Imaging of sacroiliitis: Current status, limitations and pitfalls
Introduction
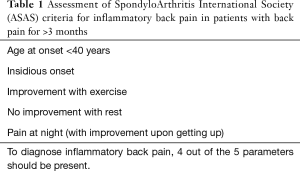
Sacroiliitis is one cause of inflammatory-type low back pain (Table 1) (1). The recognition of inflammatory-type low back pain helps segregate patients with axial spondyloarthritis (SpA) from those with more common mechanical low back pain (2). About 20% of patients with low back pain have inflammatory-type pain (3) while about 20% of these patients with inflammatory-type pain will have axial SpA. The prevalence of axial SpA is about 1% (4,5), though this prevalence does vary according to ethnicity and HLA-B27 population prevalence. For example, in German, American and Chinese populations, the population prevalence of HLA-B27 is 9%, 6% and 5%, respectively (6-8).
Full table
Early recognition and treatment of SpA can ameliorate symptoms, improve quality of life and reduce the likelihood of developing seriously impaired spinal mobility. In the USA, the diagnosis of SpA is delayed on average 14 years from symptom onset (9). One-third of these diagnoses are made by rheumatologists, and the remainder made by primary care practitioners, chiropractors, physiotherapists, orthopedic surgeons, pain physicians, and emergency care physicians (9).
SpA is diagnosed using a combination of clinical, serological and imaging criteria (Table 2). Clinical criteria include the presence of inflammatory-type low back pain and other features of SpA such as anterior uveitis. Serological criteria relate to HLA B27 positivity, and imaging criteria relate to imaging evidence of sacroiliitis and spondyloarthritis.

Full table
About half of the patients diagnosed initially with axial SpA have radiographic evidence of SpA while the remainder have non-radiographic SpA with no radiographic features of SpA (9,10). Although a small percentage of non-radiographic SpA patients will progress to radiographic SpA on follow-up, many will never develop any radiographic features of SpA. As disease activity and functional impairment is similar for patients with radiographic and non-radiographic SpA (11), the distinction does not affect prognosis or treatment, though one should nevertheless be aware that about 50% of SpA patients will not have radiographic features of the disease at presentation. In patients with radiographic SpA, sacroiliitis may take many years to become radiographically apparent (12). Radiographic recognition of mild, or even moderate, sacroiliitis is not always clear cut. As a result, magnetic resonance imaging (MRI) is increasingly utilized to enable recognition of sacroiliitis at a much earlier stage than which is possible radiographically.
Even if the radiographs are normal and axial SpA is strongly suspected clinically, MRI examination is still usually performed to detect sacroiliitis. MRI is the most sensitive imaging technique to detect sacroiliitis. It is the only imaging modality that can reliably reveal bone marrow oedema and inflammation around the sacroiliac joints and is comparable to low dose CT for demonstrating erosions and ankyloses (13). Even so, about one-third of patients with confirmed SpA on clinical grounds, will still have a normal MRI examination with no evidence of either sacroiliitis or spinal enthesopathy. Patients with significant bone marrow oedema on MRI of the sacroiliac joints generally have a good clinical response to anti-tumour necrosis factor (TNF) therapy (14) while conversely SpA patients who do not have MRI evidence of sacroiliitis or elevated c-reactive protein levels do not respond well to anti-TNF treatment (15).
Imaging, and in particular MRI, has greatly helped in the diagnosis and understanding of early SpA. It is important to remember that reactive abnormalities on MRI similar to those seen with sacroiliitis, but not due to sacroiliitis, are quite common, especially in patients with non-SpA inflammatory back pain and in athletes. There are also several other diseases that can mimic sacroiliitis on MR imaging. This review, based on our own institutional experience, illustrates the imaging appearances of common sacroiliac joint pathologies. We also discuss diagnostic or therapeutic percutaneous intervention of the sacroiliac joints.
Anatomy
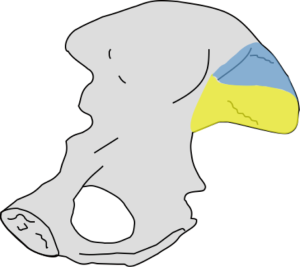
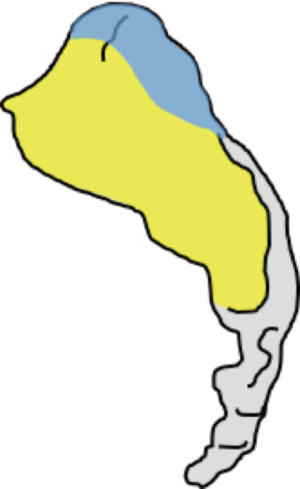
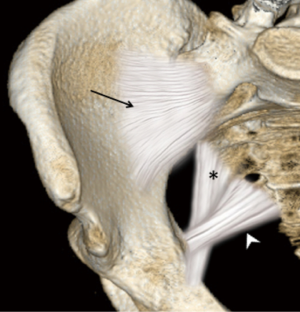
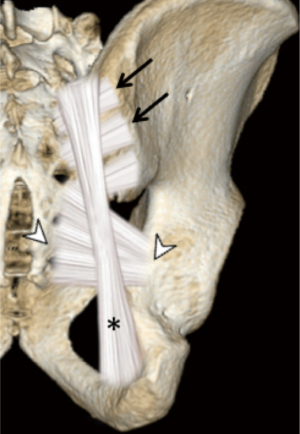
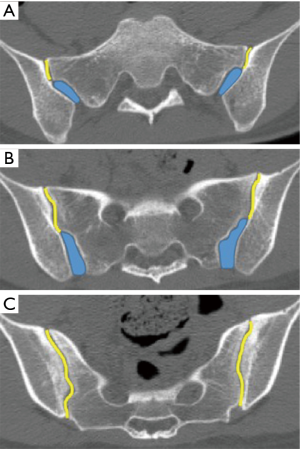
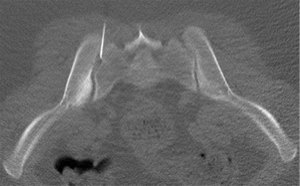
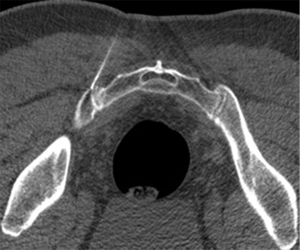
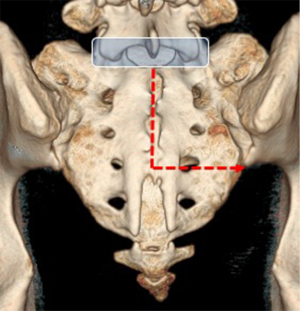
The sacroiliac joint is a large joint, shaped rather like an ear, with both synovial and ligamentous components (Figures 1,2). The joint is enclosed by the anterior and posterior sacroiliac ligaments (Figures 3,4). If the sacroiliac joint is craniocaudally divided into thirds, all of the inferior one-third is a true synovial joint. The posterior part of the middle one-third is syndesmotic while the anterior part is synovial. The posterior part of the superior one-third is syndesmotic along with the most superior aspect of the anterior part (Figure 5) (16), where the interosseous sacroiliac ligament bridges the sacrum and ilium, and blends with the posterior sacroiliac ligament. Therefore, one can appreciate that overall the sacroiliac joint is about one-third ligamentous and about two-thirds synovial. Around the time of skeletal maturity, the sacral articular surface develops a concave depression while the iliac side develops a corresponding osseous ridge (17). This depression and ridge results in interlocking of the joint which limits movement. Figure 5 shows the non-uniform orientation of the sacroiliac joint which hinders radiographic visibility. Throughout life, the sacral joint surface is lined with hyaline cartilage. In early childhood, the iliac joint surface is lined by fibrocartilage, and becomes more hyaline with increasing age (18). The sacral cartilage is overall about 3 times thicker than the iliac cartilage (18). Cartilage thickness varies across the joint, with the sacral cartilage being much thicker (6 mm) anteriorly than posteriorly (1 mm) (17). On oblique coronal imaging, the first 5–6 images from anterior to posterior will comprise the cartilaginous part of the joint, the next 1–2 images are the transitional images, with both cartilage and ligament components, and the most posterior 3–4 images comprise the ligamentous part. The ligamentous component of the sacroiliac joint stiffens and may ossify with age.
Imaging
Radiographs are still used as the first-line imaging of the sacroiliac joints (19). The irregular outline and obliquity make the sacroiliac joints difficult to fully assess radiographically. This limitation has been overcome by more sensitive imaging techniques such as computed tomography (CT) or MRI. Ultrasound is not as useful in assessing the sacroiliac joints as only the anterior and posterior margins are seen. CT is excellent for detecting erosions, bone sclerosis and ankyloses and for guiding interventional procedures although MRI is superior in detecting bone marrow oedema as a measure of sacroiliac joint inflammation (20,21). MRI is the imaging modality of choice for characterizing sacroiliac joint disease and assessing disease severity and activity. MRI has replaced nuclear medicine studies in this regard.
MRI protocol for the sacroiliac joints and spine
Careful consideration of clinical, serological and radiographic spondyloarthritic features should be performed before MRI examination is requested (22). The optimal MR protocol is now more clear. Our departmental sacroiliac joint MR protocol for suspected inflammatory sacroiliitis has become more streamlined from an earlier all-embracing, time-consuming protocol, which comprised imaging in both axial and oblique planes as well as post-contrast imaging (Figure 6).
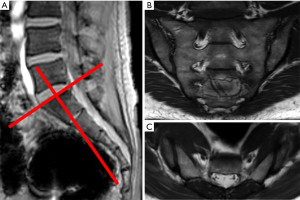
Our standard protocol when screening for sacroiliitis is now limited to oblique coronal “fat-sensitive” T1SE and “fluid-sensitive” T2-weighted fat-saturated sequences. The average time needed for this protocol is 20 minutes. This streamlined approach is based on the premise that most MR examinations undertaken for suspected inflammatory sacroiliitis show no evidence of inflammation. The additional pick-up rate of sacroiliitis on oblique axial imaging is very limited. We no longer do post-contrast imaging when evaluating suspected inflammatory sacroiliitis (22,23).
The issue of whether, how, and when to include spine imaging when screening for SpA is contentious. What clinical criteria should be met before spine imaging is undertaken? Should one routinely include the whole spine, the thoracolumbar spine or only the lumbar spine on initial MR screening evaluation? Should spine imaging comprise T1-, T2-fat-saturated and T1-fat-saturated post-contrast images? The answers to these questions are currently being worked out.
While inclusion of the spine may detect structural findings such as nerve root compression or pars defects that account for chronic low back pain (24), experience to date suggests that MRI-spine imaging has a low yield for identifying cases of SpA when the sacroiliac joints are normal (25). The addition of spinal MRI increases the false-positive diagnosis of SpA which counterbalances the limited increase in sensitivity (26). In other words, imaging of the spine in addition to the sacroiliac joints significantly increases the length of the MR examination but does not necessarily add to specificity and sensitivity.
Our current protocol is to include T1- and T2 fat-saturated sagittal imaging of the thoracic and lumbar spines at the initial presentation only in those patients with a strong clinical suspicion of spinal inflammation. We do not routinely do contrast-enhanced imaging of the spine. While contrast enhancement may increase the conspicuity of some corner lesions, whether it adds any overall diagnostic benefit is questionable (27). The current working consensus is that contrast enhanced MRI examination of the spine is not necessary for routine screening of SpA (28).
When imaging the sacroiliac joints for suspected inflammatory sacroiliitis, a phased array body coil may produce superior image quality over surface body coil imaging for bone marrow oedema (29). A single sequence of T2-weighted multipoint Dixon sequence consisting of water-only, in-phase, opposed-phase and fat-only images may potentially replace the multi-sequence protocol (30). Small field-of-view MRI of the sacroiliac joint imaging seems to confer benefit over large large-field-of-view pelvic MRI for detection of osteitis, erosions, synovitis and bone sclerosis in pediatric patients (31). We obtain all sacroiliac joint imaging with a FOV of 160 mm (craniocaudal) ×200 mm (wide), a slice thickness of 4 mm, and an interslice gap of 0.8 mm. In cases of suspected infective sacroiliitis, our imaging protocol is more extensive than that for suspected inflammatory sacroiliitis and, in this setting, we will routinely perform oblique coronal (T1SE and T2 fat-sat) and oblique axial sequences (T1SE, T2 fat-sat and T1-fat-sat post contrast) images.
Imaging of sacroiliac joint pathologies
The sacroiliac joints are prone to all of the diseases that can affect other synovial or fibrous joints and are important stress risers with transmission of force from the lower limbs to the axial skeleton. While the main clinical indication for imaging of the sacroiliac joints is to exclude or confirm an inflammatory sacroiliitis, one should remember that many diseases or stress reaction can affect the sacroiliac joints other than inflammatory sacroiliitis. In addition to the imaging appearances, the patients’ clinical and serological profile should be considered. Ninety percent of patients with axial SpA present with insidious onset low back pain before 45 years of age (32). Most mimics of inflammatory sacroiliitis do not fit this clinical profile.
While it is important to make a correct early diagnosis of inflammatory sacroiliitis and SpA in patients who have the disease, it is equally important to recognize the entities that may mimic sacroiliitis. Incorrectly over-diagnosing inflammatory sacroiliitis and labelling the patient as having SpA can have a significant negative impact on lifestyle, job prospects, health insurance, etc. (33). Potential mimics of sacroiliitis include stress-related changes, infective sacroiliitis, osteoarthritis, stress fracture, insufficiency fracture and osteitis condensans ilii (34,35).
Inflammatory sacroiliitis
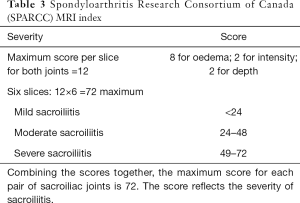
As interpretation of the sacroiliac joints on MRI is not always straightforward (12), these examinations should be interpreted by radiologists experienced in the diagnosis of sacroiliitis to minimize false positive or negative assignments. The most prevalent, reliable and diagnostic MRI feature of active sacroiliitis is bone marrow oedema (Figure 7). Bone marrow oedema is a marker of inflammation and needs to be significant in degree before a diagnosis of sacroiliitis can be made. Bone marrow oedema needs to be either ≥1 cm in depth and visible on at least two contiguous MR images, or at two separate locations on the same image before it can be considered significant (28). Semi-quantitative MRI scoring methods for assessment of sacroiliac joint bone oedema are well established. The Berlin MRI score or the Spondyloarthritis Research Consortium of Canada (SPARCC) score (36) are the most popular. Such scoring systems, although widely used in research, are not used in everyday clinical practice. They do, nevertheless, give an indication as to how sacroiliitis should be assessed and scored and overall inflammatory activity. The SPARCC scoring method is based on the presence of active inflammatory lesions in the synovial portion of the sacroiliac joints depicted on 6 consecutive coronal slices. Each sacroiliac joint is divided into 4 quadrants for scoring active inflammation with additional scores allocated for intensity of bone oedema and depth of involvement (Table 3). These scoring systems were developed as classification rather than diagnostic criteria to define homogenous cohorts for research or clinical pathways. Understandably, classification criteria use the same imaging signs as diagnostic criteria and are thus used interchangeably (12). It must be stressed that one should not use these criteria to make or refute a diagnosis of SpA. The MR appearances may be compatible with inflammatory sacroiliitis but for a diagnosis of SpA to be made, the clinical and serological criteria should also be considered.
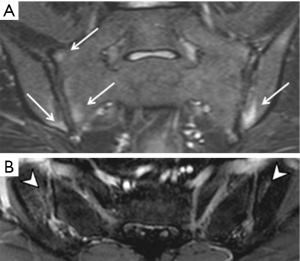
Full table
When reporting on MRI examinations of the sacroiliac joints, one needs to bear in mind that “all the glitters is not gold” (12). Bone marrow oedema can be seen in the absence of sacroiliitis. In other words, not all bone marrow oedema implies an inflammatory sacroiliitis.
Oedema of the sacroiliac joints on MRI mimicking sacroiliitis (Figure 8) may be found in almost a third of patients with non-SpA inflammatory low back pain or young athletes (20,37,38). Placing too much reliance solely on MRI findings to make an affirmative diagnosis of SpA is clearly not optimal. MRI is only one biomarker in a diagnostic process also encompassing clinical and serological testing (12). As stressed, a diagnosis of SpA should only be made after careful consideration of the clinical picture, serological tests and, where necessary, MRI findings (12).
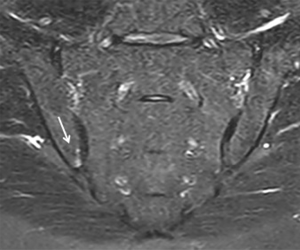
While MRI clearly has the propensity to overdiagnose sacroiliitis, there is also a question of its limited sensitivity. One-third of patients diagnosed as early axial SpA established by clinical expert opinion, have a negative MRI examination (39,40). Also, MRI lesions in SpA patients will, as expected, fluctuate over time.
In addition to bone marrow oedema, sacroiliac joint erosions should also be considered as these add specificity to the diagnosis of inflammatory sacroiliitis. T1-weighted oblique coronal MR imaging is almost as sensitive as low dose dual-energy CT examination for revealing erosions and other structural lesions such as ankyloses and sclerosis (13). Erosions, while adding specificity, are also not entirely diagnostic of sacroiliitis as they can be seen in other entities such as osteoarthritis and osteitis condensans ilii (13). Erosions are seen in ~5% of patients with non-specific low back pain though are uncommon (~1%) in healthy patients aged less than 45 years (13,41). Capsulitis (Figure 9), enthesitis (Figure 10), and synovitis (Figure 11) are less commonly encountered and are supportive, though not diagnostic, features of SpA (42). High T1-signal alongside the sacroiliac joints surrounded by sclerosis represents reparative fat metaplasia termed “backfill”, and this may be more specific to SpA than initially considered (42-45). “Backfill” may be an intermediate step between erosion and ankylosis.
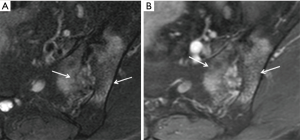
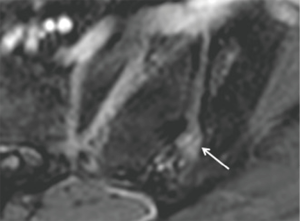
Bone marrow oedema, synovitis, capsulitis and enthesitis are the MR features of inflammation. Subchondral fat deposition (Figure 12) similar to synovitis, capsulitis and enthesitis, is a supportive rather than a primary diagnostic criterion for SpA (46). Structural changes such as subchondral sclerosis (Figure 13) and erosions (Figure 14), and joint space narrowing or ankylosis (Figure 15), are features of later disease and are more reliably detected on CT than MRI. They can also be appreciated on radiographs (Figure 16).
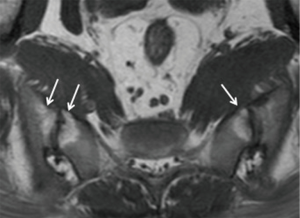
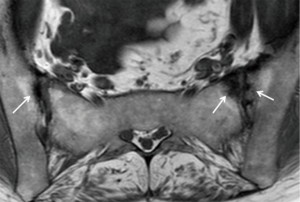
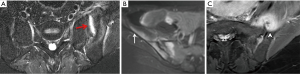
In the spine, anterior and posterior corner-based inflammatory lesions are the main features of active inflammation and are the earliest changes to be seen on MRI. These inflammatory lesions represent osteitis, are typically triangular-shaped at the corner of the vertebral bodies, and depicted as high-signal intensity on fat-suppressed T2-weighted sequences ± contrast enhancement. No strict definition exists as to what size a corner lesion should be to indicate positivity. The harder one looks, the more changes one will see at the corners of the vertebral bodies. Our policy is that all corner lesions need to be obvious, readily apparent and unequivocal. Based on the Assessment of SpondyloArthritis International Society (ASAS) consensus, anterior or posterior spondylitis at ≥3 sites in patients <50 years (47) should be present before a diagnosis of SpA can be considered. Others recommend that ≥5 fatty lesions or inflammatory lesions on the MRI-spine are needed to comply with a diagnosis of SpA with a specificity of >95% (47). In other words, if one is using osteitis as a diagnostic criterion, then osteitis should be present at ≥3 sites. If one is using both osteitis and fatty lesions, then either osteitis or fatty lesions should be present at ≥5 sites. It should be remembered, however, that osteitis, and not fatty lesions, represent active spinal inflammation and is a stronger indicator for active treatment. In the future, computerized systems for grading and standardizing the level of inflammation on MRI in SpA will become more widely used (48).
What is known as “non-infective discovertebral inflammation” or “non-infective spondylodisciitis”, infrequently affects the endplates and adjacent marrow in a more central way. Fatty deposition at the vertebral corners is a less specific, chronic sign of axial SpA and does not indicate acute inflammation. Syndesmophytes and associated ankylosis are clearly much more specific for SpA although they are a feature of chronic disease rather than active inflammation (49).
Infective sacroiliitis
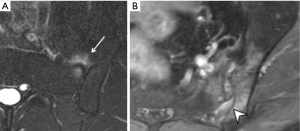
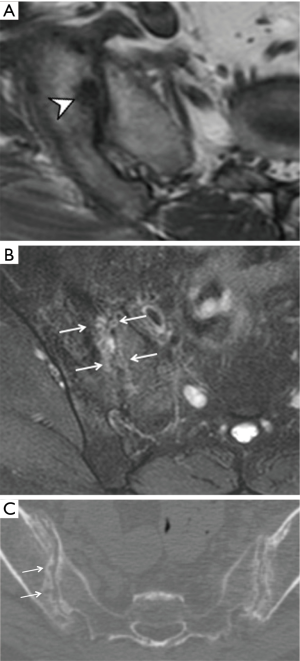
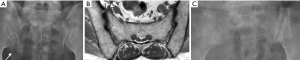
Three features of infective sacroiliitis are particularly helpful in differentiating infective from inflammatory sacroiliitis. First, bone marrow oedema in infective sacroiliitis tends to be more intense and there is more intra-articular fluid (Figure 17A). Second, inflammation in infective sacroiliitis spreads to involve the peri-articular soft tissues, particularly the iliacus and gluteal muscles (Figure 17B) (50). Third, peri-articular fluid collection or abscess is practically pathognomonic of an infective sacroiliitis (Figure 17C).
Osteoarthritis
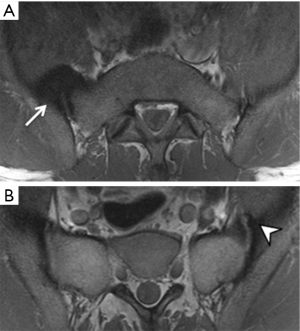
Osteoarthritis of the sacroiliac joints is common in older patients though is both under-recognized and underestimated (51). It tends to affect the iliac side of the joint more due to increased load bearing and thinner articular cartilage on this side. Features of osteoarthritis are, as expected, anterior marginal osteophytosis, joint space narrowing, joint surface irregularity with minimal subchondral sclerosis and subchondral cysts (Figure 18). Bridging osteophytosis in osteoarthritis leads to para-articular bony ankylosis that should not be mistaken for the true intra-articular bony ankylosis seen in ankylosing spondylitis (52). Bone marrow oedema is another potentially misleading feature of osteoarthritis. In osteoarthritis, bone marrow oedema tends to be milder in degree than in inflammatory sacroiliitis and tends to be confined to the immediate subchondral areas (51). Subchondral fatty change akin to Modic Type II endplate changes is a common feature of osteoarthritis that can mimic inflammatory ‘backfill’.
Diffuse idiopathic skeletal hyperostosis (DISH)
Diffuse idiopathic skeletal hyperostosis can involve the sacroiliac ligaments, mimicking sacroiliitis. Bony bridging anterior or posterior to the sacroiliac joint may be mistaken for joint ankylosis (34). It primarily affects the superior ligamentous part of the sacroiliac joint. In contrast to SpA, there are no subchondral erosions or sclerosis in DISH and the synovial joints are spared. Entheseal bridging or fusion is another feature (53). In the spine, spinal radiographs flowing ossification of at least 4 contiguous vertebrae of the thoracic spine is diagnostic of DISH (54). These osteophytes are distinguished from the thinner, more vertically oriented syndesmophytes formed over the annulus in SpA.
Stress reaction
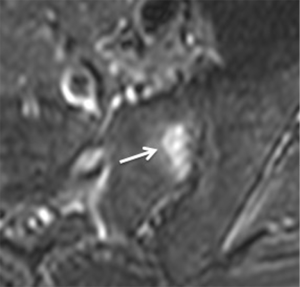
A stress-riser is an area where physical stress is concentrated during physical activity. The sacroiliac joints are a stress-riser during running and other weightbearing activities. As such, sacral stress reactions (Figure 19) are not uncommon in endurance runners (55-57). Continued physical activity will result in stress fracture (Figure 20). Chronic stress can also cause soft tissue reactive changes around the sacroiliac joint in athletes (Figure 21).
Sacral insufficiency fracture
Sacral insufficiency fracture (Figure 22) is a non-infrequent cause of low back pain in osteoporotic elderly patients. There are often, in addition, concomitant insufficiency fractures at the parasymphyseal areas of the pubic bones. MRI, and to a lesser extent CT, are more sensitive at detecting sacral insufficiency fracture than radiography. The demographic profile of patients who develop sacral insufficiency fractures is clearly very different to that of most patients with inflammatory sacroiliitis.
Osteitis condensans ilii
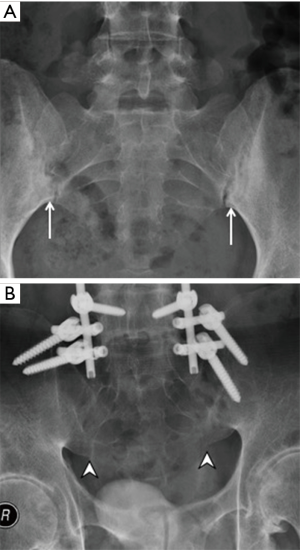
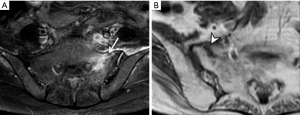
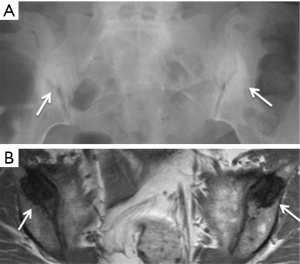
Osteitis condensans ilii is caused by bone deposition at stress areas alongside the sacroiliac joint. It is usually seen in childbearing women and is most likely related to the abnormal high stresses incurred during pregnancy. Radiography and MRI typically reveal bilateral, symmetrical, sharply circumscribed, triangular-shaped areas of subchondral sclerosis, without erosions or joint space widening, at the anteroinferior aspect of the iliac bone alongside the sacroiliac joint (Figure 23) (58,59). Less common variants of osteitis condensans ilii include sclerosis on the sacral side, sclerosis predominating in the mid-portion of the sacroiliac joint rather than the inferior, unilateral predominance, or the presence of articular erosions (60). Bone marrow oedema and fatty infiltration are recognized additional MR features of osteitis condensans ilii (60). Bone marrow oedema in osteitis condensans ilii tends to appear in a continuous pattern as an arcuate line beneath the anterior subchondral bone sclerosis in contrast to inflammatory sacroiliitis (60).
Sacroiliac joint intervention
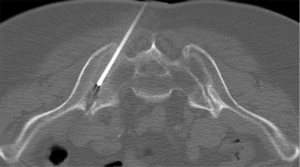
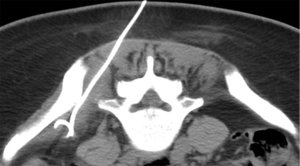
Sacroiliac joint interventions comprise biopsy, aspiration or injection. Percutaneous procedures can be performed using CT, ultrasound or fluoroscopy although CT guidance is the preferred method. In suspected infective sacroiliitis, CT can accurately guide joint aspiration of joint fluid (Figure 24). If no aspirate is obtained, flushing the joint with normal saline and aspirating this saline will increase the likelihood of obtaining a positive culture. If no sacroiliac joint fluid can be obtained even with saline flushing, as in, for example, tuberculous sacroiliitis, para-articular bone biopsy can help confirm or exclude infection (Figure 25). CT can also be used to guide pigtail insertion into abscesses related to infective sacroiliitis (Figure 26).
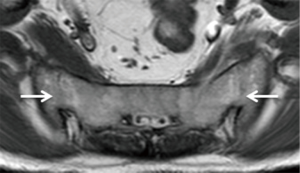
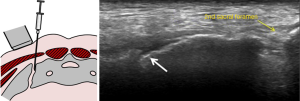
Therapeutic injection of drugs such as corticosteroids into the sacroiliac joint (Figure 27) has good clinical short-and long-term efficacy (61). It is best performed under CT-guidance though fluoroscopic or ultrasound-guided therapeutic injection is also feasible in selected thin patients (62,63). If intra-articular injection cannot be achieved, peri-articular infiltration of therapeutic agent provides similar clinical improvement (64,65). Ultrasound-guided sacroiliac joint injection allows real time guidance of joint puncture. Ultrasound-guided sacroiliac joint injection is not difficult to perform provided one can recognize the relevant anatomy. First, the transducer should be aligned transversely at the spinous process of the fifth lumbar vertebra (Figure 28). Then, it is moved caudally along the median sacral crest, which lies on the dorsal surface of sacrum, until the level of the second sacral foramen. At this level, the transducer is moved laterally when the iliac bone is also visualized. There, the hypoechoic cleft representing the lower one-third of posterior sacroiliac joint is seen and this area can be targeted for injection. A medial to lateral direction for needle placement is preferred (Figure 29) (66,67).
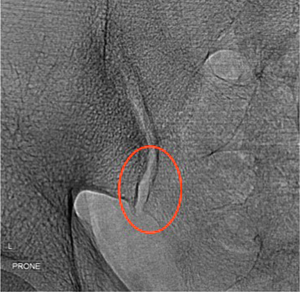
Fluoroscopic-guided sacroiliac joint injection (Figure 30) is easier to perform than ultrasound-guided injection but is challenging in patients with severely narrowed joint spaces. With the patient lying prone in an oblique position and the contralateral sacroiliac joint raised, the needle is inserted into the distal third of the sacroiliac joint angulating the needle upwards in a cephalad direction (62). Intra-articular needle tip position is confirmed by injecting a small amount of non-ionic contrast medium, prior to injection of steroid.
Conclusions
Not every sacroiliac joint abnormality is an inflammatory sacroiliitis. While it is important not to overlook inflammatory sacroiliitis, it is equally important not to over-diagnose this entity in someone who does not have sacroiliitis. This review tries to untangle some of the issues encountered in everyday practice. Sacroiliac joint intervention, though underutilized, can also positively impact patient care.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, Dougados M, Hermann KG, Landewé R, Maksymowych W, van der Heijde D. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68 Suppl 2:ii1-44. [Crossref] [PubMed]
- Weisman MH. Inflammatory back pain: the United States perspective. Rheum Dis Clin North Am 2012;38:501-12. [Crossref] [PubMed]
- Peláez-Ballestas I, Navarro-Zarza JE, Julian B, Lopez A, Flores-Camacho R, Casasola-Vargas JC, Sanin LH, Rivas L, Vázquez-Mellado J, Burgos-Vargas R. A community-based study on the prevalence of spondyloarthritis and inflammatory back pain in Mexicans. J Clin Rheumatol 2013;19:57-61. [Crossref] [PubMed]
- Weisman MH, Witter JP, Reveille JD. The prevalence of inflammatory back pain: population-based estimates from the US National Health and Nutrition Examination Survey, 2009-10. Ann Rheum Dis 2013;72:369-73. [Crossref] [PubMed]
- Reveille JD, Witter JP, Weisman MH. Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthritis Care Res (Hoboken) 2012;64:905-10. [Crossref] [PubMed]
- Bakland G, Nossent HC. Epidemiology of spondyloarthritis: a review. Curr Rheumatol Rep 2013;15:351. [Crossref] [PubMed]
- Reveille JD, Hirsch R, Dillon CF, Carroll MD, Weisman MH. The prevalence of HLA-B27 in the US: data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum 2012;64:1407-11. [Crossref] [PubMed]
- Ng SC, Liao Z, Yu DT, Chan ES, Zhao L, Gu J. Epidemiology of spondyloarthritis in the People's Republic of China: review of the literature and commentary. Semin Arthritis Rheum 2007;37:39-47. [Crossref] [PubMed]
- Danve A, Deodhar A. Axial spondyloarthritis in the USA: diagnostic challenges and missed opportunities. Clin Rheumatol 2018. Epub ahead of print. [Crossref] [PubMed]
- Baraliakos X, Braun J. Non-radiographic axial spondyloarthritis and ankylosing spondylitis: what are the similarities and differences? RMD Open 2015;1:e000053. [Crossref] [PubMed]
- Ciurea A, Scherer A, Exer P, Bernhard J, Dudler J, Beyeler B, Kissling R, Stekhoven D, Rufibach K, Tamborrini G, Weiss B, Müller R, Nissen MJ, Michel BA, van der Heijde D, Dougados M, Boonen A, Weber U. Rheumatologists of the Swiss Clinical Quality Management Program for Axial Spondyloarthritis. Tumor necrosis factor α inhibition in radiographic and nonradiographic axial spondyloarthritis: results from a large observational cohort. Arthritis Rheum 2013;65:3096-106. [Crossref] [PubMed]
- Lukas C, Cyteval C, Dougados M, Weber U. MRI for diagnosis of axial spondyloarthritis: major advance with critical limitations 'Not everything that glisters is gold (standard)'. RMD Open 2018;4:e000586. [Crossref] [PubMed]
- Diekhoff T, Hermann KG, Greese J, Schwenke C, Poddubnyy D, Hamm B, Sieper J. Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: results from the SIMACT study. Ann Rheum Dis 2017;76:1502-8. [Crossref] [PubMed]
- Haibel H, Rudwaleit M, Listing J, Heldmann F, Wong RL, Kupper H, Braun J, Sieper J. Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum 2008;58:1981-91. [Crossref]
- Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, Gensler LS, Smith JA, Borenstein D, Hiratzka J, Weiss PF, Inman RD, Majithia V, Haroon N, Maksymowych WP, Joyce J, Clark BM, Colbert RA, Figgie MP, Hallegua DS, Prete PE, Rosenbaum JT, Stebulis JA, Van Den Bosch F, Yu DT, Miller AS, Reveille JD, Caplan L. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Care Res (Hoboken) 2016;68:151-66. [Crossref] [PubMed]
- Navallas M, Ares J, Beltrán B, Lisbona MP, Maymó J, Solano A. Sacroiliitis associated with axial spondyloarthropathy: new concepts and latest trends. Radiographics 2013;33:933-56. [Crossref] [PubMed]
- Bowen V, Cassidy JD. Macroscopic and microscopic anatomy of the sacroiliac joint from embryonic life until the eighth decade. Spine (Phila Pa 1976) 1981;6:620-8. [Crossref] [PubMed]
- Vleeming A, Schuenke MD, Masi AT, Carreiro JE, Danneels L, Willard FH. The sacroiliac joint: an overview of its anatomy, function and potential clinical implications. J Anat 2012;221:537-67. [Crossref] [PubMed]
- Poddubnyy D, Gaydukova I, Hermann KG, Song IH, Haibel H, Braun J, Sieper J. Magnetic resonance imaging compared to conventional radiographs for detection of chronic structural changes in sacroiliac joints in axial spondyloarthritis. J Rheumatol 2013;40:1557-65. [Crossref] [PubMed]
- Weber U, Lambert RG, Østergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum 2010;62:3048-58. [Crossref] [PubMed]
- Taurog JD, Chhabra A, Colbert RA. Ankylosing Spondylitis and Axial Spondyloarthritis. N Engl J Med 2016;374:2563-74. [Crossref] [PubMed]
- de Hooge M, van den Berg R, Navarro-Compán V, van Gaalen F, van der Heijde D, Huizinga T, Reijnierse M. Magnetic resonance imaging of the sacroiliac joints in the early detection of spondyloarthritis: no added value of gadolinium compared with short tau inversion recovery sequence. Rheumatology (Oxford) 2013;52:1220-4. [Crossref] [PubMed]
- Giraudo C, Weber M, Puchner A, Grisar J, Kainberger F, Schueller-Weidekamm C. Which MR sequences should we use for the reliable detection and localization of bone marrow edema in spondyloarthritis? Radiol Med 2017;122:752-60. [Crossref] [PubMed]
- Jans L, Van Praet L, Elewaut D, Van den Bosch F, Carron P, Jaremko JL, Behaeghe M, Denis A, Huysse W, Lambrecht V, Verstraete K. MRI of the SI joints commonly shows non-inflammatory disease in patients clinically suspected of sacroiliitis. Eur J Radiol 2014;83:179-84. [Crossref] [PubMed]
- Ez-Zaitouni Z, Bakker PA, van Lunteren M, de Hooge M, van den Berg R, Reijnierse M, Fagerli KM, Landewé RB, Ramonda R, Jacobsson LT, Saraux A, Lenczner G, Feydy A, Pialat JB, Thévenin F, van Gaalen FA, van der Heijde D. The yield of a positive MRI of the spine as imaging criterion in the ASAS classification criteria for axial spondyloarthritis: results from the SPACE and DESIR cohorts. Ann Rheum Dis 2017;76:1731-6. [Crossref] [PubMed]
- Weber U, Zubler V, Zhao Z, Lambert RG, Chan SM, Pedersen SJ, Østergaard M, Rufibach K, Maksymowych WP. Does spinal MRI add incremental diagnostic value to MRI of the sacroiliac joints alone in patients with non-radiographic axial spondyloarthritis? Ann Rheum Dis 2015;74:985-92. [Crossref] [PubMed]
- Wang YX, Griffith JF, Deng M, Li TK, Tam LS, Lee VW, Lee KK, Li EK. Vertebral body corner oedema vs gadolinium enhancement as biomarkers of active spinal inflammation in ankylosing spondylitis. Br J Radiol 2012;85:e702-8. [Crossref] [PubMed]
- Lambert RG, Bakker PA, van der Heijde D, Weber U, Rudwaleit M, Hermann KG, Sieper J, Baraliakos X, Bennett A, Braun J, Burgos-Vargas R, Dougados M, Pedersen SJ, Jurik AG, Maksymowych WP, Marzo-Ortega H, Østergaard M, Poddubnyy D, Reijnierse M, van den Bosch F, van der Horst-Bruinsma I, Landewé R. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 2016;75:1958-63. [Crossref] [PubMed]
- Gondim Teixeira PA, Bravetti M, Hossu G, Lecocq S, Petit D, Loeuille D, Blum A. Protocol optimization of sacroiliac joint MR Imaging at 3 Tesla: Impact of coil design and motion resistant sequences on image quality. Diagn Interv Imaging 2017;98:865-71. [Crossref] [PubMed]
- Özgen A. The Value of the T2-Weighted Multipoint Dixon Sequence in MRI of Sacroiliac Joints for the Diagnosis of Active and Chronic Sacroiliitis. AJR Am J Roentgenol 2017;208:603-8. [Crossref] [PubMed]
- Wagle S, Gu JT, Courtier JL, Phelps AS, Lin C, MacKenzie JD. Value of dedicated small-field-of-view sacroiliac versus large-field-of-view pelvic magnetic resonance imaging for evaluating pediatric sacroiliitis. Pediatr Radiol 2019. Epub ahead of print. [Crossref] [PubMed]
- Sieper J, Srinivasan S, Zamani O, Mielants H, Choquette D, Pavelka K, Loft AG, Géher P, Danda D, Reitblat T, Cantini F, Ancuta C, Erdes S, Raffayová H, Keat A, Gaston JS, Praprotnik S, Vastesaeger N. Comparison of two referral strategies for diagnosis of axial spondyloarthritis: the Recognising and Diagnosing Ankylosing Spondylitis Reliably (RADAR) study. Ann Rheum Dis 2013;72:1621-7. [Crossref] [PubMed]
- Berthelot JM, Le Goff B, Maugars Y. Overdiagnosing early spondyloarthritis: what are the risks? Joint Bone Spine 2013;80:446-8. [Crossref] [PubMed]
- Antonelli MJ, Magrey M. Sacroiliitis mimics: a case report and review of the literature. BMC Musculoskelet Disord 2017;18:170. [Crossref] [PubMed]
- Slobodin G, Lidar M, Eshed I. Clinical and imaging mimickers of axial spondyloarthritis. Semin Arthritis Rheum 2017;47:361-8. [Crossref] [PubMed]
- Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M, Conner-Spady B, Palsat J, Lambert RG. Spondyloarthritis research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:703-9. [Crossref] [PubMed]
- Marzo-Ortega H, McGonagle D, O'Connor P, Hensor EM, Bennett AN, Green MJ, Emery P. Baseline and 1-year magnetic resonance imaging of the sacroiliac joint and lumbar spine in very early inflammatory back pain. Relationship between symptoms, HLA-B27 and disease extent and persistence. Ann Rheum Dis 2009;68:1721-7. [Crossref] [PubMed]
- Weber U, Hodler J, Kubik RA, Rufibach K, Lambert RG, Kissling RO, Pfirrmann CW, Maksymowych WP. Sensitivity and specificity of spinal inflammatory lesions assessed by whole-body magnetic resonance imaging in patients with ankylosing spondylitis or recent-onset inflammatory back pain. Arthritis Rheum 2009;61:900-8. [Crossref] [PubMed]
- van den Berg R, de Hooge M, van Gaalen F, Reijnierse M, Huizinga T, van der Heijde D. Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology (Oxford) 2013;52:1492-9. [Crossref] [PubMed]
- van den Berg R, Lenczner G, Thévenin F, Claudepierre P, Feydy A, Reijnierse M, Saraux A, Rahmouni A, Dougados M, van der Heijde D. Classification of axial SpA based on positive imaging (radiographs and/or MRI of the sacroiliac joints) by local rheumatologists or radiologists versus central trained readers in the DESIR cohort. Ann Rheum Dis 2015;74:2016-21. [Crossref] [PubMed]
- Weber U, Maksymowych WP, Chan SM, Rufibach K, Pedersen SJ, Zhao Z, Zubler V, Østergaard M, Lambert RG. Does evaluation of the ligamentous compartment enhance diagnostic utility of sacroiliac joint MRI in axial spondyloarthritis? Arthritis Res Ther 2015;17:246. [Crossref] [PubMed]
- Laloo F, Herregods N, Varkas G, Jaremko JL, Baraliakos X, Elewaut D, Van den Bosch F, Verstraete K, Jans L. MR signal in the sacroiliac joint space in spondyloarthritis: a new sign. Eur Radiol 2017;27:2024-30. [Crossref] [PubMed]
- Weber U, Jurik AG, Lambert RG, Maksymowych WP. Imaging in Spondyloarthritis: Controversies in Recognition of Early Disease. Curr Rheumatol Rep 2016;18:58. [Crossref] [PubMed]
- Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ. Fat metaplasia and backfill are key intermediaries in the development of sacroiliac joint ankylosis in patients with ankylosing spondylitis. Arthritis Rheumatol 2014;66:2958-67. [Crossref] [PubMed]
- Maksymowych WP, Wichuk S, Dougados M, Jones HE, Pedersen R, Szumski A, Marshall L, Bukowski JF, Lambert RG. Modification of structural lesions on MRI of the sacroiliac joints by etanercept in the EMBARK trial: a 12-week randomised placebo-controlled trial in patients with non-radiographic axial spondyloarthritis. Ann Rheum Dis 2018;77:78-84. [Crossref] [PubMed]
- Weber U, Lambert RG, Pedersen SJ, Hodler J, Østergaard M, Maksymowych WP. Assessment of structural lesions in sacroiliac joints enhances diagnostic utility of magnetic resonance imaging in early spondylarthritis. Arthritis Care Res (Hoboken) 2010;62:1763-71. [Crossref] [PubMed]
- Hermann KG, Baraliakos X, van der Heijde DM, Jurik AG, Landewé R, Marzo-Ortega H, Østergaard M, Rudwaleit M, Sieper J, Braun J. Assessment in SpondyloArthritis international Society (ASAS). Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis 2012;71:1278-88. [Crossref] [PubMed]
- Griffith JF, Wang D, Shi L, Yeung DK, Lee R, Shan TL. Computer-Aided Assessment of Spinal Inflammation on Magnetic Resonance Images in Patients With Spondyloarthritis. Arthritis Rheumatol 2015;67:1789-97. [Crossref] [PubMed]
- de Hooge M, van den Berg R, Navarro-Compán V, Reijnierse M, van Gaalen F, Fagerli K, Landewé R, van Oosterhout M, Ramonda R, Huizinga T, van der Heijde D. Patients with chronic back pain of short duration from the SPACE cohort: which MRI structural lesions in the sacroiliac joints and inflammatory and structural lesions in the spine are most specific for axial spondyloarthritis? Ann Rheum Dis 2016;75:1308-14. [Crossref] [PubMed]
- Kang Y, Hong SH, Kim JY, Yoo HJ, Choi JY, Yi M, Kang HS. Unilateral Sacroiliitis: Differential Diagnosis Between Infectious Sacroiliitis and Spondyloarthritis Based on MRI Findings. AJR Am J Roentgenol 2015;205:1048-55. [Crossref] [PubMed]
- Berthelot JM, le Goff B, Maugars Y, Laredo JD. Sacroiliac joint edema by MRI: Far more often mechanical than inflammatory? Joint Bone Spine 2016;83:3-5. [Crossref] [PubMed]
- Resnick D, Niwayama G, Goergen TG. Degenerative disease of the sacroiliac joint. Invest Radiol 1975;10:608-21. [Crossref] [PubMed]
- Leibushor N, Slonimsky E, Aharoni D, Lidar M, Eshed I. CT Abnormalities in the Sacroiliac Joints of Patients With Diffuse Idiopathic Skeletal Hyperostosis. AJR Am J Roentgenol 2017;208:834-7. [Crossref] [PubMed]
- Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology 1976;119:559-68. [Crossref] [PubMed]
- Hod N, Ashkenazi I, Levi Y, Fire G, Drori M, Cohen I, Bernstine H, Horne T. Characteristics of skeletal stress fractures in female military recruits of the Israel defense forces on bone scintigraphy. Clin Nucl Med 2006;31:742-9. [Crossref] [PubMed]
- Johnson AW, Weiss CB Jr, Stento K, Wheeler DL. Stress fractures of the sacrum. An atypical cause of low back pain in the female athlete. Am J Sports Med 2001;29:498-508. [Crossref] [PubMed]
- Ahovuo JA, Kiuru MJ, Visuri T. Fatigue stress fractures of the sacrum: diagnosis with MR imaging. Eur Radiol 2004;14:500-5. [Crossref] [PubMed]
- Mitra R. Osteitis Condensans Ilii. Rheumatol Int 2010;30:293-6. [Crossref] [PubMed]
- Thompson M. Osteitis condensans ilii and its differentiation from ankylosing spondylitis. Ann Rheum Dis 1954;13:147-56. [Crossref] [PubMed]
- Ma L, Gao Z, Zhong Y, Meng Q. Osteitis condensans ilii may demonstrate bone marrow edema on sacroiliac joint magnetic resonance imaging. Int J Rheum Dis 2018;21:299-307. [Crossref] [PubMed]
- Braun J, Bollow M, Seyrekbasan F, Häberle HJ, Eggens U, Mertz A, Distler A, Sieper J. Computed tomography guided corticosteroid injection of the sacroiliac joint in patients with spondyloarthropathy with sacroiliitis: clinical outcome and followup by dynamic magnetic resonance imaging. J Rheumatol 1996;23:659-64. [PubMed]
- Soneji N, Bhatia A, Seib R, Tumber P, Dissanayake M, Peng PW. Comparison of Fluoroscopy and Ultrasound Guidance for Sacroiliac Joint Injection in Patients with Chronic Low Back Pain. Pain Pract 2016;16:537-44. [Crossref] [PubMed]
- Jee H, Lee JH, Park KD, Ahn J, Park Y. Ultrasound-guided versus fluoroscopy-guided sacroiliac joint intra-articular injections in the noninflammatory sacroiliac joint dysfunction: a prospective, randomized, single-blinded study. Arch Phys Med Rehabil 2014;95:330-7. [Crossref] [PubMed]
- Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci 2007;12:274-80. [Crossref] [PubMed]
- Hartung W, Ross CJ, Straub R, Feuerbach S, Schölmerich J, Fleck M, Herold T. Ultrasound-guided sacroiliac joint injection in patients with established sacroiliitis: precise IA injection verified by MRI scanning does not predict clinical outcome. Rheumatology (Oxford) 2010;49:1479-82. [Crossref] [PubMed]
- Klauser A, De Zordo T, Feuchtner G, Sögner P, Schirmer M, Gruber J, Sepp N, Moriggl B. Feasibility of ultrasound-guided sacroiliac joint injection considering sonoanatomic landmarks at two different levels in cadavers and patients. Arthritis Rheum 2008;59:1618-24. [Crossref] [PubMed]
- Chang WH, Lew HL, Chen CP. Ultrasound-guided sacroiliac joint injection technique. Am J Phys Med Rehabil 2013;92:278-9. [Crossref] [PubMed]