Percutaneous ultrasound-guided balloon-assisted embolization of iatrogenic femoral artery pseudoaneurysms with Glubran®2 cyanoacrylate glue: safety, efficacy and outcomes
Introduction
Iatrogenic pseudoaneurysm (PA) is a classical complication of arterial percutaneous diagnostic angiography or interventional procedures which occurs in 0.2–2.6% of cases (1,2).
Several therapeutic options have been described for the treatment of PAs, including surgery, ultrasound (US)-guided compression, coil embolization, stent-graft placement, or percutaneous thrombin injection. US-guided compression is effective in a large proportion of patient but is associated with pain and discomfort (3-6). Thrombin injection is more effective but at risk of distal spillage in limb arteries and anaphylaxis. Furthermore, thrombin is quite expensive (5-7) and not available everywhere. Surgical interventions are more invasive but still have a role to play in case of failure of others techniques or in patients presenting with mass effect (8).
N-butyl cyanoacrylate methacryloxy sulfolane (NBCA-MS) is a well-known glue comprising a proprietary comonomer as approved by the European Community for internal human use (9,10). This synthetically derived glue shows rapid polymerization (1–5 s), but complete sealing occurs in approximately 5 min. Additionally, NBCA-MS presents high echogenicity and for this reason it is easily detectable by US when injected in vessels or tissues. Lastly, the efficacy of glue is not affected by anticoagulant or antiplatelet therapy. Aytekin et al. (11) first described the use of glue injection as first-line therapy of iatrogenic PAs and Del Corso et al. (12) reported a high success rate of microinjections of cyanoacrylate glue after US-guided PAs compression. However, the main risk of glue injection by direct puncture of a pulsating femoral mass is distal spillage of glue in the native artery through the pseudoaneurysmal neck (13). To minimize such complications, US-guided compression of the PA’s neck before NBCA-MS injection into the sac may be performed but remains difficult, and is often times ineffective and painful because of the presence of hematoma. Percutaneous US-guided glue injection into the sac with a balloon-assisted technique might be a good option in order to avoid distal spillage of glue in limb arteries.
In this study, we evaluated the safety, efficacy and utility of direct percutaneous injection of NBCA-MS with balloon occlusion for embolization of iatrogenic femoral PAs.
Methods
Study population
Retrospective study of 23 iatrogenic PAs treated with percutaneous US-guided balloon-assisted NBCA-MS glue embolization in 23 consecutive patients between July 2013 and November 2017 in two tertiary centers. Inclusion criteria were the presence of post-arterial catheterization iatrogenic PA at the femoral puncture site, whatever the size of the sac, after failed or contraindicated US-guided compression, or not.
Due to the retrospective nature of this study, our Ethics Committee waived the requirement for informed patient consent.
Imaging evaluation
In all 23 patients, the diagnosis of PA was confirmed by color Doppler US. For each lesion, the size of the aneurysmal sac was measured and the relationship to the artery from which it originated was noted. Pre-procedural US evaluation also included assessment of the neck length, PA chamber diameter, presence of arteriovenous fistula, and diameter of the native artery.
Embolization procedure
All patients underwent diagnostic angiography under local anesthesia by contralateral femoral approach through a long 45 cm × 6.0-French sheath (Terumo, Tokyo, Japan) by crossover technique. Selective cannulation of the feeding artery was then performed. A 0.035’’ MustangTM balloon dilatation catheter of appropriate size (5 to 7 mm in diameter, 4 cm in length) (Boston Scientific, San Jose, CA) was inflated in front of the PA neck to prevent distal embolization due to escape of the material from the aneurysmal cavity. No heparin was given during the procedure. After local anesthesia just superficial to the PA, US-guided puncture of the PA sac was performed with a 17-G metallic needle previously flushed with 5% dextrose solution, and already connected to a 5 mL luer-lock syringe loaded with a 1:1 ratio mixture of NBCA-MS (Glubran®2, General Enterprise Marketing, Viareggio, Italy) and Lipiodol® Ultra-Fluide (Guerbet, Aulnay-sous-Bois, France). Then, injection of the mixture into the aneurysmal cavity was slowly performed under fluoroscopy guidance to visualize the glue distribution until complete filling of the sac (Figure 1). The glue was allowed to polymerize for a few seconds after injection, and then the needle was withdrawn. Overall, 1.5 to 5 mL of the mixture was injected for each patient. Balloon catheter was remained inflated for 3 minutes after injection to allow complete and definitive polymerization of NBCA-MS. Effectiveness of the procedure was assessed by arteriogram. If the PA sac was not totally filled by glue with persistent perfusion, further injections were administered, repeating each step above until complete occlusion was achieved. Since the needle became blocked with glue each time, a new one was used for each injection. In case of impossibility to totally exclude the lesion despite several injections for anatomical reasons, a Viabahn® stent-graft (W.L. Gore & Associates, Newark, DE) was then deployed in front of the PA neck during the same procedure. A FemoSeal™ (Terumo) or AngioSeal™ (St. Jude Medical) vascular closure device was systematically used at the contralateral femoral puncture site. Color-Doppler US examination was performed at 1 day and again at 1, 3, and 12 months after the procedure.
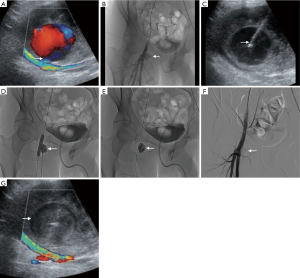
Data collection
In the framework of this study, we retrospectively collected each patient’s demographic data, clinical presentation, underlying etiology and interventional data. Demographic data included age and gender, whereas interventional data included endovascular treatment, the nature of the procedure, the culprit embolized vessel, the angiographic success, the patency of the native artery, the US features at 1 day, the complications and recurrence within 1 month following the procedure.
Immediate success was defined as a complete occlusion of the targeted pseudoaneurysmal sac on final angiography. Primary clinical success was defined as immediate success with glue injection alone. Stent-assisted clinical success was defined as immediate success after glue injection and additional stent-graft placement. All complications were recorded and classified as minor or major complications according to the Society of Interventional Radiology classification system. Procedural time was measured from the induction of local anesthesia until the last glue injection.
Statistical analysis
Descriptive statistics and parameters, such as frequencies and percentages, were used and provided in order to accurately describe our experience regarding the embolization of PAs using Glubran®2. Values are presented as means ± SDs for variables with normal distribution.
Results
Patient characteristics
Twenty-three patients (12 females, 11 males; mean age, 73.0±13.4 years; range, 18–93 years) were treated with US-guided balloon-assisted NBCA-MS glue embolization in the present study. Mean time to treatment was 7.2±7.4 days (range, 1–30 days). Sixteen (69.6%) of the 23 patients had groin hematoma. Eleven patients (47.8%) were under anticoagulant therapy and twelve (52.2%) were under antiplatelet therapy. Common femoral artery, superficial femoral artery, and deep femoral artery were involved in 47.8%, 47.8%, and 4.3%, respectively. The mean PA size was 33.0±10.7 mm (range, 17–60 mm). Nineteen PAs had 1 lobe (82.6%), and four (17.4%) were polylobulate. Neck length ranged from 0 to 6 mm (mean, 2.1±1.7 mm). Characteristics of treated PAs are listed in Table 1.
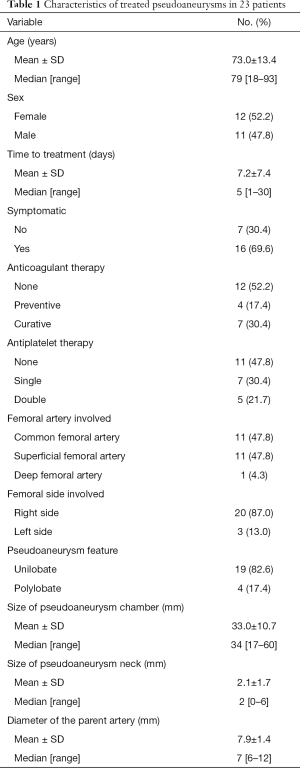
Full table
None of the 23 patients presented with neuropathy or soft-tissue necrosis. In 1 case (4.3%), an arterial-venous fistula was associated with PA and treated with additional arterial stent-graft. The mean volume of glue injected per patient was 2.1±1.6 mL, but the amounts ranged from 1.5 to 5 mL. In 15 patients (65.2%), the PAs were successfully occluded with a single injection. The other 8 lesions required additional glue injections (range, 2–4). Needle occlusion occurred in 2 cases (8.7%) and required needle substitution.
Procedural outcomes
Mean time of treatment was 41.8±18.9 min (range, 17–87 min). Twenty (86.9%) of the 23 PAs were successfully treated with glue injection alone. The three remaining patients (13.1%) required additional arterial stent-graft placement because of incomplete occlusion of the sac despite several injections (2 patients) or because of the presence of an arterial-venous fistula associated with PA (1 patient). Stent-graft was placed in the common femoral artery in 2 patients and in the superficial femoral artery in 1 patient, without compromising the deep femoral artery patency. All the PAs were finally occluded 1 day after the procedure and the 1-month clinical success was 100%. None of the patients developed recurrence in 2–73 months (mean, 15.4±15.2 months). In all cases, Doppler US confirmed that the patency of the femoral artery immediately after and 1 day after the procedure. Two patients (9%) complained of temporary mild pain after the intervention, successfully managed with symptomatic analgesic treatment. Outcomes of embolization are reported in Table 2.
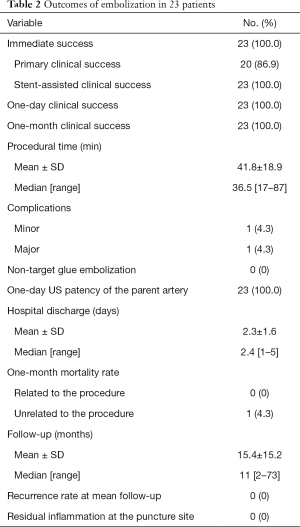
Full table
Complications
All procedures were performed under local anesthesia, with no need for anesthesiology support. No distal spillage of glue or thrombotic material in the native artery or limb arteries was noted during or after the procedure. No bleeding, non-target glue embolization or leakage through the balloon, native artery stenosis or thrombosis, allergic reaction, infection, skin ischemia, or PA rupture were observed. Balloon dilatation catheter was deflated in all patients without any issue as gluing or damage. No secondary surgical treatment was needed.
Minor complication occurred in 1 patient (4%) who had contralateral iatrogenic PA <5 mm at the femoral puncture site, but spontaneously resolved within 2 days, with no prolonged hospital stay. Major complication was reported in 1 other patient (4%) who presented a 6-cm groin hematoma at the contralateral femoral puncture. The patient needed prolonged hospital stay for surveillance but surgery was not required. One 73-year-old patient with several comorbidities died within 1 month from unrelated cause to the procedure.
Discussion
In the present study, US-guided balloon-assisted NBCA-MS glue injection alone into the aneurysmal sac proved to be safe and effective in the large majority of patients with iatrogenic femoral PAs.
Femoral artery PAs usually develop in the groin after catheterization for endovascular procedures. These lesions are more and more frequent due to the increasing number of angiographic procedures and patients who are on anticoagulant therapy. Natural history of PAs is spontaneous resolution within 2 weeks, at least for the smallest ones. However, waiting for spontaneous thrombosis may lead to longer hospital stay, decubitus complication and increased cost. Moreover, PAs are frequently symptomatic and source of discomfort for patients. For these reasons, the current trend is to treat PAs once diagnosed.
The gold standard treatment for PAs is surgery but it can lead to local complications and prolonged hospitalization. Several nonsurgical approaches have been then described. US-guided compression is an effective treatment for femoral PAs, with success rates of ranging from 70% to 90% in patients without anticoagulation therapy (14,15). Nevertheless, this method has significant limitations. It is painful and time-consuming (14,16-18). It also gives poorer results in patient who are taking anticoagulants. Lastly, this procedure may be impossible or challenging to perform in obese patients, and is usually uncomfortable for both patients and physicians. As an alternative, direct percutaneous embolization of the PA sac with different embolic materials has been reported. Thrombin is the most popular embolic agent used for percutaneous occlusion of femoral PAs because it causes fast and efficient thrombosis in the aneurysmal sac without filling it with foreign material (3,6,19-23). However, some complications may occur with this agent, including allergic reaction and distal thrombosis of the parent artery by leakage through the PA neck (24). Kurzawski et al. (7) prospectively studied 353 patients treated by thrombin injection and reported 53 (15%) arterial micro-embolizations and 1 (0.3%) pulmonary embolism in a case of concomitant arterial-venous fistula. Also, thrombin is not available everywhere, as is the situation in France, and is quite expensive.
NBCA glue is a potential alternative to thrombin but is rarely used for treating femoral PAs. Indeed, only two series have reported results of glue embolization for PAs by direct puncture of the sac (11,12). Aytekin et al. (11) employed the glue as an alternative to thrombin to induce thrombosis of the PA cavity. In contrast, Del Corso et al. (12) induced collapse of the PA chamber and then stuck its superior and inferior walls together with glue. They thus obtained the disappearance of the PA chamber. Regarding authorized glues, NBCA-MS (Glubran®2) is a well-known surgical glue in which NBCA is combined with another monomer, metacryloxysulpholane, to produce a more pliable and stable polymer whose milder exothermic reaction (45 °C) results in less inflammation and histotoxicity than Histoacryl® or Trufill®, which are respectively not allowed for endovascular purpose and not available in Europe (24). Glubran2® has the advantage of offering fast and permanent embolization, independently of coagulation disorders. It has also the advantage of being cheap in comparison with thrombin. No allergic reaction has been described (25). NBCA-MS is naturally resorbed in soft tissue with time and does not act as foreign material like coils. However, the main risk of direct percutaneous embolization with NBCA glue is distal embolization due to escape of the material from the pseudoaneurysmal sac before it is completely polymerized. To prevent this complication, Aytekin et al. (11) and Del Corso et al. (12) compressed the neck of the PA with the US probe during injection, and held the probe in place until polymerization was complete. Indeed, it is important to make sure that the flow between the aneurysm and the parent artery is stopped by compression before injection of NBCA glue. However, the compression is often times impossible, painful or not sufficient to cease flow. In that case, NBCA should be used with balloon protection. This is the technique we used in the current study as first treatment of iatrogenic femoral artery PAs. In this technique, an insufflated balloon is placed in the vessel to occlude the neck of the PA and avoid reflux of glue into the native artery.
Finally, unlike the technique described by Del Corso et al. (12), we mixed cyanoacrylate glue with Lipiodol to make it radiopaque. Indeed, even if glue presents high echogenicity, it is radiotransparent and cannot be visualized under fluoroscopy guidance without contrast medium. In our modified technique, US was only used for puncture of the PA sac but injection of glue was performed under fluoroscopy. It is then easier to check any leakage thanks to contrast medium. The addition of Lipiodol increases polymerization time. For this reason, we used a 50:50 dilution because this corresponds to 2 to 3 seconds polymerization time (26), which is adequate for occluding an aneurysm.
This balloon-assisted method is well tolerated and may be easily and promptly performed even in weak patients with painful groin hematoma, potentially reducing hospital stay. The risk of leakage into the limb arteries is then reduced without risk of gluing the balloon wall. Only two patients complained of temporary mild pain after the intervention, but none during the follow-up. Furthermore, this approach may be useful in case of failure of filling of the PA sac with glue, the contralateral arterial access then allowing final treatment of the PA at the same time by stent-graft placement in front of the neck, as it was performed for three patients in the present study. However, the main drawback of our technique is the need for contralateral arterial access that can lead to the same kind of complication, with PA formation, as we reported in 1 patient, which spontaneously resolved.
Our study had some limitations. First, conclusions must be drawn with caution because of the retrospective nature of the current study and the small number of patients included. Second, data on factors predisposing to PAs as sheath diameter, use of vascular closure devices, hypertension, and obesity were not available for most of initial endovascular procedures. The last limitation is the lack of a control arm. Future larger studies are needed to confirm the safety and efficacy of this intervention with glue and to compare it with other standard percutaneous treatments, especially with thrombin injection.
In conclusion, our study demonstrates that balloon-assisted NBCA-MS glue embolization is a safe and effective alternative for US-guided direct percutaneous occlusion of iatrogenic femoral artery PAs. This is the first study describing the injection of glue under fluoroscopy guidance. It offers complete exclusion of the PA in most cases and avoid the need for surgery. This technique is especially suitable to prevent distal embolization thanks to balloon occlusion of the native artery. Furthermore, the low cost of glue in comparison with thrombin may have significant economic impact in the current management of patients with iatrogenic PAs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was performed in compliance with the requirements of the institutional review board and approved by the institution ethical committee. Informed consent was waived.
References
- Katzenschlager R, Ugurluoglu A, Ahmadi A, Hülsmann M, Koppensteiner R, Larch E, Maca T, Minar E, Stümpflen A, Ehringer H. Incidence of pseudoaneurysm after diagnostic and therapeutic angiography. Radiology 1995;195:463-6. [Crossref] [PubMed]
- Kresowik TF, Khoury MD, Miller BV, Winniford MD, Shamma AR, Sharp WJ, Blecha MB, Corson JD. A prospective study of the incidence and natural history of femoral vascular complications after percutaneous transluminal coronary angioplasty. J Vasc Surg 1991;13:328-33; discussion 333-5. [Crossref] [PubMed]
- Grewe PH, Mügge A, Germing A, Harrer E, Baberg H, Hanefeld C, Deneke T. Occlusion of pseudoaneurysms using human or bovine thrombin using contrast-enhanced ultrasound guidance. Am J Cardiol 2004;93:1540-2. [Crossref] [PubMed]
- Kang SS, Labropoulos N, Mansour MA, Baker WH. Percutaneous ultrasound guided thrombin injection: a new method for treating postcatheterization femoral pseudoaneurysms. J Vasc Surg 1998;27:1032-8. [Crossref] [PubMed]
- Pope M, Johnston KW. Anaphylaxis after thrombin injection of a femoral pseudoaneurysm: recommendations for prevention. J Vasc Surg 2000;32:190-1. [Crossref] [PubMed]
- Taylor BS, Rhee RY, Muluk S, Trachtenberg J, Walters D, Steed DL, Makaroun MS. Thrombin injection versus compression of femoral artery pseudoaneurysms. J Vasc Surg 1999;30:1052-9. [Crossref] [PubMed]
- Kurzawski J, Sadowski M, Janion-Sadowska A. Complications of percutaneous thrombin injection in patients with postcatheterization femoral pseudoaneurysm. J Clin Ultrasound 2016;44:188-95. [Crossref] [PubMed]
- Savolainen H, Baumgartner I, Schmidli J, Heller G, Do DD, Willenberg T. Femoral pseudoaneurysms requiring surgical treatment. Trauma Mon 2012;16:194-7. [Crossref] [PubMed]
- Stoesslein F, Ditscherlein G, Romaniuk PA. Experimental studies on new liquid embolization mixtures (histoacryl-lipiodol, histoacryl-panthopaque). Cardiovasc Intervent Radiol 1982;5:264-7. [Crossref] [PubMed]
- Abdulmalak G, Chevallier O, Falvo N, Di Marco L, Bertaut A, Moulin B, et al. Safety and efficacy of transcatheter embolization with Glubran®2 cyanoacrylate glue for acute arterial bleeding: a single-center experience with 104 patients. Abdom Radiol (NY) 2018;43:723-33. [Crossref] [PubMed]
- Aytekin C, Firat A, Yildirim E, Kirbas I, Boyvat F. Ultrasound-guided glue injection as alternative treatment of femoral pseudoaneurysms. Cardiovasc Intervent Radiol 2004;27:612-5. [Crossref] [PubMed]
- Del Corso A, Vergaro G. Percutaneous treatment of iatrogenic pseudoaneurysms by cyanoacrylate-based wall-gluing. Cardiovasc Intervent Radiol 2013;36:669-75. [Crossref] [PubMed]
- Mittal R, Stephen E, Keshava SN, Moses V, Agarwal S. Percutaneous cyanoacrylate glue embolization for peripheral pseudoaneurysms: an alternative treatment. Indian J Surg 2012;74:483-5. [Crossref] [PubMed]
- Fellmeth BD, Roberts AC, Bookstein JJ, Freischlag JA, Forsythe JR, Buckner NK, Hye RJ. Postangiographic femoral artery injuries: nonsurgical repair with US-guided compression. Radiology 1991;178:671-5. [Crossref] [PubMed]
- Lange P, Houe T, Helgstrand UJ. The efficacy of ultrasound-guided compression of iatrogenic femoral pseudo-aneurysms. Eur J Vasc Endovasc Surg 2001;21:248-50. [Crossref] [PubMed]
- Coley BD, Roberts AC, Fellmeth BD, Valji K, Bookstein JJ, Hye RJ. Postangiographic femoral artery pseudoaneurysms: further experience with US-guided compression repair. Radiology 1995;194:307-11. [Crossref] [PubMed]
- Hajarizadeh H, LaRosa CR, Cardullo P, Rohrer MJ, Cutler BS. Ultrasound-guided compression of iatrogenic femoral pseudoaneurysm failure, recurrence, and long-term results. J Vasc Surg 1995;22:425-30. [Crossref] [PubMed]
- Cox GS, Young JR, Gray BR, Grubb MW, Hertzer NR. Ultrasound-guided compression repair of postcatheterization pseudoaneurysms: results of treatment in one hundred cases. J Vasc Surg 1994;19:683-6. [Crossref] [PubMed]
- Sultan S, Nicholls S, Madhavan P, Colgan MP, Moore D, Shanik G. Ultrasound guided human thrombin injection. A new modality in the management of femoral artery pseudo-aneurysms. Eur J Vasc Endovasc Surg 2001;22:542-5. [Crossref] [PubMed]
- Liau CS, Ho FM, Chen MF, Lee YT. Treatment of iatrogenic femoral artery pseudoaneurysm with percutaneous thrombin injection. J Vasc Surg 1997;26:18-23. [Crossref] [PubMed]
- Ergun O, Çeltikçi P, Güneş Tatar İ, Yılmaz M, Hekimoğlu B. Percutaneous thrombin injection treatment of a femoral artery pseudoaneurysm with simultaneous arterial balloon occlusion: Case report and review of the literature. Turk Kardiyol Dern Ars 2016;44:684-9. [PubMed]
- Weinmann EE, Chayen D, Kobzantzev ZV, Zaretsky M, Bass A. Treatment of postcatheterisation false aneurysms: ultrasound-guided compression vs ultrasound-guided thrombin injection. Eur J Vasc Endovasc Surg 2002;23:68-72. [Crossref] [PubMed]
- Cope C, Zeit R. Coagulation of aneurysms by direct percutaneous thrombin injection. AJR Am J Roentgenol 1986;147:383-7. [Crossref] [PubMed]
- Loffroy R. N-butyl cyanoacrylate glue: the best hemostatic embolic agent for patients with acute arterial bleeding. Cardiovasc Intervent Radiol 2017;40:1290-1. [Crossref] [PubMed]
- Loffroy R, Gui B, Cercueil JP, Krausé D. Endovascular therapeutic embolization: an overwiew of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol 2009;7:250-63. [Crossref] [PubMed]
- Berthod PE, Chevallier O, Latournerie M, Gehin S, Falvo N, Midulla M, Loffroy R. Atypical use of ALN inferior vena cava filters as protection devices prior to embolization of a large portosystemic shunt with Amplatzer Vascular Plugs and Glubran 2 cyanoacrylate glue. Quant Imaging Med Surg 2018;8:452-6. [Crossref] [PubMed]


