacoustic signal when imaged at 730 nm and were monitored for up to 14 days in vivo (42).
Cell labeling
Gold nanoparticles in the form of spheres (43), rods (44), and star shapes (45) have been commonly used to track stem cells in vivo. Nam et al. showed the longitudinal monitoring of stem cell behaviors up to one week using gold nanosphere labeled MSCs captured in the PEGylated fibrin gel injected into the limb of a Lewis rat (43). Jokerst et al. showed the increased uptake of gold nanorods by 5-fold into the MSCs by coating with silica. Increased signal-to-noise ratio allowed visualization of up to 100,000 cells. No cytotoxicity or changes in cell proliferation were observed (44). Due to the high energy absorption of gold, it can act as a photothermal therapy agent as well as a contrast agent. Liang et al. conjugated gold nanostars with CD44v6 monoclonal antibodies to target gastric cancer stem cells (GCSCs) for PA imaging and photothermal therapy. CD44v6-Gold nanostars actively targeted the GCSCs up to 4 h post-injection (45).
Endoscopic probe
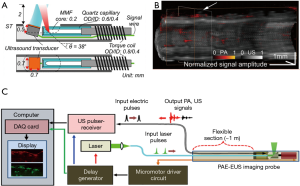
The photoacoustic endoscope (PAE) integrates a light-emitting optical fiber, ultrasound receiver, and circumferential scanning mechanism. Flexible shaft-based proximal rotation is a commonly used scanning mechanism in the PAE (Figure 3A,B) (48-50). This scanning mechanism does not have a mechanical scanning device at the distal portion of the probe, and thus, the probe can be miniaturized. However, the downside of this rotation scheme is the non-uniform distortion (NURD) and the slow speed of the rotation. Another type of PAE uses a micromotor for the circumferential scanning at the distal end of the imaging probe (Figure 3C). The ring ultrasound transducer and the optical fiber are co-aligned with each other (47,51). Recently, an all-optical photoacoustic probe has been developed based on the Fabry-Perot (FP) sensor (Figure 4A,B) (50). Since the optical ultrasound sensor can be much smaller than the electrical ultrasound transducer, the PAE can be miniaturized using an all-optical design.
Advantages and limitations
The advantage of the PAE is the penetration depth. It can image deeper than fluorescence endoscopy, two-photon (2P) endoscopy, and optical coherence tomography (OCT). Also, compared to ultrasound which only provides structural information, the PAE can give information on optical contrast and thermoelastic contrast with a higher spatial resolution (51). However, the limitation is the large size of the imaging probe since it has to fit both the ultrasound transducer and optical fiber. For an application such as intravascular imaging, the probe size has to be no more than 1 mm.
Multimodality endoscopic imaging
Since each imaging technology has unique advantages and disadvantages, researchers have developed reporters, genes, and probes that can be imaged with multiple imaging modalities (52,53). Multimodality imaging can minimize the drawbacks of using each imaging tool alone, increase the signal specificity and sensitivity, and gain a complete picture of stem cell behavior (52). Nam et al. combined ultrasound (US) and photoacoustic tomography (PAT) to monitor stem cell behaviors in vivo longitudinally (43). The mesenchymal stem cells (MSCs) labeled with gold nanotracers (AuNTs) were injected in the lower limb of a Lewis rat and imaged with US/PAT for up to 10 days. Taking advantage of the high spatial resolution and molecular-specific contrast of PAT and deep penetration depth of the US, this study demonstrated the feasibility and benefits of using multimodal imaging for stem cell imaging.
Cell labeling
In one study by Wang et al., polyethylene glycol (PEG) functionalized single-walled carbon nanotubes (PEG-SWNT) were used to label human MSCs for in vivo Raman/MRI/PA triple-modal imaging (54). SWNTs have strong inherent resonance Raman scattering that provides ultrasensitive Raman imaging (55). They also have a strong optical absorption coefficient that allows deep tissue imaging through photoacoustic imaging (56). The metallic nanoparticles can be conjugated with carbon nanotubes to serve as the T2-contrast for MRI (54). In another study, Zhang et al. demonstrated two-photon/photoacoustic dual-modality imaging using MSCs labeled with gold nanocages (57).
Endoscopic probe
While few studies have shown stem cell tracking using multimodality endoscopic probes, a lot of literature has reported multimodality endoscopic imaging probes with different resolutions and sensitivities. A miniature endoscopic probe that integrates OCT, US, and PAI has been developed and demonstrated for imaging of human arteries and ovarian tissue (Figure 5A,B) (58,60). Similar to the PA endoscopic probe, an optical fiber and ultrasound transducer are placed at the distal end of the endoscope. OCT allows the visualization of tissue structure 2 mm below the surface and PAI gives molecular contrast and blood vessel information. US provides information on deeper tissue structure than OCT. In a recent study, Li et al. showed an OCT/FI endoscope for imaging the gastrointestinal tract (Figure 5C,D) (59). FI provided molecular contrast with sub-cellular resolution. This probe also used a Micro-Electro-Mechanical Systems (MEMS) micromotor for scanning OCT and the FI beam. These imaging platforms are complementary to magnetic resonance imaging (MRI) which assessed the structural integrity of adipose-derived mesenchymal stem cell (MSC)-based tissue engineering for arthroscopic rotator cuff repair with the 28 months of follow-up (61), offering a new landscape for stem cell therapy.
Advantages and limitations
The advantages of multimodality imaging are that it can provide comprehensive information on implanted stem cells and the microenvironment. By combining a high-resolution imaging modality, such as fluorescence imaging, multi-photon microscopy (MPM), and Raman imaging, with techniques that allow large-area scannings, such as OCT, MRI, PAI, and US, we can track the stem cells at different scales. On the other hand, combining multiple imaging modalities can increase the cost, complexity, and the size of the endoscopic probe.
Conclusions
Stem cell therapy is a rapidly growing field in medical research as well as in the clinic, demanding effective imaging to track down the fate of implanted stem cells. Many molecular imaging techniques can be translated into an endoscope to provide high-resolution longitudinal monitoring of implanted stem cells. Fluorescence/confocal endomicroscopy and MPM endoscopy have a high potential to monitor stem cells in vivo with cellular resolution using endogenous fluorophores, exogenous fluorophores, or genetically-modified cells. Photoacoustic endoscopy is the new imaging modality that can image much deeper into the tissue and provide a molecular contrast using a contrast agent such as gold nanoparticles.
Although endoscopic imaging has its limitations, such as a small field of view and shallow imaging depth, compared to CT, MRI, and US imaging, we believe specific stem cell therapies will significantly benefit from functional endoscopic imaging. For example, adult tracheobronchial stem cells have recently been demonstrated to be an effective therapeutic option to cure airway disease, repair damaged airway tissue, and replace malfunctioning cells (62). Functional endoscopy allows tracking and monitoring of those implanted stem cells in situ with cellular resolution. Also, most of the endoscopic imaging probe can be combined with a conventional bronchoscope to provide multimodal imaging. Traditional cell tracking techniques, such as MRI and CT, will likely be combined with cellular-resolution endoscopic imaging in the future to monitor the fate of implanted stem cells effectively. The ultimate goal of relevant stem cell therapy imaging lies in the spatiotemporal determination of the ideal “therapeutic window” (63) for tracking subclonal evolution at the single cell level (64) in prevention and a “wait-and-watch” approach by continuous biomarker profiling of diseases during an entire lifetime (65).
Acknowledgements
Funding: This work was supported by the National Institutes of Health under grant P41-EB015890 and in part by the CHOC Children’s Foundation, CHOC-UCI Joint Research Awards (2014, 2015, 2016).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Baldari S, Di Rocco G, Piccoli M, Pozzobon M, Muraca M, Toietta G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int J Mol Sci 2017;18:E2087. [Crossref] [PubMed]
- Zucca E, Corsini E, Galbiati V, Lange-Consiglio A, Ferrucci F. Evaluation of amniotic mesenchymal cell derivatives on cytokine production in equine alveolar macrophages: an in vitro approach to lung inflammation. Stem Cell Res Ther 2016;7:137. [Crossref] [PubMed]
- Li SC, Tachiki LM, Luo J, Dethlefs BA, Chen Z, Loudon WG. A biological global positioning system: considerations for tracking stem cell behaviors in the whole body. Stem Cell Rev 2010;6:317-33. [Crossref] [PubMed]
- Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell 2014;14:431-44. [Crossref] [PubMed]
- Perkel JM. Special news feature: What lies beneath: in vivo stem cell imaging -BioTechniques Focus translational Tools. BioTechniques 2011;50:223-7. [PubMed]
- Paré G, Lebel R, Perez J, Chagnon F, Bonin MA, Bibeau C, El Naqa I, Marsault E, Lepage M, Lesur O. Emerging applications of intra-vital smart micro-imaging: from bench-tobedside. In: Méndez-Vilas A. editor. Microscopy and imaging science: practical approaches to applied research and education. Spain: Formatex Research Center, 2017:120-33.
- Pan Y, Volkmer JP, Mach KE, Rouse RV, Liu JJ, Sahoo D, Chang TC, Metzner TJ, Kang L, van de Rijn M, Skinner EC, Gambhir SS, Weissman IL, Liao JC. Endoscopic molecular imaging of human bladder cancer using a CD47 antibody. Sci Transl Med 2014;6:260ra148. [Crossref] [PubMed]
- Ahn J, Choe K, Wang T, Hwang Y, Song E, Kim KH, Kim P. In vivo longitudinal cellular imaging of small intestine by side-view endomicroscopy. Biomed Opt Express 2015;6:3963-72. [Crossref] [PubMed]
- Brown S, Greco V. Stem cells in the wild: understanding the World of stem cells through intravital imaging. Cell Stem Cell 2014;15:683-6. [Crossref] [PubMed]
- Lo Celso C, Lin CP, Scadden DT. In vivo imaging of transplanted hematopoietic stem and progenitor cells in mouse calvarium bone marrow. Nat Protoc 2011;6:1-14. [Crossref] [PubMed]
- Mortensen LJ, Levy O, Phillips JP, Stratton T, Triana B, Ruiz JP, Gu F, Karp JM, Lin CP. Quantification of Mesenchymal Stem Cell (MSC) delivery to a target site using in vivo confocal microscopy. PLoS One 2013;8:e78145. [Crossref] [PubMed]
- Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, Wang W, Yerebakan C, Li W, Gaebel R, Li RK, Vollmar B, Steinhoff G, Ma N. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res 2009;77:370-6. [Crossref] [PubMed]
- Perez JR, Ybarra N, Chagnon F, Serban M, Lee S, Seuntjens J, Lesur O, El Naqa I. Tracking of Mesenchymal Stem Cells with Fluorescence Endomicroscopy Imaging in Radiotherapy-Induced Lung Injury. Sci Rep 2017;7:40748. [Crossref] [PubMed]
- Lin B, Matthews DL, Demos SG, Urayama S, Saroufeem RM. Endomicroscopy imaging of epithelial structures using tissue autofluorescence. J Biomed Opt 2011;16:046014. [Crossref] [PubMed]
- Rakotomamonjy A, Petitjean C, Salaün M, Thiberville L. Scattering features for lung cancer detection in fibered confocal fluorescence microscopy images. Artif Intell Med 2014;61:105-18. [Crossref] [PubMed]
- Danilevskaya O, Averyanov A, Klimko N, Lesnyak V, Sorokina A, Sazonov D, Zabozlaev F. The case of diagnostics of invasive pulmonary aspergillosis by in vivo probe-based confocal laser endomicroscopy of central and distal airways. Med Mycol Case Rep 2014;5:35-9. [Crossref] [PubMed]
- Zhang Z, Li M, Chen F, Li L, Liu J, Li Z, Ji R, Zuo X, Li Y. Probe-based confocal laser endomicroscopy for imaging TRAIL-expressing mesenchymal stem cells to monitor colon xenograft tumors in vivo. PLoS One 2016;11:e0162700. [Crossref] [PubMed]
- Oh G, Chung E, Yun SH. Optical fibers for high-resolution in vivo microendoscopic fluorescence imaging. Optical Fiber Technology 2013;19:760-71. [Crossref]
- James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev 2012;92:897-965. [Crossref] [PubMed]
- Lecchi M, Ottobrini L, Martelli C, Del Sole A, Lucignani G. Instrumentation and probes for molecular and cellular imaging. Q J Nucl Med Mol Imaging 2007;51:111-26. [PubMed]
- Leblond F, Davis SC, Valdés PA, Pogue BW. Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications. J Photochem Photobiol B 2010;98:77-94. [Crossref] [PubMed]
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science 1990;248:73-6. [Crossref] [PubMed]
- Lefort C. A review of biomedical multiphoton microscopy and its laser sources. J Phys D: Appl Phys 2017;50:423001. [Crossref]
- Hoover EE, Squier JA. Advances in multiphoton microscopy technology. Nature Photonics 2013;7:93-101. [Crossref] [PubMed]
- Phillips JA, Mortensen LJ, Ruiz JP, Sridharan R, Kumar S, Torres M, Sharma P, Lin CP, Karp JM, Hauschka PV. Advances in single-cell tracking of mesenchymal stem cells (MSCs) during musculoskeletal regeneration. Orthop J Harv Med Sch 2012;14:22-8. [PubMed]
- Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 2012;487:496-9. [Crossref] [PubMed]
- Qu X, Wang J, Zhang Z, Koop N, Rahmanzadeh R, Huettmann G. Imaging of cancer cells by multiphoton microscopy using gold nanoparticles and fluorescent dyes. J Biomed Opt 2008;13:031217. [Crossref] [PubMed]
- Rane TD, Armani AM. Two-photon microscopy analysis of gold nanoparticle uptake in 3D cell spheroids. PLoS One 2016;11:e0167548. [Crossref] [PubMed]
- Porcaro F, Miao Y, Kota R, Haun J, Polzonetti G, Battocchio C, Gratton E. Fluctuation Spectroscopy Analysis of Glucose Capped Gold Nanoparticles. Langmuir 2016;32:13409-17. [Crossref] [PubMed]
- Farrer RA, Butterfield FL, Chen VW, Fourkas JT. Highly efficient multiphoton-absorption-induced luminescence from gold nanoparticles. Nano Lett 2005;5:1139-42. [Crossref] [PubMed]
- Drobizhev M, Makarov NS, Tillo SE, Hughes TE, Rebane A. Two-photon absorption properties of fluorescent proteins. Nat Methods 2011;8:393-9. [Crossref] [PubMed]
- Collins MC, Gunst PR, Cascio WE, Kypson AP, Muller-Borer BJ. Labeling and imaging mesenchymal stem cells with quantum dots. Nanoparticles in Biology and Medicine. Springer, 2012:199-210.
- König K, Uchugonova A, Gorjup E. Multiphoton fluorescence lifetime imaging of 3D-stem cell spheroids during differentiation. Microsc Res Tech 2011;74:9-17. [Crossref] [PubMed]
- Lee HS, Teng SW, Chen HC, Lo W, Sun Y, Lin TY, Chiou LL, Jiang CC, Dong CY. Imaging human bone marrow stem cell morphogenesis in polyglycolic acid scaffold by multiphoton microscopy. Tissue Eng 2006;12:2835-41. [Crossref] [PubMed]
- Rice WL, Kaplan DL, Georgakoudi I. Two-photon microscopy for non-invasive, quantitative monitoring of stem cell differentiation. PLoS One 2010;5:e10075. [Crossref] [PubMed]
- Liu G, Kieu K, Wise FW, Chen Z. Multiphoton microscopy system with a compact fiber-based femtosecond-pulse laser and handheld probe. J Biophotonics 2011;4:34-9. [Crossref] [PubMed]
- Liu G, Xie T, Tomov IV, Su J, Yu L, Zhang J, Tromberg BJ, Chen Z. Rotational multiphoton endoscopy with a 1 microm fiber laser system. Opt Lett 2009;34:2249-51. [Crossref] [PubMed]
- Ripoll CB, Bunnell BA. Comparative characterization of mesenchymal stem cells from eGFP transgenic and non-transgenic mice. BMC Cell Biol 2009;10:3. [Crossref] [PubMed]
- Liang W, Hall G, Messerschmidt B, Li MJ, Li X. Applications. Nonlinear optical endomicroscopy for label-free functional histology in vivo. Light Sci Appl 2017;6:e17082. [Crossref] [PubMed]
- Duan X, Li H, Qiu Z, Joshi BP, Pant A, Smith A, Kurabayashi K, Oldham KR, Wang TD. MEMS-based multiphoton endomicroscope for repetitive imaging of mouse colon. Biomed Opt Express 2015;6:3074-83. [Crossref] [PubMed]
- Li W, Chen X. Gold nanoparticles for photoacoustic imaging. Nanomedicine (Lond) 2015;10:299-320. [Crossref] [PubMed]
- Kim T, Lemaster JE, Chen F, Li J, Jokerst JV. Photoacoustic Imaging of Human Mesenchymal Stem Cells Labeled with Prussian Blue-Poly(l-lysine) Nanocomplexes. ACS Nano 2017;11:9022-32. [Crossref] [PubMed]
- Nam SY, Ricles LM, Suggs LJ, Emelianov SY. In vivo ultrasound and photoacoustic monitoring of mesenchymal stem cells labeled with gold nanotracers. PLoS One 2012;7:e37267. [Crossref] [PubMed]
- Jokerst JV, Thangaraj M, Kempen PJ, Sinclair R, Gambhir SS. Photoacoustic imaging of mesenchymal stem cells in living mice via silica-coated gold nanorods. ACS Nano 2012;6:5920-30. [Crossref] [PubMed]
- Liang S, Li C, Zhang C, Chen Y, Xu L, Bao C, Wang X, Liu G, Zhang F, Cui D. CD44v6 Monoclonal Antibody-Conjugated Gold Nanostars for Targeted Photoacoustic Imaging and Plasmonic Photothermal Therapy of Gastric Cancer Stem-like Cells. Theranostics 2015;5:970-84. [Crossref] [PubMed]
- Piao Z, Ma T, Li J, Wiedmann MT, Huang S, Yu M, Kirk Shung K, Zhou Q, Kim CS, Chen Z. High speed intravascular photoacoustic imaging with fast optical parametric oscillator laser at 1.7 µm. Appl Phys Lett 2015;107:083701. [Crossref] [PubMed]
- Yang JM, Favazza C, Yao J, Chen R, Zhou Q, Shung KK, Wang LV. Three-dimensional photoacoustic endoscopic imaging of the rabbit esophagus. PLoS One 2015;10:e0120269. [Crossref] [PubMed]
- Yang JM, Li C, Chen R, Zhou Q, Shung KK, Wang LV. Catheter-based photoacoustic endoscope. J Biomed Opt 2014;19:066001. [Crossref] [PubMed]
- Cao Y, Hui J, Kole A, Wang P, Yu Q, Chen W, Sturek M, Cheng JX. High-sensitivity intravascular photoacoustic imaging of lipid-laden plaque with a collinear catheter design. Sci Rep 2016;6:25236. [Crossref] [PubMed]
- Ansari R, Zhang EZ, Desjardins AE, Beard PC. All-optical forward-viewing photoacoustic probe for high-resolution 3D endoscopy. Light Sci Appl 2018;7:75. [Crossref] [PubMed]
- Yang JM, Maslov K, Yang HC, Zhou Q, Shung KK, Wang LV. Photoacoustic endoscopy. Opt Lett 2009;34:1591-3. [Crossref] [PubMed]
- Chao F, Shen Y, Zhang H, Tian M. Multimodality molecular imaging of stem cells therapy for stroke. Biomed Res Int 2013;2013:849819. [Crossref] [PubMed]
- Santelli J, Lechevallier S, Baaziz H, Vincent M, Martinez C, Mauricot R, Parini A, Verelst M, Cussac D. Multimodal gadolinium oxysulfide nanoparticles: a versatile contrast agent for mesenchymal stem cell labeling. Nanoscale 2018;10:16775-86. [Crossref] [PubMed]
- Wang C, Ma X, Ye S, Cheng L, Yang K, Guo L, Li C, Li Y, Liu Z. Protamine functionalized single-walled carbon nanotubes for stem cell labeling and in vivo raman/magnetic resonance/photoacoustic triple-modal imaging. Adv Funct Mater 2012;22:2363-75. [Crossref]
- Hong H, Gao T, Cai W. Molecular Imaging with Single-Walled Carbon Nanotubes. Nano Today 2009;4:252-61. [Crossref] [PubMed]
- De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi J, Smith BR, Ma TJ, Oralkan O, Cheng Z, Chen X, Dai H, Khuri-Yakub BT, Gambhir SS. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol 2008;3:557-62. [Crossref] [PubMed]
- Zhang YS, Wang Y, Wang L, Wang Y, Cai X, Zhang C, Wang LV, Xia Y. Labeling human mesenchymal stem cells with gold nanocages for in vitro and in vivo tracking by two-photon microscopy and photoacoustic microscopy. Theranostics 2013;3:532-43. [Crossref] [PubMed]
- Dai X, Yang H, Shan T, Xie H, Berceli SA, Jiang H. Miniature Endoscope for Multimodal Imaging. ACS Photonics 2017;4:174-80. [Crossref]
- Li Y, Jing J, Yu J, Zhang B, Huo T, Yang Q, Chen Z. Multimodality endoscopic optical coherence tomography and fluorescence imaging technology for visualization of layered architecture and subsurface microvasculature. Opt Lett 2018;43:2074-7. [Crossref] [PubMed]
- Yang Y, Li X, Wang T, Kumavor PD, Aguirre A, Shung KK, Zhou Q, Sanders M, Brewer M, Zhu Q. Integrated optical coherence tomography, ultrasound and photoacoustic imaging for ovarian tissue characterization. Biomed Opt Express 2011;2:2551-61. [Crossref] [PubMed]
- Kim YS, Sung CH, Chung SH, Kwak SJ, Koh YG. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am J Sports Med 2017;45:2010-8. [Crossref] [PubMed]
- Ghosh M, Ahmad S, White CW, Reynolds SD. Transplantation of Airway Epithelial Stem/Progenitor Cells: A Future for Cell-Based Therapy. Am J Respir Cell Mol Biol 2017;56:1-10. [Crossref] [PubMed]
- Li SC, Han YP, Dethlefs BA, Loudon WG. Therapeutic Window, a Critical Developmental Stage for Stem Cell Therapies. Curr Stem Cell Res Ther 2010;5:297-303. [PubMed]
- Li SC, Lee KL, Luo J. Control dominating subclones for managing cancer progression and posttreatment recurrence by subclonal switchboard signal: implication for new therapies. Stem Cells Dev 2012;21:503-6. [Crossref] [PubMed]
- Li SC, Kabeer MH. Spatiotemporal switching signals for cancer stem cell activation in pediatric origins of adulthood cancer: Towards a watch-and-wait lifetime strategy for cancer treatment. World J Stem Cells 2018;10:15-22. [Crossref] [PubMed]


