Radiological signs associated with pulmonary multi-drug resistant tuberculosis: an analysis of published evidences
Introduction
Pulmonary tuberculosis (TB) is the ninth leading cause of death worldwide and the leading cause from a single infectious agent, ranking above HIV/AIDS. In 2016, there were an estimated 1.3 million TB deaths among HIV-negative patients (down from 1.7 million in 2000) and an additional 374,000 deaths among HIV-positive patients. An estimated 10.4 million people fell ill with TB in 2016, with 56% in five countries: India, Indonesia, China, the Philippines and Pakistan (1). During anti-TB treatment, there is a selection pressure on the population of Mycobacterium tuberculosis (M.tb) resulting in the occurrence of spontaneous resistance-causing mutations in susceptible bacilli, which then gradually increase to become the dominant strain (2). However, the frequency of these single mutations is sufficiently low that if the appropriate combination chemotherapy is administered and reliably ingested. Multidrug-resistant tuberculosis (MDR-TB) refers to TB infection resistant to at least two most powerful anti-TB drugs, isoniazid and rifampicin. Extensively drug-resistant TB (XDR-TB) is defined as TB that has evolved resistance to rifampin and isoniazid, as well as to any member of the quinolone family and at least one of the second line injectable drugs: kanamycin, amikacin and capreomycin. Of MDR-TBs, XDR-TB accounts for 4–20% of these infections (3,4). In 2016, there were 600,000 new cases with resistance to rifampicin, the most effective first-line drug, of which 490,000 had MDR-TB. Almost half (47%) of these cases were in India, China and the Russian Federation. There were 476,774 reported cases of HIV(+) TB (1). Erratic and inappropriate use of medications and HIV/TB co-infection contribute to the concerns. When resistant mutants arise during treatment with anti-TB drugs, it is considered acquired resistance (previously treated MDR-TB, ptMDR-TB). People who are infected with an already drug-resistant strain could develop primary resistance (new MDR-TB, nMDR-TB), which is observed in newly diagnosed TB patients. It has been estimated that globally 3.5% (which can be much higher in some regions) of newly diagnosed TB patients, and 20.5% of previously treated patients had MDR-TB (5). For already existing strains of drug-resistant M.tb, it is vital to halt their transmission in community or hospital.
The treatment success rate of patients with drug-resistance to one of the first-line anti-TB drugs, particularly isoniazid mono-resistant TB, is similar to that of TB patients who are drug-sensitive (DS) to all first-line anti-TB drugs, but the treatment success rate of patients with MDR-TB was only around 48% and the mortality rate ranged from 1% to 30% among patients with MDR-TB (6). Prevention and early detection of MDR-TB are priorities for proper MDR-TB control. Successful treatments outcomes in MDR-TB patients are related to the use of second line anti-TB drugs, use of greater number of effective drugs, and the longer treatment (20–24 months) as compared with DS-TB (6 months) (7). For the first time in 40 years, new anti-TB drugs have been introduced recently. New TB drugs, bedaquiline and delamanid, were recommended for programmatic management of MDR-TB by World Health Organization (WHO) in 2013 and 2014 respectively (8,9). However, despite that wide range of geno- and phenotypic tests are available to detect M.tb strains and their susceptibility/resistance to drugs used, delay of appropriate MDR-TB treatment is common and sometime can take several weeks (10,11). The suspicion of MDR/XDR-TB by chest imaging can further guide and even intensify diagnostic process for MDR-TB. In this article, we describe the results of our literature review on radiological (imaging) signs associated with pulmonary MDR/XDR-TB.
Methods
We performed a literature search using PubMed (https://www.ncbi.nlm.nih.gov/pubmed) on January 29, 2018. The search words combination was “((extensive* drug resistant tuberculosis) OR (multidrug-resistant tuberculosis)) AND (CT or radio-graph or imaging or X-ray or computed tomography)”. This search generated 427 titles. The titles and abstracts of these papers were screened, and only English literatures were retrieved. We searched for articles describing radiological signs associated pulmonary MDR-TB (as compared to DS-TB), particularly we looked for radiological signs which may offer differentiation between pulmonary MDR-TB and DS-TB. It has already been known that cavitary lesion can be seen in both DS-TB and MDR-TB, and with higher prevalence in MDR-TB (12-16). However, cavitary lesion alone does not offer differential diagnosis of MDR-TB, thus articles merely reported the differences of cavitary lesion prevalence in DS-TB and MDR-TB were not analyzed in this review. Since one of the important determinants of radiologic patterns of parenchymal abnormalities in TB patients is patient’s own immunity (17,18), during the analysis we divided the reported pulmonary MDR-TB cases into four categories: (I) previously treated MDR-TB (ptMDR-TB) in HIV(−) adults; (II) new MDR-TB (nMDR-TB) in HIV(−) adults; (III) MDR-TB in HIV(+) adults; and (IV) MDR-TB in child patients. Previously treated MDR-TB, referred as ‘acquired’ or ‘secondary’ MDR-TB in some publications, was termed to the patients with TB who had a prior history of treatment with anti-TB drugs for >1 month; new MDR-TB, referred as ‘primary’ MDR-TB in some publications, was termed to the patients with TB who had never received treatment for TB or patients with TB who had taken any anti-TB drugs for less than 1 month (19).
Results
General findings and limitation of the reported studies
With the criteria defined in the method section, 17 articles were included for analysis (20-36). The commonly reported imaging findings of pulmonary MDR-TB included centrilobular small nodules, branching linear and nodular opacities (tree-in-bud sign), patchy or lobular areas of consolidation, cavitation, and bronchiectasis (20-36). The centrilobular small nodules and tree-in-bud sign reflect the presence of endobronchial spread and are due to the presence of caseous necrosis and granulomatous inflammation filling surrounding terminal and respiratory bronchioles and alveolar ducts. These signs are considered a marker of the activity of the pathological process (37,38), rather than being MDR-TB specific. Signs such as bronchiectasis and calcified granulomas may be associated with chronicity of infections (37,38). The imaging findings from the reported studies (20-36) which offer potential diagnosis of MDR-TB are summarized and tabulated in Tables 1-8.

Full table
Full table

Full table
Full table

Full table

Full table
Full table
There are many limitations of the reported studies. Many studies reported no more than 50 cases of patients, and were retrospective. These studies might have patient selection bias. Most studies reported CT data, while patients with the characteristic chest X-ray (CXR) findings of TB might not to have undergone chest CT, and CT scans tend to be performed in patients with more severe symptoms and signs. For cases reported from tertiary referral hospitals, patients with more severe symptoms or cases that were more complicated might have been selectively included and reported. For comparative studies, the durations of TB infection tended to be not well controlled for patients with MDR-TB or DS-TB. The variation in imaging manifestations across the studies could be a consequence of differential time intervals between disease onset and chest imaging examination, which could have led to varied progression of imaging manifestation (29,31). Sometimes, the exact time between symptom onset and an imaging study were not available. In some studies the majority of patients in the DS-control group were patients who did not have a history of previously treated pulmonary TB, while ptMDR patients developed drug-resistance after receiving anti-TB drugs, and also ptMDR patients tends to have longer disease history. In some studies, DS-control group were composed of a mixture of previous treated and untreated cases. The duration of TB disease may have had an effect on the likelihood of cavity lesion formation. Some studies only reported CXR results, while CT is more sensitive than CXR in the detection and characterization of subtle diseases. CT helps the detection of small foci of cavitation in areas of confluent pneumonia and in areas of dense nodularity and scarring.
In the following results section, the description of imaging signs is based on CT unless specified as based on CXR.
Imaging signs of pulmonary ptMDR-TB in HIV(−) adults
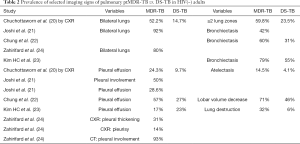
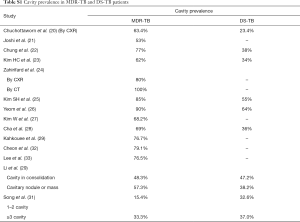
For pulmonary ptMDR-TB adults without HIV infection, the results from five studies are summarized in Tables 1,2. Joshi et al. (21) reported 50 ptMDR-TB patients. Chung et al. (22) reported 35 MDR-TB patients (31 ptMDR-TB, and 4 nMDR-TB) and 113 DS-TB patients as controls. Kim HC et al. (23) reported 47 MDR patients (45 ptMDR, 2 nMDR) and 47 DS-TB patients as controls. Zahirifard et al. (24) reported 35 MDR-TB patients (all with XCR, and 15 with CT), with 33 (94%) being ptMDR-TB and 2 (6%) being nMDR-TB. Chuchottaworn et al. (20) reported CXR findings of 145 patients with MDR-TB (4.8% HIV+), including 140 (96.6%) ptMDR-TB and 5 (3.4%) nMDR-TB; and the controls were 145 DS-TB patients [2.8% HIV(+)]. Note these studies also included a small portion of nMDR-TBs, but the majority of cases were ptMDR-TB. Chuchottaworn et al. further concluded that the characteristics of cavities, including having a maximum diameter ≥30 mm, number of cavities ≥3, and the presence of cavities in ≥2 lung zones, were associated with lung MDR-TB (20).
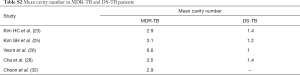
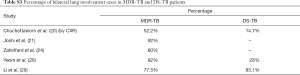
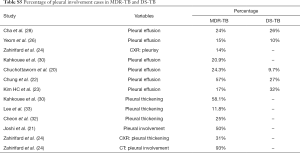
Table 1 suggests that cavity lesion occurs in approximately 70% of ptMDR-TB patients, and in approximately 30% of DS-TB patients. Cavities in MDR-TB are more likely to be multiple, and larger in size. Multiple cavities are common in MDR-TB patients. The probability of MDR-TB is high when three or more cavities exit. Table 2 shows MDR-TBs are more likely to have bilateral involvement, and more likely to have bronchiectasis which may be related to the chronic history of many ptMDR patients. However, high proportion of DS-TB patients also has bronchiectasis. Pleural effusion is more common in ptMDR than in DS-TB.
Imaging signs of pulmonary nMDR-TB in HIV(−) adults
For pulmonary nMDR-TB adults without HIV infection, the results from five studies are summarized in Table 3. Kim SH et al. (25) reported 40 nMDR-TB patients and 40 DS-TB patients as controls. Yeom et al. (26) reported 39 nMDR-TB patients and 39 DS-TB patients as controls. Kim W et al. (27) reported 44 nMDR-TB patients. Cha et al. (28) reported 65 nMDR-TB patients (CXR available in 53 patients and CT available in 42 patients) and compared with 141 DS-TB patients as controls (both CXR and CT available for all patients). Li et al. (29) reported 89 nMDR-TB patients and 89 DS-TB as controls. Cha et al.’s study also confirmed the superiority of CT over CXR in characterizing the details of pulmonary TB lesions.
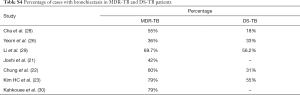
Table 3 suggests cavity occurs in approximately 75% of ptMDR patients, while in approximately 45% of the DS-TB patients (which are slightly higher than the cavity prevalence in the DS controls in Table 1). Multiple cavities are more common in MDR-TB than in DS-TB. Taking together the results of Tables 1,3, it can be seen that there may be not much difference in cavity prevalence among nMDR and ptMDR patients. Tables 2,4 suggest that while nMDR lesion may tend to be more extensive, pleural effusion or bronchiectasis do not allow for differentiation between nMDR-TB and DS-TB.
Li et al. (29) reported that calcification and calcified lymph nodes were more frequently seen in DS-TB than in nMDR-TB. This suggests that many their DS-TB patients might have had a chronic disease course, which is also reflected by the high bronchiectasis rate (Table 4).
Imaging signs of pulmonary mixed nMDR-TB and ptMDR-TB in HIV(−) adults
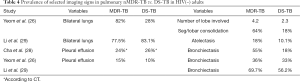
A few studies reported mixed nMDR-TB and ptMDR-TB patients, or MDR-TB patients with unknown being primary or secondary cause. The results from these studies are summarized in Table 5. Kahkouee et al. (30) reported 43 MDR-TB patients. Song et al. (31) reported 39 MDR-TB patients with Type 2 diabetes mellitus, 46 DS-TB patients with Type 2 diabetes mellitus, and 72 DS-TB without Type 2 diabetes mellitus (as controls). Cheon et al. (32) reported 72 MDR-TB (30 nMDR-TB, and 42 ptMDR-TB) patients.
Kahkouee et al. (30) noted that cavities in MDR-TB were thick-walled in the background of consolidation, while non-tuberculosis mycobacterium (NTM) cavities were more likely thin-walled. The results of Table 5 tend to confirm the observations in Tables 1-4. Dholakia et al. (16) reported that there was a significant association between a sensitive or mono-resistant DST profile and unilateral cavities compared with an MDR (mixed nMDR-TB and ptMDR-TB) profile with bilateral cavities, and MDR-TB was specifically associated with bilateral cavitation.
Song et al. (31) reported cavity lesion may be as common in DS-TB patients as in MDR patients. Actually, both the two studies from China reported relative lower cavity prevalence in MDR-TB patients, and a relative higher cavity prevalence in DS-TB patients (29,31). It remains unknown the results were due to sampling bias. However, it is possible for a developing country like China, some DS-TB patients might have had a longer pre-treatment history, and their TB diagnosis might have been initially delayed.
Comparison of imaging signs of pulmonary MDR-TB and XDR-TB
Cheon et al. (32) reported 50 MDR-TB patients and 25 XDR-TB patients who were matched by applying 2:1 propensity score matching. Variables such as presence of cavities, number of cavities, number of involved lobes, signs of tree-in-bud pattern, consolidation, pleural effusion, and lymphadenopathy were not significantly different between two groups. The statistically significant imaging findings between MDR- and XDR-TB were the cavity wall thickness and cavity size. The mean thickness of cavities was 8.3 mm in MDR-TB group and 11.5 mm in XDR-TB group; while the mean size of cavities was 21 mm in MDR-TB group and 36 mm in XDR-TB group (Table 6).
Cha et al. (28) compared 65 nMDR-TB (CXR available in 53 patients and CT available in 42 patients) and 17 XDR-TB (mixed new and post-treatment XDR; CXR available in 15 patients and CT available in 7 patients), and reported the mean number of cavities for MDR-TB and XDR-TB patients was 2.45 and 4.86, respectively. Cha et al. suggested that imaging findings were not different between patients with MDR-TB and XDR-TB. However, CT was available in only 7 of their XDR patients. Lee et al. (33) reported 20 XDR-TB patients and 85 MDR-TB patients (assumed to be a mixture of nMDR-TB and ptMDR-TB), and concluded that CT findings of pulmonary XDR-TB were similar to those of non-XDR MDR-TB; however, XDR-TB tends to have more extensive consolidation and tree-in-bud appearance. Thus, XDR-TB may overall appear even more aggressive than MDR-TB, with more number of cavities, larger cavities, and cavities of thicker wall. However, XDR-TB is relatively less common clinically, more radiological studies are necessary.
Imaging signs of pulmonary MDR-TB in HIV(+) adults
The imaging signs of lung MDR-TB in HIV(+) adults are till now primarily based on a 1998 CXR based report by Fishman et al. (34). In that study, Fishman et al. described 33 HIV(+) nMDR-TB, 13 ptMDR-TB (69% HIV+), and 60 DS-TB patients. The majority of HIV(+) nMDR patients show non-cavitary consolidations, pleural effusions (26%, 6/23), and lymphadenopathy, and with lower prevalence of cavity lesions (Table 7). For MDR-TB as well as DS-TB, severe immune suppression may limit the development of a radiographically observable response to TB infection, as cavitation may require some degree of immunocompetence (17,18). However, Table 7 shows in HIV(+) patients cavitary disease is still common for ptMDR cases.
These points in Fishman et al.’s study have been supported by other reports. Nunes et al. (35) reported that there was no cavity in their 10 pulmonary MDR-TB HIV(+) patients. Kiyan et al. (39) retrospectively analyzed the medical records of 63 non-AIDS immunocompromised patients and 80 immunocompetent patients with pulmonary TB. In immunocompromised patients, TB was more frequently disseminated (23.8% vs. 3.8%), and lung infiltrations were more often lobar or segmental consolidation (20.6% vs. 0%) and miliary lesions (17.5% vs. 3.8%) than in the control patients. Hilar and/or mediastinal adenopathy was also more frequently documented in immunocompromised patients (14.3% vs. 2.5%). Cavitation is slightly less common in immunocompromised patients (20.6%) than in the immunocompetent patients (31.3%). In a small HIV(+) cohort, Dholakia et al. (16) reported HIV positivity was associated with mediastinal adenopathy and miliary TB with borderline significance. Long et al. (40) and Pitchenik et al. (41) also reported that cavitation occur less frequently in HIV(+) TB patients.
Imaging signs of pulmonary MDR-TB in child patients
The identification of MDR-TB is equally important in the pediatric population, as a child with TB is as likely as an adult to have MDR-TB (5). Studies on pulmonary MDR-TB in child patients remained limited. The CXR study of Manikkam et al. (36) involving 45 pulmonary MDR-TB children (all <12 years, median age: 6 years) is the largest report till now (Table 8). Among their subjects, 48.9% were HIV(−) and 44.4% were HIV(+). The most common CXR finding was consolidation (53.5%), followed by lymphadenopathy (35.6%), bronchopneumonic opacification (33.3%) and cavities (31.1%). There were no statistically significant differences in any of the CXR patterns in HIV(−) children compared with HIV(+) children. Cavities were more common in ptMDR than nMDR. Manikkam et al. suggested that the development of cavitation in CXR of children with TB could raise concern for the possibility of MDR-TB, and prompt further testing (36). However, Manikkam et al. did not separately report cases of both nMDR and HIV(+), so it could not be known from their report that whether child patients with both nMDR and HIV(+) had less prevalence of cavity lesion.
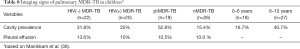
The cavity lesion prevalence of in Manikkam et al.’s report was similar to other reports of South African child patients of pulmonary MDR-TB [mixed ptMDR/nMDR, and mixed HIV(+/−)], where cavities have been described in 35–38% of the patients (42,43). The 31.1% prevalence was lower than those in adult MDR-TB patients (estimated to 70%). However, this may be higher than DS-TB child patients. The prevalence of cavity lesion is considered to be 14.0–16.2% in South African child patients without MDR (44,45). Note cavities were found more frequently in the 6–12-year age group than the <6-year group (40.7% in older children compared with 16.7% in younger children) (Table 8), thus cavity lesion prevalence may be even lower in very young child MDR-TB patients.
Discussion
It was reported that imaging findings of pulmonary MDR-TB do not differ from those of DS-TB (37). Our literature analysis demonstrates that, while overall pulmonary MDR-TBs tend to have more extensive disease, more likely to be bilateral, to have pleural involvement, and to have bronchiectasis (Tables S1-S5), based on imaging findings alone these signs are insufficient to differentiate MDR-TB from DS-TB. The only sign which may offer good specificity, though maybe at the cost of low sensitivity, will be thick-walled multiple cavities, particularly, if the cavity number is ≥3, cavity size is ≥30 mm (20). While it can be postulated that ptMDR-TB will have higher cavity prevalence than nMDR-TB, our literature analysis shows for adult HIV(−) patients, nMDR appeared to have no lower prevalence of cavity compared with ptMDR, both estimated to have a prevalence of 70%, as opposed to the estimated cavity prevalence of 30–40% in DS-TB (Table S1). In a large cohort of 1,219 MDR-TB patients [mostly HIV(−)], Kurbatova et al. (46) reported a cavity prevalence of 76.9% (937/1,219). In a high TB incidence area, by restriction fragment length polymorphism (RFLP) analysis Van Rie et al. (47) reported that even in some cases of ptMDR-TB, the MDR M.tb strain was actually transmitted. However, studies in children by Manikkam et al. and in HIV(+) adults by Fishman et al. did suggest that ptMDR patients have higher prevalence of cavity than nMDR patients (34,36). Literatures also showed that compared with cavity in MDR-TB, cavities in XDR-TB may have even thicker wall and even larger size (32), and nMDR in HIV(+) patients may show low prevalence of cavity (34). Until now most of the reported studies did not describe cavity size, cavity wall thickness, and cavity wall morphology in details. As thick-walled multiple cavities may present the most promising imaging sign for differential diagnosis of MDR-TB, it is warranted that for future studies cavity lesion characters should be quantified in sufficient details.
Full table
Full table
Full table
Full table
Full table
Lung cavity is believed to be the biological foundation for MDR/XDR-TB (48). In addition to that cavitary lesion is a key means of disease transmission, the lining of the cavity reduce the amount of drug that can penetrate from the bloodstream. The increased risk associated with cavitary disease could be explained by the increased bacillary burden within cavitary lesions, in which the likelihood of spontaneous mutations associated with drug resistance is greater, and/or the existence of subpopulations of bacilli that survive either due to metabolic dormancy or exposure to sub-inhibitory drug concentrations (49-51). High bacillary titers in cavities increase the probability of establishing drug-resistant bacterial populations (52,53). It is therefore understandable that thicker walls and larger cavities are more likely to be associated with MDR-TB and XDR-TB (20,32). Additionally, it is also possible that some strains of M.tb are more likely to be associated with cavity lesion. Chatterjee et al. (54) reported that strains of M.tb from Western Maharashtra, India exhibit a strain-specific associations with drug resistance, cavitary disease and treatment failure.
If the liquefying contents of a cavity escape into the bronchial tree, the bacilli can become widely dispersed to other parts of both lungs, including previously unaffected areas of the lung, partly by gravity and coughing. Therefore, bilateral involvement and a greater number of lobes affected by parenchymal lesions are more likely to occur in MDR-TB with cavities. MDR-TB patients tend to have a significantly higher sputum smear-positivity rate than those with mono-resistant or DS-TB. Metcalfe et al. (55) reported that sputum smear-positivity rate was 80.0% in MDR-TB patients, and 53.3% in DS-TB patients. Perrin et al. (56) described the presence of cavities is associated with heavier mycobacterial load. Chuchottaworn et al. (20) showed that a sputum AFB smear-positive score of 3+ is an independent risk factor for MDR-TB. Patients with radiological cavities are typically more infectious than patients without cavitary disease (57). Pulmonary TB with cavitary lesion is associated with poor outcomes (58-63). Holtz et al. (64) reported that bilateral cavitation on CXR was an independent predictors of a longer sputum culture conversion time for MDR-TB patients. Pulmonary TBs with cavitary lesion are also associated with the development of XDR-TB during MDR-TB treatment (65). These observations suggest that one of the important roles of imaging for TB patients’ management, including MDR-TB patients, is to find cavities and follow-up the change of cavities. If there are cavities which are with very thick-walled or too big which may inhibit anti-TB drug penetration, or no response of cavities to treatment, then individualized treatment such as aggressive medication or surgery, rather than standard regimens, may be considered. In a meta-analysis Bastos et al. (66) demonstrated that the pooled treatment success for MDR-TB patients that received individualized regimens was significantly higher when compared with patients who received standardized regimens (64% vs. 52%).
Of course, imaging is not meant to compete for MDR-TB diagnostics with microbiological and genomics methods. However, in some cases, sputum is not ideal material for testing, especially at the end of the treatment, or for some groups of patients such as children. It is difficult to standardize across patients and may be not representative especially for patients with significant cavities acting like “safe heaven” for pathogen. Imaging may offer significant insight when sputum samples no longer show the presence of active TB such as when at the end of the treatment. If thick-walled cavities do not diminishing in size during treatment, it may sometimes call for surgical treatment and removal of most stubborn resources of infection (67,68). Statistics of drug resistance in repeat TB patients suggests that significant number of such patients were not fully cured in the course of their initial drug treatment. Even though according to some guidelines patients with negative sputum tests are deemed “cured”, the statistics of repeat MDR patients perhaps call for a re-evaluation of these guidelines. To help recognizing the lungs still harboring the active pathogen with high probability of re-infection, we would need to collect large collections of lung images of patients that were released from hospitals after TB treatment, but came back with relapse (in time most consistent with relapse but not another infection). Comparing such images (based on expert radiologist annotation, or using deep learning and other artificial intelligence methods) with similar cohort of patients that did not experience a relapse can help identifying factors and characteristics useful for improved diagnosis.
In addition to radiological study, the diagnosis of MDR-TB should be combined with history and other tests. The management of prior episodes of pulmonary TB is an important factor in the development of MDR-TB. Most commonly, the development of acquired drug resistance occurs when there is a large multiplying bacillary population such as what occurred in pulmonary cavities when an inadequate drug regimen (inappropriate drugs, insufficient dosages and duration, etc.) is prescribed, or when there is a combined failure of both the patient and the provider to ensure that an adequate therapeutic regimen is taken. Rarely, malabsorption of one or more anti-TB drugs may account for acquired resistance. Patients with ptMDR-TB were reported to be associated with lower educational level and economic status, and less compliance with treatment than those with primary drug-resistance (13,15). It is of great importance that early diagnosis of drug resistance, prompt therapy with appropriate second-line drugs, and strategies to ensure treatment adherence are implemented.
In conclusion, this literature analysis shows that until now the research on imaging signs of pulmonary MDR-TB remain limited. The prevalence of cavity lesions in MDR-TB patients may be around 70%, and the mean cavity number in cavity positive MDR-TB patients may be ≥3. nMDR-TB appears to have no lower cavity prevalence compared with ptMDR-TB. Further studies into the detailed morphology of cavity lesions are warranted in order to better define diagnostic imaging signs for MDR/XDR-TB.
Acknowledgements
Funding: This study is partially supported by China TB Portal (OISE-17-63315-1), Grant Assistance Program/FOCUS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Global tuberculosis report 2017. Available online: http://www.who.int/tb/publications/global_report/en/
- Chiang CY, Centis R, Migliori GB. Drug-resistant tuberculosis: past, present, future. Respirology 2010;15:413-32. [Crossref] [PubMed]
- Raviglione MC, Smith IM. XDR tuberculosis–implications for global public health. N Engl J Med 2007;356:656-9. [Crossref] [PubMed]
- Aziz MA, Wright A, Laszlo A, De Muynck A, Portaels F, Van Deun A, Wells C, Nunn P, Blanc L, Raviglione M. WHO/International Union Against Tuberculosis And Lung Disease Global Project on Anti-tuberculosis Drug Resistance Surveillance. Epidemiology of antituberculosis drug resistance (the Global Project on Anti-tuberculosis Drug Resistance Surveillance): an updated analysis. Lancet 2006;368:2142-54. [Crossref] [PubMed]
- World Health Organization. Global tuberculosis report. 2014. Available online: http://www.who.int/tb/publications/global_report/en/
- Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, Gandhi NR, Galvani AP. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis 2009;9:153-61. [Crossref] [PubMed]
- Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, Becerra MC, Benedetti A, Burgos M, Centis R, Chan ED, Chiang CY, Cox H, D'Ambrosio L, DeRiemer K, Dung NH, Enarson D, Falzon D, Flanagan K, Flood J, Garcia-Garcia ML, Gandhi N, Granich RM, Hollm-Delgado MG, Holtz TH, Iseman MD, Jarlsberg LG, Keshavjee S, Kim HR, Koh WJ, Lancaster J, Lange C, de Lange WC, Leimane V, Leung CC, Li J, Menzies D, Migliori GB, Mishustin SP, Mitnick CD, Narita M, O'Riordan P, Pai M, Palmero D, Park SK, Pasvol G, Peña J, Pérez-Guzmán C, Quelapio MI, Ponce-de-Leon A, Riekstina V, Robert J, Royce S, Schaaf HS, Seung KJ, Shah L, Shim TS, Shin SS, Shiraishi Y, Sifuentes-Osornio J, Sotgiu G, Strand MJ, Tabarsi P, Tupasi TE, van Altena R, Van der Walt M, Van der Werf TS, Vargas MH, Viiklepp P, Westenhouse J, Yew WW, Yim JJ. Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 2012;9:e1001300. [Crossref] [PubMed]
- World Health Organization. The use of bedaquiline in the treatment of multidrug-resistant tuberculosis: World Health Organization, 2013. Available online: http://apps.who.int/iris/bitstream/10665/84879/1/9789241505482_eng.pdf
- World Health Organization. The use of delamanid in the treatment of multidrug-resistant tuberculosis: interim policy guidance. Available online: http://apps.who.int/iris/bitstream/10665/137334/1/WHO_HTM_TB_2014.23_eng.pdf
- Lam E, Nateniyom S, Whitehead S, Anuwatnonthakate A, Monkongdee P, Kanphukiew A, Inyaphong J, Sitti W, Chiengsorn N, Moolphate S, Kavinum S, Suriyon N, Limsomboon P, Danyutapolchai J, Sinthuwattanawibool C, Podewils LJ. Use of drug-susceptibility testing for management of drug-resistant tuberculosis, Thailand, 2004–2008. Emerg Infect Dis 2014;20:400-8. [Crossref] [PubMed]
- American Thoracic Society, Centers of Disease Control and Prevention. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000;161:1376-95. [Crossref] [PubMed]
- Keane VP, de Klerk N, Krieng T, Hammond G, Musk AW. Risk Factors for the Development of non-response to first-line treatment for tuberculosis in southern Vietnam. Int J Epidemiol 1997;26:1115-20. [Crossref] [PubMed]
- Mulu W, Mekonnen D, Yimer M, Admassu A, Abera B. Risk factors for multidrug resistant tuberculosis patients in Amhara National Regional State. Afr Health Sci 2015;15:368-77. [Crossref] [PubMed]
- Zhang L, Pang Y, Yu X, Wang Y, Lu J, Gao M, Huang H, Zhao Y. Risk factors for pulmonary cavitation in tuberculosis patients from China. Emerg Microbes Infect 2016;5:e110. [Crossref] [PubMed]
- Balaji V, Daley P, Anand AA, Sudarsanam T, Michael JS, Sahni RD, Chordia P, George IA, Thomas K, Ganesh A, John KR, Mathai D. Risk factors for MDR and XDR-TB in a tertiary referral hospital in India. PLoS One 2010;5:e9527. [Crossref] [PubMed]
- Dholakia YN, D'souza DT, Tolani MP, Chatterjee A, Mistry NF. Chest X-rays and associated clinical parameters in pulmonary tuberculosis cases from the National Tuberculosis Programme, Mumbai. Infect Dis Rep 2012;4:e10. [Crossref] [PubMed]
- Geng E, Kreiswirth B, Burzynski J, Schluger NW. Clinical and radiographic correlates of primary and reactivation tuberculosis: a molecular epidemiology study. JAMA 2005;293:2740-5. [Crossref] [PubMed]
- Rozenshtein A, Hao F, Starc MT, Pearson GD. Radiographic appearance of pulmonary tuberculosis: dogma disproved. AJR Am J Roentgenol 2015;204:974-8. [Crossref] [PubMed]
- World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis -2011 update. 2011. Available online: http://www.who.int/tb/challenges/mdr/programmatic_guidelines_for_mdrtb/en/index.html
- Chuchottaworn C, Thanachartwet V, Sangsayunh P, Than TZ, Sahassananda D, Surabotsophon M, Desakorn V. Risk Factors for Multidrug-Resistant Tuberculosis among Patients with Pulmonary Tuberculosis at the Central Chest Institute of Thailand. PLoS One 2015;10:e0139986. [Crossref] [PubMed]
- Joshi AR, Mishra S, Sankhe AP, Bajpai AR, Firke V. HRCT Spectrum of Pulmonary Multidrug-Resistant Tuberculosis in HIV Negative Patients: A Study in Indian Population. International Journal of Science and Research 2017;6:596-600.
- Chung MJ, Lee KS, Koh WJ, Kim TS, Kang EY, Kim SM, Kwon OJ, Kim S. Drug-sensitive tuberculosis, multidrug-resistant tuberculosis, and nontuberculous mycobacterial pulmonary disease in non-AIDS adults: comparisons of thin-section CT findings. Eur Radiol 2006;16:1934-41. [Crossref] [PubMed]
- Kim HC, Goo JM, Lee HJ, Park SH, Park CM, Kim TJ, Im JG. Multidrug-resistant tuberculosis versus drug-sensitive tuberculosis in human immunodeficiency virus-negative patients: computed tomography features. J Comput Assist Tomogr 2004;28:366-71. [Crossref] [PubMed]
- Zahirifard S, Amiri MV, Bakhshayesh Karam M, Mirsaeidi SM, Ehsanpour A, Masjedi MR. The radiological spectrum of pulmonary multidrug-resistant tuberculosis in HIV-negative patients. Iran J Radiol 2003;1:161-6.
- Kim SH, Min JH, Lee JY. Radiological Findings of Primary Multidrug-resistant Pulmonary Tuberculosis in HIV-seronegative Patients. Hong Kong J Radiol 2014;17:4-8. [Crossref]
- Yeom JA, Jeong YJ, Jeon D, Kim KI, Kim CW, Park HK, Kim YD. Imaging findings of primary multidrug-resistant tuberculosis: a comparison with findings of drug-sensitive tuberculosis. J Comput Assist Tomogr 2009;33:956-60. [Crossref] [PubMed]
- Kim W, Lee KS, Kim HS, Koh WJ, Jeong BH, Chung MJ, Jang HW. CT and microbiologic follow-up in primary multidrug-resistant pulmonary tuberculosis. Acta Radiol 2016;57:197-204. [Crossref] [PubMed]
- Cha J, Lee HY, Lee KS, Koh WJ, Kwon OJ, Yi CA, Kim TS, Chung MJ. Radiological findings of extensively drug-resistant pulmonary tuberculosis in non-AIDS adults: Comparisons with findings of multidrug-resistant and drug-sensitive tuberculosis. Korean J Radiol 2009;10:207-16. [Crossref] [PubMed]
- Li D, He W, Chen B, Lv P. Primary multidrug-resistant tuberculosis versus drug-sensitive tuberculosis in non-HIV-infected patients: Comparisons of CT findings. PLoS One 2017;12:e0176354. [Crossref] [PubMed]
- Kahkouee S, Esmi E, Moghadam A, Karam MB, Mosadegh L, Salek S, Tabarsi P. Multidrug resistant tuberculosis versus non-tuberculous mycobacterial infections: a CT-scan challenge. Braz J Infect Dis 2013;17:137-42. [Crossref] [PubMed]
- Song Q, Zhang G, Jiang H, Ren Y, Lu X. Imaging Features of Pulmonary CT in Type 2 Diabetic Patients with Multidrug-Resistant Tuberculosis. PLoS One 2016;11:e0152507. [Crossref] [PubMed]
- Cheon H. Comparison of CT findings of between MDR-TB and XDR-TB: A propensity score matching study. Imaging Med 2017;9:125-9.
- Lee ES, Park CM, Goo JM, Yim JJ, Kim HR, Lee HJ, Lee IS, Im JG. Computed tomography features of extensively drug-resistant pulmonary tuberculosis in non-HIV-infected patients. J Comput Assist Tomogr 2010;34:559-63. [Crossref] [PubMed]
- Fishman JE, Sais GJ, Schwartz DS, Otten J. Radiographic findings and patterns in multidrug-resistant tuberculosis. J Thorac Imaging 1998;13:65-71. [Crossref] [PubMed]
- Nunes EA, Capitini EM, Coelho E, Joaquim OA, Figueiredo IRO, Cossa AM, Panunto AC, Carvalho-Ramos M. Patterns of anti-tuberculosis drug resistance among HIV-infected patient in Maputo, Mozambique, 2002-2003. Int J Tuberc Lung Dis 2005;9:494-500. [PubMed]
- Manikkam S, Archary N, Bobat R. Chest X-ray patterns of pulmonary multidrug-resistant tuberculosis in children in a high HIV-prevalence setting. SA Journal of Radiology 2016;20:1-6.
- Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol 2008;191:834-44. [Crossref] [PubMed]
- Leung AN. Pulmonary tuberculosis: the essentials. Radiology 1999;210:307-22. [Crossref] [PubMed]
- Kiyan E, Kilicaslan Z, Gurgan M, Tunaci A, Yildiz A. Clinical and radiographic features of pulmonary tuberculosis in non-AIDS immunocompromised patients. Int J Tuberc Lung Dis 2003;7:764-70. [PubMed]
- Long R, Maycher B, Scalcini A, Manfreda J. The chest roentgenogram in pulmonary tuberculosis patients seropositive for human immunodeficiency virus type I. Chest 1991;99:123-7. [Crossref] [PubMed]
- Pitchenik AE, Rubinson HA. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis 1985;131:393-6. [PubMed]
- Seddon JA, Hesseling AC, Willemse M, Donald PR, Schaaf HS. Culture-confirmed multidrug-resistant tuberculosis in children: clinical features, treatment, and outcome. Clin Infect Dis 2012;54:157-66. [Crossref] [PubMed]
- Schaaf HS, Shean K, Donald PR. Culture confirmed multidrug resistant tuberculosis: diagnostic delay, clinical features, and outcome. Arch Dis Child 2003;88:1106-11. [Crossref] [PubMed]
- Lamont AC, Cremin BJ, Pelteret RM. Radiological patterns of pulmonary tuberculosis in the paediatric age group. Pediatr Radiol 1986;16:2-7. [Crossref] [PubMed]
- Donald PR, Ball JB, Burger PJ. Bacteriologically confirmed pulmonary tuberculosis in childhood. Clinical and radiological features. S Afr Med J 1985;67:588-90. [PubMed]
- Kurbatova EV, Gammino VM, Bayona J, Becerra MC, Danilovitz M, Falzon D, Gelmanova I, Keshavjee S, Leimane V, Mitnick CD, Quelapio MI, Riekstina V, Taylor A, Viiklepp P, Zignol M, Cegielski JP. Predictors of sputum culture conversion among patients treated for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2012;16:1335-43. [Crossref] [PubMed]
- Van Rie A, Warren R, Richardson M, Gie RP, Enarson DA, Beyers N, Van Helden PD. Classification of drug-resistant tuberculosis in an epidemic area. Lancet 2000;356:22-5. [Crossref] [PubMed]
- Long R. Drug-resistant tuberculosis. CMAJ 2000;163:425-8. [PubMed]
- Yew WW, Chau CH. Drug-resistant tuberculosis in the 1990s. Eur Respir J 1995;8:1184-92. [Crossref] [PubMed]
- Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 2004;84:29-44. [Crossref] [PubMed]
- Connolly LE, Edelstein PH, Ramakrishnan L. Why is long-term therapy required to cure tuberculosis? PLoS Med 2007;4:e120. [Crossref] [PubMed]
- Blanchard JS. Molecular mechanisms of drug resistance in Mycobacterium tuberculosis. Annu Rev Biochem 1996;65:215-39. [Crossref] [PubMed]
- David HL. Probability of distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol 1970;20:810-4. [PubMed]
- Chatterjee A, D'Souza D, Vira T, Bamne A, Ambe GT, Nicol MP, Wilkinson RJ, Mistry N. Strains of Mycobacterium tuberculosis from western Maharashtra, India, exhibit a high degree of diversity and strain-specific associations with drug resistance, cavitary disease, and treatment failure. J Clin Microbiol 2010;48:3593-9. [Crossref] [PubMed]
- Metcalfe JZ, Makumbirofa S, Makamure B, Sandy C, Bara W, Mungofa S, Hopewell PC, Mason P. Drug-resistant tuberculosis in high-risk groups, Zimbabwe. Emerg Infect Dis 2014;20:135-7. [Crossref] [PubMed]
- Perrin FM, Woodward N, Phillips PP, McHugh TD, Nunn AJ, Lipman MC, Gillespie SH. Radiological cavitation, sputum mycobacterial load and treatment response in pulmonary tuberculosis. Int J Tuberc Lung Dis 2010;14:1596-602. [PubMed]
- Madhi F, Fuhrman C, Monnet I, Atassi K, Poirier C, Housset B, Delacourt C. Transmission of tuberculosis from adults to children in a Paris suburb. Pediatr Pulmonol 2002;34:159-63. [Crossref] [PubMed]
- Kritski AL, Rodrigues de Jesus LS, Andrade MK, Werneck-Barroso E, Vieira MA, Haffner A, Riley LW. Retreatment tuberculosis cases: factors associated with drug resistance and adverse outcomes. Chest 1997;111:1162-7. [Crossref] [PubMed]
- Yew WW, Chan CK, Chau CH, Tam CM, Leung CC, Wong PC, Lee J. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest 2000;117:744-51. [Crossref] [PubMed]
- Huang Q, Yin Y, Kuai S, Yan Y, Liu J, Zhang Y, Shan Z, Gu L, Pei H, Wang J. The value of initial cavitation to predict re-treatment with pulmonary tuberculosis. Eur J Med Res 2016;21:20. [Crossref] [PubMed]
- Ahmad N, Javaid A, Basit A, Afridi AK, Khan MA, Ahmad I, Sulaiman SA, Khan AH. Management and treatment outcomes of MDR-TB: results from a setting with high rates of drug resistance. Int J Tuberc Lung Dis 2015;19:1109-14. [Crossref] [PubMed]
- Umanah TA, Ncayiyana JR, Nyasulu PS. Predictors of cure among HIV co-infected multidrug-resistant TB patients at Sizwe Tropical Disease Hospital Johannesburg, South Africa. Trans R Soc Trop Med Hyg 2015;109:340-8. [Crossref] [PubMed]
- Basit A, Ahmad N, Khan AH, Javaid A, Syed Sulaiman SA, Afridi AK, Adnan AS. Haq Iu, Shah SS, Ahadi A, Ahmad I. Predictors of two months culture conversion in multidrug-resistant tuberculosis: findings from a retrospective cohort study. PLoS One 2014;9:e93206. [Crossref] [PubMed]
- Holtz TH, Sternberg M, Kammerer S, Laserson KF, Riekstina V, Zarovska E, Skripconoka V, Wells CD, Leimane V. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med 2006;144:650-9. [Crossref] [PubMed]
- Shin SS, Keshavjee S, Gelmanova IY, Atwood S, Franke MF, Mishustin SP, Strelis AK, Andreev YG, Pasechnikov AD, Barnashov A, Tonkel TP, Cohen T. Development of extensively drug-resistant tuberculosis during multidrug-resistant tuberculosis treatment. Am J Respir Crit Care Med 2010;182:426-32. [Crossref] [PubMed]
- Bastos ML, Lan Z, Menzies D. An updated systematic review and meta-analysis for treatment of multidrug-resistant tuberculosis. Eur Respir J 2017;49:1600803. [Crossref] [PubMed]
- World Health Organization. The role of surgery in the treatment of pulmonary TB and multidrug- and extensively drug-resistant TB. 2014. Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/259691/The-role-of-surgery-in-the-treatment-of-pulmonary-TB-and-multidrug-and-extensively-drug-resistant-TB.pdf?ua=1
- Kang MW, Kim HK, Choi YS, Kim K, Shim YM, Koh WJ, Kim J. Surgical treatment for multidrug-resistant and extensive drug-resistant tuberculosis. Ann Thorac Surg 2010;89:1597-602. [Crossref] [PubMed]



