Monitoring reperfused myocardial infarction with delayed left ventricular systolic dysfunction in rabbits by longitudinal imaging
Introduction
Myocardial infarction (MI) is the leading cause of morbidity, mortality, and disability worldwide and remains one of the greatest challenges in biomedical research (1). The evaluation of cardiac dysfunction after MI is essential for the prediction and diagnosis of heart failure (HF). The common fundamental defect in cardiac dysfunction after MI is a gradually decreased ability of the heart to provide sufficient cardiac output to support the normal functions of organs due to impaired ejection of the left ventricle and blood perfusion to tissues. Severe left ventricle systolic dysfunction (LVSD) will lead to irreversible congestive HF. Therefore, LVSD is most frequently studied to elucidate the physiological and pathophysiological mechanisms of HF and to develop new diagnostic and therapeutic approaches for HF after MI (2-5). Several factors such as infarct size, LV remodeling, stunned or hibernating myocardium, and mechanical complications, may influence the appearance of LVSD after MI (6-8). Among these factors, ventricular remodeling is the most important (8). The process of ventricular remodeling refers to alterations in ventricular architecture, associated with an increased volume and an altered chamber configuration, which are driven by a combination of pathologic hypertrophy and apoptosis of myocytes, myofibroblast proliferation, and interstitial fibrosis (9). Ventricular remodeling is directly implicated in post-infarction development of ventricular dilation, a process that can influence LV function and survival outcomes (10). Multiple imaging approaches have been used to better understand the structural and molecular changes that underlie the progression of LV remodeling from myocardial injury to MI and, ultimately, to congestive HF. Advances in cardiovascular imaging have come at a rapid pace over the last several years (11). Cardiac magnetic resonance imaging (cMRI) and speckle-tracking imaging with ultrasound echocardiography (Echo) have been widely used to monitor LV remodeling after MI. These techniques can (I) provide specific quantitation of cardiac function, accurate chamber volumes and structure, myocardial viability and coronary perfusion in both acute and chronic settings and, (II) exclude mechanical complications (11-14), though often lacking gold standard histopathology proofs.
In translational cardiology, development of appropriate animal models for non-invasive evaluation of cardiac function is important to understand mechanisms of cardiac remodeling, and to provide new therapeutic strategies. Surgical complete coronary ligation to induce irreversible myocardium damage and subsequent remodeling has been extensively used and described (15-17), but often with high mortality due to severe ventricular tachycardia and ventricular fibrillation in the acute phase (18-20). While small animal MI models in rats and mice (19,21) display marked differences compared to the human heart (22), larger animals such as the dog, sheep and swine are very labor intensive and expensive (23,24). We experienced that the rabbit coronary occlusion/reperfusion model meets the requirements of the ideal experimental model (25). A medium sized rabbit heart has many similarities to the human heart in terms of cardiovascular anatomy, ventricular performance, cardiac metabolism, electrophysiology, coronary artery distribution, and collateralization after an acute event. In addition, with the lower phylogenetic scale, longer life span, strain-specific characteristics and low cost, the rabbit is a suitable species for cardiac research (13,25,26). Technically, the dimension of a rabbit heart is large enough to study with clinical imaging scanners and small enough to just fit in a standard glass slide for entire-heart-slice histomorphologic studies, both of great translational significance.
In the clinic, approximately 40% of MI patients suffer from LVSD (8). However, a good animal model of LVSD has not been proposed thus far. Most experiments have focused on acute or subacute MI over hours and days, but not on a MI model that systematically progresses to chronic LVSD. In this study, we established a rabbit model of reperfused MI that evolves to chronic LVSD over the time course of 7 weeks, and developed an imaging platform for longitudinal monitoring and evaluation of cardiac morphology and contractile function. This experimental platform combines in vivo cardiac MRI and echocardiography with postmortem immunohistochemical analysis.
Methods
Experimental protocol
All animal procedures were approved by the Ethical Committee of the KU Leuven. Fifty-five New Zeeland white male rabbits (3–4 kg) were obtained from the Animal Center of KU Leuven (Heverlee, Belgium). Animals were randomized into two groups: one group was subjected to open-chest surgery without manipulation of the heart (sham group), while the other group received induction of MI (MI group). To monitor cardiac function, cMRI and echocardiography were performed at baseline, 48 h (cMRI only), 1 week (echo only) and 7 weeks post MI on each animal. After 7 weeks, rabbits were killed for further multiple histological processions.
MI model
All surgical procedures were performed in a sterile manner in the animal operating suites. The rabbit model of acute reperfused MI was previously described in details (13). Briefly, rabbits were sedated, endotracheally intubated and mechanically ventilated. After intravenous (iv) access was established, rabbits received 40 mg/kg/h sodium pentobarbital to maintain anesthesia. After disinfection of the chest, skin and subcutaneous tissues were cut open by layers along the left sternal border. Subsequently, the 4th and 5th intercostal space cartilages were cut and the pericardium was opened to expose the left circumflex artery branch (LCx). The LCx was ligated by a detachable knot using 2-0 silk at 2 mm below the left atrial appendage, of which the pullable end was left outside the thorax after closure of the thoracic cavity by layered sutures. Reperfusion was induced by pulling the exteriorized end of the suture in a closed-chest condition after 1 h of coronary occlusion. Similar procedures were applied for sham-operated animals, except for the LCx ligation. In the event of sustained ventricular fibrillation during coronary occlusion or reperfusion, animals were given 2% XYLOCAINE (1 mg/kg iv; lidocain, Eurovet Animal Health B.V.). After reperfusion, animals were allowed to recover on a warming blanket and were ventilated further until their own respiration took over.
Serum cardiac troponin T (cTnT) measurements
To determine the optimal time point of peak cTnT concentrations, serum samples at different time points post MI (baseline, 30 min, 1, 2, 4, 8 and 24 h) from a rabbit were collected from the ear vein without anesthesia. Serum cTnT levels were evaluated using a routine laboratory assay.
Cardiac magnetic resonance imaging
Using a 16-channel phased array knee coil, cMRI was performed on the anesthetized rabbit at a 3.0T clinical Siemens MRI scanner (Trio, Siemens, Erlangen, Germany) with a maximum gradient capability of 45 mT/m, triggered by ECG and gated by respiration using a small animal monitoring and gating system (SA Instruments, Inc. Stony Brook, NY, USA). The two ECG electrodes were attached to the shaved thorax skin with an apical pulse and to the left leg. The respiration sensor was attached to abdomen of the rabbit, which was placed supinely in a holder and gas-anesthetized with 2% isoflurane in the mixture of 20% oxygen and 80% room air, through a mask connected via a tube to a ventilation instrument (Harvard Apparatus, Holliston, MA, USA). All images were acquired during free breathing of the animal. Eight short-axial slices of the heart were collected with a slice thickness of 3.0 mm without gap to cover the entire LV. Turbo spin echo sequence of black blood imaging was applied for cardiac morphology with the parameters: TR of 621–750 ms, TE of 15–74 ms, FOV of 240×195 mm2, FA of 180°, and in-plane resolution of 0.9×0.9 mm2. The cine-MRI images were acquired on gradient echo in the short-axis, vertical long-axis and horizontal long-axis planes for displaying cardiac contraction. Each cine-MRI consisted of 25 frames/cycle, and the scan parameters: TR of 23 ms, TE of 3.7 ms, FOV of 240×195 mm2, FA of 12° and spatial resolution of 1.3×0.9 mm2. The delayed contrast enhancement (CE) images were acquired by a 3D segmented k-space inversion recovery turbo fast low angle shot sequence 20 minutes after an iv bolus injection of meglumine gadoterate (Gd-DOTA, Dotarem®, Guerbet, France) at 0.2 mmol/kg with parameters: TR of 396 ms, TE of 1.54 ms, TI of 360 ms, FOV of 240×180 mm2, FA of 15° and in-plane resolution of 1.1×0.8 mm2.
Analysis of cMRI
cMRI images were read using an off-line workstation with dedicated software (SyngoMR A30, Siemens). The assessment and quantification of MI size and global LV function in CE and Cine-MRI images were made using the software SEGMENT (Medviso AB, Lund, Sweden). The endocardial and epicardial borders were manually traced in the end-diastolic and end-systolic short-axis Cine images. Papillary muscles were included in the myocardium. Global LV functions including end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), ejection fraction (EF), cardiac output (CO) and mass were measured according to standard methods (4). Regional LV function was also assessed by measuring wall thickening from end-diastolic phase to end-systolic phase in six clockwise sectors on the mid-ventricle section of Cine images.
Transthoracic echocardiography
Transthoracic echocardiographic examinations were performed on anesthetized rabbits using a 10S transducer (4.4–10 Mhz) (GE Healthcare, Machelen, Belgium) on a Vivid 7 ultrasound machine (GE Healthcare). LV internal diameter at end-diastolic (LVIDd) and end-systolic phase (LVIDs), muscle thickness in diastole (IVSd) and in systole (IVSs) and LV posterior wall thickness in end-diastole (LVPWd) and end-systole (LVPWs) were measured at three levels: at the level of the mitral valve (mv), papillary muscle (pm) and apex (ap). In addition, the long axis diameter in end-diastole (LAXd) and systole (LAXs) was obtained. EDV [EDV = (LVIDd_mv2 + LVIDd_pm2 + LVIDd_ap2) ×LAXd×Π/18] and ESV [ESV = (LVIDs_mv2 + LVIDs_pm2 + LVIDs_ap2) ×LAXs×Π/18], EF {EF = [(EDV – EDV)/EDV] ×100}, and SV (SV = EDV – ESV) were calculated (27). The measurements for all parameters at 1 and 7 weeks post-surgery are reported as a percentage of the baseline measurement for each animal. Subsequently, an average per group was calculated for each parameter.
Histomorphology
After in vivo data acquisition, rabbits were euthanized with an overdose of sodium pentobarbital. The isolated heart and left lung were photographed, and then fixed with 10% formalin for 24 h. The fixed heart was cut into 3 mm short-axis sections, which were paraffin-embedded and cut into 5 µm slices. The heart slices were mounted entirely on standard glass slides, followed by hematoxylin-eosin (HE) and Masson Trichrome (MT) staining for necrosis and chronic fibrosis evaluation. Myocardial macrophage infiltration was evaluated by RAM-11 staining, using a mouse monoclonal anti-rabbit macrophage clone (1/50; M0633; DAKO, Leuven, Belgium) in Tris-NaCl-blocking buffer (TNB) (Perkin Elmer, Boston, USA). RAM-11 signals were subsequently detected with the Tyramide Signal Amplification kit (Perkin Elmer). In addition, the dissected lung was photographed and a small portion of fixed pulmonary tissue was paraffin-embedded and cut into 5 µm slices for HE staining to observe the presence or absence of HF-induced pulmonary congestion.
Statistical analysis
Data are shown as means ± SD for the number of animals studied. Differences between all groups were analyzed using the parametric one-way ANOVA. If a statistical difference was detected (P<0.05), the difference between the individual groups was determined using the Tukey’s multiple comparison test. Correlation analyses between serum cTnT levels and LV infarct sizes and between EF measurements of MRI and Echo were performed using the non-parametric Pearson correlation test. All statistical analyses were performed with GraphPad Prism 6 software (GraphPad, La Jolla, CA, USA).
Results
General characteristics of the rabbits
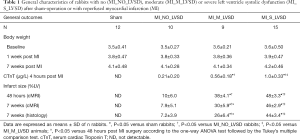
In 48 out of 55 rabbits, surgery was successful (87.3%). Mortality rate was 12.7% (7/55) or 3.6% (2/55) for the acute or chronic stage respectively. Four groups were distinguished: sham (n=12), MI_NO_LVSD (n=10), MI_M (moderate)_LVSD (n=9), and MI_S (severe)_LVSD (n=15). LVSD and severe LVSD developed in 71% (24/34) and 44% (15/34) respectively of MI animals after 7 weeks.
Body weight was not significantly different between the groups at the start, at 1 and 7 weeks post-surgery (Table 1). Serum cTnT levels were the highest in MI_S_LVSD rabbits and were significantly different from that of sham, MI_NO_LVSD and MI_M_LVSD animals (Table 1). Serum cTnT levels correlated positively with LV infarct size at the acute stage (r=0.89; P<0.0001), while they were negatively correlated with EF (r=0.95; P<0.01).
Full table
Cardiac MRI
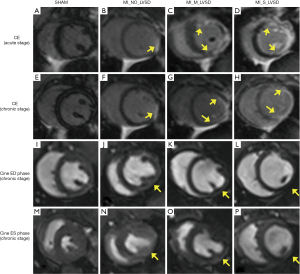
MI sizes were less than 20% of the LV in MI_No_LVSD, 20–42% of the LV in MI_M_LVSD and 43–54% of the LV in MI_S_LVSD (Table 1). Chronic MI sizes by cMRI were smaller than acute MI sizes, and were better correlated with MI sizes determined by histology (r=0.93 versus r=0.86, respectively). As shown in Figure 1, longitudinal evaluation by in vivo CE-MRI of mid-ventricular slices at 48 hrs and at 7 weeks post MI (Figure 1A,B,C,D,E,F,G,H) revealed that the hyper-enhanced MI region in the MI_S_LVSD animals extended to the anterior, lateral and posterior wall at the acute stage (Figure 1D). This range persisted, however the wall became thinner 7 weeks (Figure 1H) post-surgery for MI_S_LVSD rabbits in comparison to the other groups. The corresponding cine images for the MI_S_LVSD (Figure 1L,P) rabbits indicated that the LV dilated and wall motion decreased, as can be demonstrated vividly by the videos of cine cMRI (Figure S1).

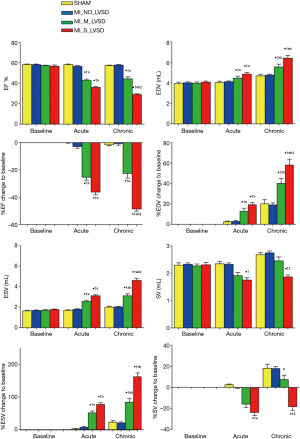
For global cardiac functions as shown in Figure 2, cMRI parameters did not show significant differences (either as raw data or expressed as % change of baseline) between sham and MI_NO_LVSD animals at either acute (48 hrs) or chronic (7 weeks) post MI stages. MI_M_LVSD rabbits at the acute and chronic stage showed an unrecovered reduction in cardiac function (EF decreased from 57% to 43% and to 44%, respectively) and functional changes (%EF decreased by 25% and 23%, respectively). In MI_S_LVSD rabbits this reduction was more pronounced (EF decreased from 58% to 36% and to 28%, respectively; %EF decreased by 36% and 49%, respectively). MI size was significantly correlated with EF for all groups (r=0.95, P<0.01). In contrast to sham rabbits, EDV and ESV in MI_M_LVSD animals increased by 10% and 45% respectively at the acute stage and by 20% and 60% respectively at the chronic stage; in MI_S_LVSD rabbits EDV and ESV were increased by 17% and 70% respectively at the acute stage and by 27% and 130% respectively at the chronic stage (Figure 2). SV in MI_M_LVSD rabbits decreased by 17% and 11% at the acute and chronic stage, respectively; the decrease of SV in MI_S_LVSD animals was more pronounced (25% and 36% at the acute and chronic stage respectively, suggesting decompensation of the heart), in comparison to the sham group.
For regional cardiac function measured by six segments, there were no significant changes of LV wall thickening between sham and MI_NO_LVSD animals at both experimental stages (Table 2). Wall thickening significantly decreased to about 44% and 42% at the acute and chronic stage, respectively (P<0.05 for each) in only two cardiac segments (inferolateral and anterolateral) of MI_M_LVSD rabbits. But, wall thickening significantly decreased (25–38% at the acute stage; 25–41% at the chronic stage; P<0.05 for each) in up to four cardiac segments (anterior, inferior, inferolateral and anterolateral) of MI_S_LVSD rabbits, as compared to other groups (Table 2).

Full table
Echocardiography
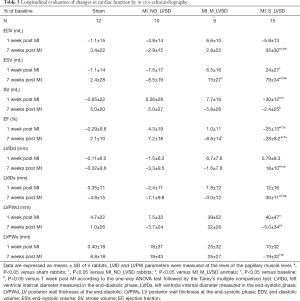
Echocardiographic analysis revealed that rabbits with the largest LV infarct size (MI_S_LVSD group) developed a severe cardiac dysfunction 1 and 7 weeks post MI, as indicated by a significant EF reduction as compared to the other groups (Table 3). Acutely, hearts of these rabbits showed a significant increase in ESV as compared to MI_NO_LVSD rabbits, while EDV was not different. This resulted in a significant reduction of the SV in rabbits with severe LVSD versus the other three groups (Table 3). At the chronic stage both EDV and ESV were increased and EF significantly reduced in MI_S_LVSD rabbits as compared to the other animals. A strong positive correlation was found between EF determined by echocardiography and by cMRI at both time points, but the correlation was even stronger at 7 weeks as compared to 1 week post MI surgery (Figure 3).
Full table
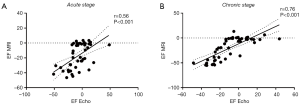
For MI_S_LVSD rabbits LVID in both phases was also significantly enhanced as compared to the other groups (Table 3). In addition, these animals had significant thinning of the posterior wall at the papillary muscle level in the chronic phase as compared to the other groups; however, this change was more pronounced in the end-systolic phase as compared to the end-diastolic phase (Table 3).
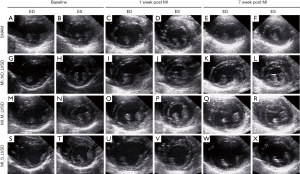
Figure 4 illustrates the echocardiographic changes as shown in Table 3. For sham-operated and MI_NO_LVSD animals, LV volume and wall thickness did not change over time. Hypokinesis and akinesis of the posterior wall was observed for rabbits with a large infarct area (MI_M_LVSD and MI_S_LVSD) at the acute stage. At the chronic stage hypokinesis and akinesis was more severe for the MI_S_LVSD group. MI_S_LVSD rabbits showed the highest dilation of the heart and extreme thinning of the posterior wall in the end-diastolic (Figure 4W) and end-systolic phase (Figure 4X) as compared to the other groups at the chronic stage.
Postmortem histomorphology
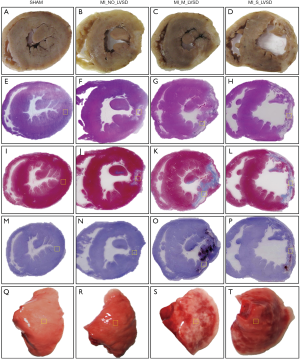
Postmortem heart sections of the four animal groups revealed ventricular remodeling to different degrees among three MI groups (Figure 5). The most extensive infarct lesion was present in MI_S_LVSD animals (whitish region in Figure 5D). The infarct lesion was substantially smaller for animals with moderate (Figure 5C) or no LVSD (Figure 5B) relative to no infarct in sham animal (Figure 5A). The LV cavity was dilated, the thickness of lateral wall was decreased and the papillary muscles were atrophied in rabbits with MI_S_LVSD (Figure 5D) relative to the other groups. The transverse heart slices (Figure 5A,B,C,D), size of the infarct lesion, dilation of the LV cavity and lateral wall thickness corresponded well in-between the macroscopic views of the HE (Figure 5E,F,G,H), MT (Figure 5I,J,K,L) and RAM-11 (Figure 5M,N,O,P) stained heart sections. The isolated wet lung lobes gradually turned dark red from sham animals to rabbits with MI_S_LVSD due to a different degree of pulmonary congestion (Figure 5Q,R,S,T), confirming our findings in the heart.
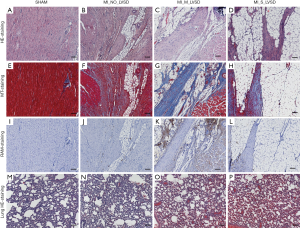
Microscopically (Figure 6), MI_NO_LVSD hearts had more fibrosis (Figure 6F) and more adipocyte (Figure 6B,F,J) and macrophage (Figure 6J) infiltration as compared to the heart of sham animals (Figure 6A,E,I). Heart sections of MI_M_LVSD rabbits showed most severe infiltration of inflammatory macrophages (Figure 6K) and interstitial collagen deposition (Figure 6C,G) and moderate presence of adipocytes (Figure 6C,G,K). Hearts of rabbits with MI_S_LVSD were characterized by excessive presence of adipocytes (Figure 6D,H,L), enriched fibrotic collagen (Figure 6H) and less macrophage infiltration (Figure 6L), resulting in post-MI scar formation. In addition, microscopically, lung sections (Figure 6M,N,O,P) demonstrated that the capillaries in the alveolar walls were congested with red blood cells in MI versus sham rabbits, with the highest degree of congestion in the lung of rabbits with MI_S_LVSD.
Discussion
In this study we report, for the first time, an occlusion/reperfusion rabbit model that evolved from the acute MI into chronic stage with four different groups (sham, MI_NO_LVSD, MI_M_LVSD, and MI_S_LVSD) by using a combination of cMRI, echocardiography, serum biomarker cTnT test and histomorphology. The highest degree of LV remodeling was observed with the largest infarct size in MI_S_LVSD models at 1 and 7 weeks post MI surgery. EF measured by cMRI in these chronic MI_S_LVSD rabbits was less than 35%, which is consistent with clinical results (28,29). This change was accompanied by a severe dilation of the heart as evidenced by an increase of EDV and ESV by 50% and 150%, respectively after 7 weeks post MI. Among all MI models, 44% displayed severe LVSD, which is close to the clinical finding that 40% of MI patients suffer from LVSD following MI (7,8). The different degrees of chronic LVSD could be distinguished from sham and MI_NO_LVSD rabbits via imaging and histology.
The different symptoms and/or signs with HF are often subjective, and the threshold for diagnosis may vary widely among clinicians. However, the presence and degree of LVSD can be measured objectively by cardiac imaging techniques. To induce a rabbit model of LVSD is technically challenging and only rarely reported with a less advanced imaging methods (30). In our study, we induced acute MI with ST-segment elevation by abrupt surgical occlusion of the LCx artery for 1 hour followed by coronary reperfusion. As a result, the myocardium distal to the occlusion becomes irreversibly necrotic instead of reversibly ischemic due to the prolonged ischemia (>30 minutes). Unrelieved ischemia causes permanent damage to the myocardium previously supplied by the occluded LCx artery. Moreover, the destroyed myocardium is replaced by fibrous scar tissue in the MI area and non-MI myocardium may develop hypertrophy over time. Because scar tissue does not contribute to myocardial contractility, global LV contractile function becomes impaired when the scar is large, resulting in progressive chronic LVSD. To establish a successful LVSD model, we had to induce the largest possible MI size in order to significantly impair cardiac function. This however bears risks: animals may die during or shortly after surgery due to a too large MI, severe arrhythmia, postoperative infection, etc. To avoid these risks: (I) the LCx was accurately ligated to ensure successful surgery and to reduce animal mortality; (II) the entire surgery was performed under a sterile environment to avoid infection; and (III) xylocaine was applied to prevent/stop arrhythmias during the LCx ligation-reperfusion stage. Finally, ventilation was properly maintained and each animal was kept at an optimal oxygenation level until it completely woke up. These conditions are based on our previous studies in which rabbit MI models were utilized (4,13,25,26).
So far most of the animal experiments on MI have focused on acute or subacute MI over hours and days, but the follow-up by cMRI and echocardiography over months have not been reported. Using a non-invasive imaging platform, MI from acute through chronic stage has been systematically investigated in this study. MI sizes after surgery were determined by delayed contrast-enhanced cMRI in vivo, and cardiac contractility was evaluated both by cine cMRI and echocardiography at the acute and chronic stages. We found that cardiac contractile function strongly correlated with MI sizes. According to cardiac function measurements, serum troponin T level analysis and histology, we distinguished four different groups after surgery: sham, MI_NO_LVSD, MI_M_LVSD and MI_S_LVSD rabbits. Acute serum levels of cardiac troponin T, a marker for myocardial injury used in the diagnosis of acute MI (31), correlated well with infarct sizes. EF, the most common parameter of global cardiac performance in clinical practice, is more influenced by the degree of LV remodeling than by any other factors (6). Therefore, we focused on EF changes for evaluation of global cardiac function and remodeling. At present, echocardiography remains the predominant clinically applicable non-invasive test of choice, based on a broader availability. The 2D echocardiography is well established and has emerged as an important non-invasive clinical tool for the assessment of LV systolic and diastolic function after MI (30,32). However, non-invasive 2D echocardiography on small animals is challenging. Although echocardiography on rabbits has been reported, most studies focused on the complete occlusion coronary artery model (30,32-34). Only one study reported the regional function in a rabbit ischemia-reperfusion model, but the authors used open chest invasive echocardiography (35). In our study, we longitudinally evaluated global LV function using non-invasive echocardiography in a rabbit ischemia-reperfusion model. We showed high-quality 2D cardiac images of rabbits with different MI sizes, which correlated well with cMRI at both acute and chronic stages.
Postmortem histology confirmed the different degrees of LV remodeling in the 3 MI groups. All animals with a large extended transmural infarction belonged to the MI group with severe LVSD, while the MI_M_LVSD rabbits showed smaller infarct areas without transmurality. This may explain why some rabbits with initially a high reduction in EF and a large infarct size at the acute stage, do not develop severe LVSD in the end. At 7 weeks post MI, the LV architecture changed in the infarcted regions, resulting in different degrees of fibrosis or collagen deposition, as well as adipocyte and macrophage infiltration as characterized using H&E and RAM-11 stainings. We found the largest quantities of macrophages mixed with fibrotic collagen and normal myocytes in the moderate LVSD heart, while the MI region in the severe LVSD heart was replaced by scar tissue enriched with adipose cells and fibrotic collagen, but with less macrophages. Larger transmural infarcts were associated with more infiltrating adipocytes, more collagen deposition/fibrosis and fewer macrophages as compared to smaller non-transmural infarcts as the MI transitioned from acute into chronic stage. In the severe LVSD heart, a large amount of adipose cells and fibrotic collagen predominantly formed the infarct scar region during the remodeling process, which resulted in elongation and thinning of the infarcted LV. These changes provoked a progressive decline in ventricular performance. In turn, the LV chamber became enlarged and the shape of the heart shifted from an elliptical to a more spherical chamber configuration. No statistically significant differences were seen in septal wall thickness between the four groups, which may indicate that cardiomyocyte size may not have been affected. The reason may be that seven weeks post MI in rabbits are not long enough to induce the compensatory hypertrophy of normal myocardium. To confirm the different degrees of ventricular damage in our model, we analyzed postmortem wet lungs as indirect evidence. A bigger LV infarct size resulted in LV dysfunction, and ultimately led to accumulation of red blood cells in the capillaries of the pulmonary alveoli. The MI_S_LVSD group did indeed show the highest level of blood congestion as evidenced by both macro- and microscopic findings. This is the first study to report such findings in the rabbit.
MI induced by left anterior descending (LAD) coronary artery ligation results in more uniform infarct sizes, but the mortality is more than 50% due to severe ventricular tachycardia during and after the MI induction (22). In our study, we show that ligation of the LCx in rabbits reduced the mortality rate to 12.7% and 3.6% at the acute and chronic stage, respectively. Although we always ligated the LCx at the same location (2 mm below the left atria), the infarct extent is quite variable. This may be due to the anatomy of the rabbit coronary artery system. This not only differs between species, but may also vary significantly within a single species. In our study, different infarct sizes were obtained, which in turn developed into different degrees of LV dysfunction, thus more closely resembling structural and functional characteristics of the dysfunction in human patients. Combined with the advanced clinical cardiac imaging techniques (36-40), this experimental LVSD platform in rabbits can be easily applied in clinically relevant imaging studies on translational cardiology and can help strengthen drug development and clinical research for the management of cardiovascular diseases. Recently, this platform has already been successfully applied in ongoing cardiac animal experiments (41,42).
There exist certain limitations in this study. The mortality could become higher at the acute stage if the infarct area was made too big. To reduce the mortality we choose LCx instead of LAD ligation, it was difficult to control the progress of MI, thus not all MIs turned transmural and further became cases of S_LVSD. The plasma concentrations of N terminal pro B type natriuretic peptide (NT-proBNP) after MI provide an alternative method of assessing cardiac function, but we could not detect NT-proBNP in our rabbit LVSD models, the reasons might be that the kit is not sensitive to rabbit serum, or seven weeks post MI are not long enough for NT-proBNP detection.
Conclusions
Our study provides a rabbit model with different degrees of chronic LVSD, which could be distinguished from sham and MI_NO_LVSD rabbits via in vivo cMRI, echocardiography and postmortem histology. Our model matches the pathophysiology of systolic dysfunction after MI in human patients and is highly reproducible and cost-effective. It may also be useful for the preclinical testing of treatments targeting myocardial damage following MI and satisfy the needs in preclinical or translational cardiac imaging research.
Acknowledgements
We thank Christine Vranckx, Inge Vorsters for expert technical assistance, and Jie Yu for making histological preparations. We are grateful to Prof. Paul Herijgers and Mieke Ginckels for access to the surgery rooms and the Vivid 7 ultrasound machine.
Funding: This work was supported by a grant from the ‘Agentschap voor Innovatie door Wetenschap en Technologie Onderzoek & Ontwikkeling’ (ICD 668894), awarded to the groups of Prof. Ni and Prof. Lijnen and to Dr. Lu and Dr. Gallacher from Janssen Pharmaceutical NV.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
Ethical Statement: All animal procedures were approved by the Ethical Committee of the KU Leuven.
References
- Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 2016;4:256. [Crossref] [PubMed]
- Raya TE, Gay RG, Lancaster L, Aguirre M, Moffett C, Goldman S. Serial changes in left ventricular relaxation and chamber stiffness after large myocardial infarction in rats. Circulation 1988;77:1424-31. [Crossref] [PubMed]
- Raya TE, Gay RG, Aguirre M, Goldman S. Importance of venodilatation in prevention of left ventricular dilatation after chronic large myocardial infarction in rats: a comparison of captopril and hydralazine. Circ Res 1989;64:330-337. [Crossref] [PubMed]
- Feng Y, Chen F, Yin T, Xia Q, Liu Y, Huang G, Zhang J, Oyen R, Ni Y. Pharmacologic Effects of Cannabidiol on Acute Reperfused Myocardial Infarction in Rabbits: Evaluated With 3.0T Cardiac Magnetic Resonance Imaging and Histopathology. J Cardiovasc Pharmacol 2015;66:354-363. [Crossref] [PubMed]
- Monnet E, Chachques JC. Animal models of heart failure: what is new? Ann Thorac Surg 2005;79:1445-53. [Crossref] [PubMed]
- Zornoff LA, Paiva SA, Duarte DR, Spadaro J. Ventricular remodeling after myocardial infarction: concepts and clinical implications. Arq Bras Cardiol 2009;92:150-64. [PubMed]
- Cleland JG, Torabi A, Khan NK. Epidemiology and management of heart failure and left ventricular systolic dysfunction in the aftermath of a myocardial infarction. Heart 2005;91 Suppl 2:ii7-13; discussion ii31, ii43-18.
- Minicucci MF, Azevedo PS, Polegato BF, Paiva SA, Zornoff LA. Heart failure after myocardial infarction: clinical implications and treatment. Clin Cardiol 2011;34:410-4. [Crossref] [PubMed]
- Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging 2011;4:98-108. [Crossref] [PubMed]
- Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 2000;101:2981-8. [Crossref] [PubMed]
- Flachskampf FA, Schmid M, Rost C, Achenbach S, DeMaria AN, Daniel WG. Cardiac imaging after myocardial infarction. Eur Heart J 2011;32:272-83. [Crossref] [PubMed]
- Weaver JC, Ramsay DD, Rees D, Binnekamp MF, Prasan AM, McCrohon JA. Dynamic Changes in ST Segment Resolution After Myocardial Infarction and the Association with Microvascular Injury on Cardiac Magnetic Resonance Imaging. Heart Lung Circ 2011;20:111-8. [Crossref] [PubMed]
- Feng Y, Xie Y, Wang H, Chen F, Ye Y, Jin L, Marchal G, Ni Y. A modified rabbit model of reperfused myocardial infarction for cardiac MR imaging research. Int J Cardiovasc Imaging 2009;25:289-98. [Crossref] [PubMed]
- Symons R, Claus P, Marchi A, Dresselaers T, Bogaert J. Quantitative and qualitative assessment of acute myocardial injury by CMR at multiple time points after acute myocardial infarction. Int J Cardiol 2018;259:43-6. [Crossref] [PubMed]
- Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ. American Heart Association Council on Basic Cardiovascular Sciences CoCC, Council on Functional G, Translational B. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 2012;111:131-50. [Crossref] [PubMed]
- Gupta S, Sen S. Animal models for heart failure. Methods Mol Med 2006;129:97-114. [PubMed]
- Kuster DW, Merkus D, Kremer A, van Ijcken WF, de Beer VJ, Verhoeven AJ, Duncker DJ. Left ventricular remodeling in swine after myocardial infarction: a transcriptional genomics approach. Basic Res Cardiol 2011;106:1269-81. [Crossref] [PubMed]
- Gao XM, Dart AM, Dewar E, Jennings G, Du XJ. Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovasc Res 2000;45:330-8. [Crossref] [PubMed]
- Saleh MG, Sharp SK, Alhamud A, Spottiswoode BS, van der Kouwe AJW, Davies NH, Franz T, Meintjes EM. Long-Term Left Ventricular Remodelling in Rat Model of Nonreperfused Myocardial Infarction: Sequential MR Imaging Using a 3T Clinical Scanner. J Biomed Biotechnol 2012;2012. [Crossref] [PubMed]
- Iwanaga K, Takano H, Ohtsuka M, Hasegawa H, Zou Y, Qin Y, Odaka K, Hiroshima K, Tadokoro H, Komuro I. Effects of G-CSF on cardiac remodeling after acute myocardial infarction in swine. Biochem Biophys Res Commun 2004;325:1353-9. [Crossref] [PubMed]
- Nahrendorf M, Hiller KH, Hu K, Ertl G, Haase A, Bauer WR. Cardiac magnetic resonance imaging in small animal models of human heart failure. Med Image Anal 2003;7:369-75. [Crossref] [PubMed]
- Klocke R, Tian W, Kuhlmann MT, Nikol S. Surgical animal models of heart failure related to coronary heart disease. Cardiovasc Res 2007;74:29-38. [Crossref] [PubMed]
- Dubi S, Arbel Y. Large animal models for diastolic dysfunction and diastolic heart failure-a review of the literature. Cardiovasc Pathol 2010;19:147-52. [Crossref] [PubMed]
- Geens JH, Trenson S, Rega FR, Verbeken EK, Meyns BP. Ovine models for chronic heart failure. Int J Artif Organs 2009;32:496-506. [Crossref] [PubMed]
- Feng Y, Bogaert J, Oyen R, Ni Y. An overview on development and application of an experimental platform for quantitative cardiac imaging research in rabbit models of myocardial infarction. Quant Imaging Med Surg 2014;4:358-75. [PubMed]
- Feng Y, Chen F, Ma Z, Dekeyzer F, Yu J, Xie Y, Cona MM, Oyen R, Ni Y. Towards stratifying ischemic components by cardiac MRI and multifunctional stainings in a rabbit model of myocardial infarction. Theranostics 2013;4:24-35. [Crossref] [PubMed]
- Stypmann J, Engelen MA, Troatz C, Rothenburger M, Eckardt L, Tiemann K. Echocardiographic assessment of global left ventricular function in mice. Lab Anim 2009;43:127-37. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Stevenson WG, Yancy CW. 2013 ACCF/AHA Guideline for the Management of Heart Failure: Executive Summary A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:1810-52. [Crossref] [PubMed]
- Verma A, Meris A, Skali H, Ghali JK, Arnold JMO, Bourgoun M, Velazquez EJ, McMurray JJV, Kober L, Pfeffer MA, Califf RM, Solomon SD. Prognostic Implications of Left Ventricular Mass and Geometry Following Myocardial Infarction The VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging 2008;1:582-591. [Crossref] [PubMed]
- Pennock GD, Yun DD, Agarwal PG, Spooner PH, Goldman S. Echocardiographic changes after myocardial infarction in a model of left ventricular diastolic dysfunction. Am J Physiol 1997;273:H2018-29. [PubMed]
- Christenson E, Christenson RH. Characteristics of cardiac troponin measurements. Coron Artery Dis 2013;24:698-704. [Crossref] [PubMed]
- Edwards R, Yousef Z, Rakhit R, Wright M, Rosenthal E, Redwood S, Marber M. A model of closed chest regional myocardial infarction in the rabbit: a clinically relevant in vivo assay system of post-infarction remodelling. Basic Res Cardiol 2002;97:374-83. [Crossref] [PubMed]
- Wang YT, Popovic ZB, Efimov IR, Cheng YN. Longitudinal Study of Cardiac Remodelling in Rabbits Following Infarction. Can J Cardiol 2012;28:230-8. [Crossref] [PubMed]
- Wu H, Li L, Niu P, Huang X, Liu J, Zhang F, Shen W, Tan W, Wu Y, Huo Y. The Structure-function remodeling in rabbit hearts of myocardial infarction. Physiol Rep 2017.5. [PubMed]
- Liu YJ, Leng XP, Du GQ, Wang XD, Tian JW, Ren M. Two-dimensional longitudinal strains and torsion analysis to assess the protective effects of ischemic postconditioning on myocardial function: a speckle tracking echocardiography study in rabbits. Ultrasonics 2015;56:344-53. [Crossref] [PubMed]
- Al Musa T, Uddin A, Swoboda PP, Garg P, Fairbairn TA, Dobson LE, Steadman CD, Singh A, Erhayiem B, Plein S, McCann GP, Greenwood JP. Myocardial strain and symptom severity in severe aortic stenosis: insights from cardiovascular magnetic resonance. Quant Imaging Med Surg 2017;7:38-47. [Crossref] [PubMed]
- Gupta S, Li H, Keshavamurthy JH, Sharma GK, Polimenakos AC. Case-based approach to demonstrate utility of cardiac magnetic resonance imaging (MRI) for planning biventricular repair with inconclusive echo: illustration of two cases. Quant Imaging Med Surg 2017;7:732-5. [Crossref] [PubMed]
- Lau JM, Zheng J. Disease-specific cardiovascular positron emission tomography/magnetic resonance imaging: a brief review of the current literature. Quant Imaging Med Surg 2016;6:297-307. [Crossref] [PubMed]
- Likhite D, Suksaranjit P, Adluru G, Wilson B, DiBella E. Estimating extraction fraction and blood flow by combining first-pass myocardial perfusion and T1 mapping results. Quant Imaging Med Surg 2017;7:480-95. [Crossref] [PubMed]
- Nemes A, Dezsi L, Domsik P, Kalapos A, Forster T, Vecsei L. Left ventricular deformation abnormalities in a patient with calpainopathy-a case from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Quant Imaging Med Surg 2017;7:685-90. [Crossref] [PubMed]
- Hemmeryckx B, Feng Y, Frederix L, Lox M, Trenson S, Vreeken R, Lu HR, Gallacher D, Ni YC, Lijnen HR. Evaluation of cardiac arrhythmic risks using a rabbit model of left ventricular systolic dysfunction. Eur J Pharmacol 2018;832:145-55. [Crossref] [PubMed]
- Hemmeryckx B, Feng Y, Frederix L, Lox M, Trenson S, Vreeken R, Lu HR, Gallacher D, Ni YC, Lijnen HR. Prolonged High-Fat Diet Feeding of WHHLMI Rabbits does Not Aggravate Metabolic or Cardiac Dysfunction. J diab Obes 2018;5:10-7.
- Feng Y, Hemmeryckx B, Frederix L, et al. Videos of in vivo cine cMRI display mid-ventricular slices at baseline without MI, at 48 hrs of acute MI phase and at 7 weeks of chronic MI phase on the rabbits of moderate (first line) and severe (second line) LVSD. Asvide 2018;5. Available online: http://www.asvide.com


