Osteoporosis: what the clinician needs to know?
Introduction
Osteoporosis is a common bone metabolic disease characterized by loss of bone strength, due to modifications in bone turnover, with the subsequent increased risk of fracture (1). It is a very common condition which mostly affects post-menopausal women and men aged over 50 years (2), rare (but still important) its occurrence in pediatric population (3). Osteoporosis can be distinguished as primary or secondary (4). Primary osteoporosis is caused by changes in normal bone turnover; these can be secondary to reduction of bone matrix production due to low osteoblastic activity, as it happens in postmenopausal women (following the loss of estrogen protection on bone matrix) (4), or in adult population for the aging of cortical and cancellous bone (5); otherwise, primary osteoporosis can be secondary to increased osteoblastic activity, as may result during corticosteroid treatments (2,6)
Secondary osteoporosis can be associated to several conditions, such as congenital (i.e., osteogenesis imperfecta, Ehlers-Danlos syndrome, Marfan syndrome), malnutrition (i.e., vitamin D deficiency, low calcium intake), metabolic, endocrine or iatrogenic diseases (3,5).
Bone strength depends on both bone quantity, which can be expressed in terms of bone mineral density (BMD), and bone quality, which reflects bone microarchitecture (2); these components can be well evaluated on different types of imaging methods.
Imaging techniques
Dual-energy X-ray absorptiometry (DXA)
DXA is the referring technique in the evaluation of osteoporosis, and it consists in a radiogenic tube which delivers an X-ray fan beam with two alternative energy levels (7). BMD is expressed in terms of bone mass per cm2 (g/cm2) and can be easily assessed using DXA (8). World Health Organization (WHO) proposed to classify osteoporosis according to BMD values and the difference [expressed as standard deviation (SD)] from those of a referred population, in terms of T-score or Z-score. T-score is referred to the mean density value measured in 30 years young adults (peak mineral density), while Z-score to the mean density measured in a sample of same age, sex and shape people; osteopenia is defined if the measured BMD value are within −1 and −2.5 SD, osteoporosis if BMD are below 2.5 SD (8,9).
Main sites for BMD evaluation are lumbar spine (L1–L4), femur (femoral neck and total hip) and distal third of the radius (which is mostly composed of cortical bone and its evaluation is relevant in primary and secondary hyperparathyroidism) (2,8) (Figure 1).
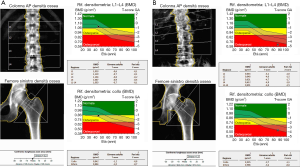
Even if DXA examination has several advantages, such as the low dose of radiation (1–6 µSv) delivered, the evaluation of anatomical regions which are sensible site of fracture and the short time of acquisition (less the 5 minutes for district) (2,4,10), it cannot distinguish between cortical and trabecular bone and between changes secondary to bone structure or bone density (4,10).
Trabecular bone score (TBS)
BMD calculated with DXA examination, even if is one the most important factor in the determination of bone strength, some patients with fragility fractures may have a normal or osteopenic BMD value (11). For this reason, other factors must be implied in bone strength, such as bone microarchitecture.
TBS consists in measuring the difference pixel by pixel of grey-level texture on DXA image of lumbar spine; even if it does not directly represent the bone microstructure, it is dependent from the three dimensional structure of the vertebral body, which include the trabecular number, the separation between one another and the connectivity density (12): high TBS value indicates a dense, and stronger, bone architecture, while a low value means a fragile bone with an increased risk of fracture. TBS values seem to decrease with aging, more in women than in men, similarly to BMD values (11,12). For postmenopausal women, TBS range above 1.350 is considered normal; if values are between 1.350 and 1.200, TBS is considered compatible with partial microstructural degradation; while a range lower below 1.200 is considered as the degraded microstructure. Considering that TBS has been found a predictor of risk fracture, it may be used with BMD for the selection of patient at high risk of fracture (11).
Quantitative computed tomography (QCT)
QCT allows a volumetric estimation of bone density and also permits separate measurement of cortical and trabecular bone (2). QCT is generally performed at the lumbar spine (so called axial QCT) and it well correlates with the bone volume fraction, over the total volume, and the trabecular spacing, while it is poorly correlated with trabecular number and thickness; the result can be represented in terms of absolute T-score and Z-score values or expressed as g/cm3 (13) (Figure 2): a BMD ranging from 80 to 110 mg/cm3 is associated to mild risk of fracture, a BMD value between 80 and 50 mg/cm3 is associated with a moderate risk of fracture, while a BMD value lower than 50 mg/cm3 is associated to severe risk of fracture (14). QCT has shown a great ability in the prediction of fracture risk and importance in the treatment follow-up, but as it delivers a high dose of radiation and also several other bone marrow changes may affect the measurements, its application in clinical use has been narrowed (2,14).

Vertebral morphometry
As previously stated, osteoporosis is a condition on which lays an increased risk of fracture. Theoretically, every bone site is at risk, but fractures generally occur at the level of spine, hip or distal radius. In particular, vertebral fractures are the most common type of fractures, for which a semi-quantitative grading system have been developed by Genant et al. (15): according to the reduction in the vertebral anterior, middle or posterior height with respect to the normal adjacent vertebra, fractures can be defined as grade 1 (mild) if the reduction is 20–25%, grade 2 (moderate) if reduction ranges between 25–40% or grade 3 (severe) if it is higher than 40% (Figure 3).
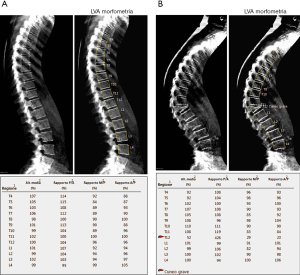
Evaluation of vertebral fractures can be performed on both lateral standard radiograms or lateral DXA images of the thoracolumbar spine (from T4 to L4) and the evaluation of vertebral fractures is made accordingly to Genant’s criteria (16). In addition, a semi-quantitative method may be used in order to make the evaluation of vertebral fractures clearer and more reproducible among observers: in this view, six points are placed within the vertebral body, four at the margins and two at the middle of vertebral endplates, for the measurement of the anterior, middle and posterior height (16,17). Even if both kinds of imaging evaluation reported a similar detection of vertebral fractures, the assessment on DXA might be preferred for at least two reasons: it delivers a low radiogenic dose than lateral conventional radiogram and also vertebral deformations, due to other spine deformations not related to vertebral fractures, are less common than in standard lateral radiograms, for the image acquisition is performed with a parallel photon beam (16,18).
Quantitative ultrasound (QUS)
QUS is a novel technique which provides information not only on bone mass but also mechanical and microstructural properties; it uses sound waves with a length ranging from 0.5 and 1.25 kHz (2,19). The rationale of this methodic is based on the modification in shape, intensity and speed of the wave while passing through the bone and soft tissues. In particular, the two main parameters evaluated are the speed of sound (SOS) and the broadband ultrasound (intensity) attenuation which have been shown to be related to bone microstructure and density, but other more complex ones [such as stiffness index (SI), QUS index (QUI) and amplitude dependent speed of sound (AD-SoS)], derived from their combination, may help to distinguish patients at increased risk of fracture (2,19-21).
QUS is performed on peripheral sites, such as phalanges, radius, tibia and calcaneus. In particular, this latter well lends itself to the evaluation because it is easily accessible, is predominantly composed of trabecular bone and delimited by the medial and lateral aspects which are flat and run almost parallel one to another, characteristics that make this site very suitable for the evaluation (19,20,22-24).
Other techniques
Recent studies have reported that some other imaging modalities may play in the quantification of bone mass and its quality (14).
Multidetector CT (MDCT)
In the recent literature, some studies have proposed that early detection of osteoporosis, the patients who underwent MDCT for other reasons, may be achieved calculating the BMD from the same scan (25-27). In fact, CT findings were found in correlation to bone microstructure changes in some diseases and during some therapy regimens (28-31).
High-resolution (HR) peripheral QCT
HR-peripheral QCT is a novel imaging modality that has been implemented for the evaluation of bone structure (32); it also may play a role in monitoring therapies (33). The examination is performed at the level of radius, tibia and metacarpal bones and permits the quantification of BMD in both cortical and trabecular bone, together or separately (10,20,34,35). Even if it delivers a low radiation dose in comparison to QCT, it does not evaluate the lumbar spine or the hip, which are sensible sites at risk of fracture (14).
Magnetic resonance imaging (MRI)
In the recent years, the role of MRI in the evaluation of osteoporosis has been spreading. Basing on the assumption that during osteoporosis there is an adipose involution of bone marrow, different methods have been proposed to quantify the amount of fat fraction, such as T1-weighted images, the Dixon method (which provide fat and water images), diffusion weighted imaging (DWI) or metabolic evaluation using proton magnetic resonance spectroscopy (1H-MRS) (36-40); perfusion studies have also demonstrated a reduced blood supply to the bone marrow, in course of osteoporosis (39,41). Moreover, some research studies have shown that HR imaging sequences, scanning peripheral sites such as tibia or calcaneus, may be helpful in the evaluation of bone microstructure and correlate with spinal BMD (42,43). Functional imaging, such as diffusion tensor imaging (DTI), may also provide further information on bone density and structure (44).
Qualitative evaluation
Radiological evaluation of osteoporosis, with conventional methods, it’s often the first step in the radiological work-up of the disease and still has its validity.
Changes in bone density do not become evident on standard radiograms until a certain amount of bone mass is loss (estimated around 30%). Findings can be better appreciated in the axial skeleton and at the proximal portion of long bones and consist in increased bone radiolucency, changes of trabecular pattern network (at the beginning resorption involves mainly the horizontal trabeculae with relative accentuation of vertical ones, in later stages resorption extends also to the vertical trabeculae) and cortical thinning (4,45). These features may be better evaluated with CT scan.
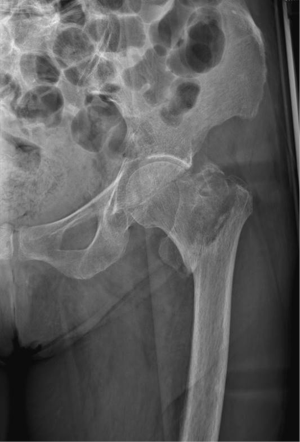
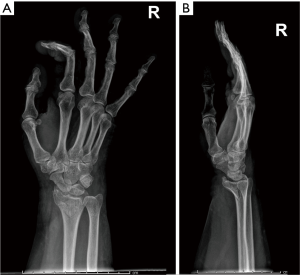
Complications of osteoporosis comprehend bone fractures (compression fractures), which may be even secondary to minor traumas and often involves the spine; other sites are hip (Figure 4), sacrum and distal radius (Figure 5) (4,45). As previous stated, vertebral fractures may at first be assessed with lateral conventional radiology and be described according to Genant’s. On anteroposterior projection, the fracture is usually symmetrical on both sides; the integrity of the posterior must be also checked, for it is lost it may be a sign of malignancy (4,45) and need further investigations. A characteristic vertebral fracture sign, which is strongly indicative of compression fractures, is the Kümmel phenomenon, that represents the avascular necrosis of the collapsed vertebral body and appears as intravertebral vacuum on X-ray images (46), while on MRI has a fluid signal (47).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785-95. [PubMed]
- Adams JE. Advances in bone imaging for osteoporosis. Nat Rev Endocrinol 2013;9:28-42. [Crossref] [PubMed]
- Mäkitie O. Causes, mechanisms and management of paediatric osteoporosis. Nat Rev Rheumatol 2013;9:465-75. [Crossref] [PubMed]
- Guglielmi G, Muscarella S, Bazzocchi A. Integrated Imaging Approach to Osteoporosis: State-of-the-Art Review and Update. Radiographics 2011;31:1343-64. [Crossref] [PubMed]
- Sözen T, Özışık L, Başaran NC. An overview and management of osteoporosis. Eur J Rheumatol 2017;4:46-56. [Crossref] [PubMed]
- Komori T. Glucocorticoid Signaling and Bone Biology. Horm Metab Res 2016;48:755-63. [Crossref] [PubMed]
- Bazzocchi A, Ponti F, Albisinni U, Battista G, Guglielmi G. DXA: Technical aspects and application. Eur J Radiol 2016;85:1481-92. [Crossref] [PubMed]
- Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2013;8:136. [Crossref] [PubMed]
- WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser 1994;843:1-129. [PubMed]
- Guglielmi G, Nasuto M, Avery LY, Cheng X. Bone densitometry: current status and future trends. J Genet Genomics 2016;64:97-103.
- Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 2014;29:518-30. [Crossref] [PubMed]
- Harvey NC, Glüer CC, Binkley N, McCloskey EV, Brandi ML, Cooper C, Kendler D, Lamy O, Laslop A, Camargos BM, Reginster JY, Rizzoli R, Kanis JA. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015;78:216-24. [Crossref] [PubMed]
- Griffith JF, Genant HK. New advances in imaging osteoporosis and its complications. Endocrine 2012;42:39-51. [Crossref] [PubMed]
- Link TM. Osteoporosis Imaging: State of the Art and Advanced. Radiology 2012;263:3-17. [Crossref] [PubMed]
- Genant HK, Wu CY, van Kuijk C, Nevitt M. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137-48. [Crossref] [PubMed]
- Diacinti D, Guglielmi G. Vertebral morphometry. Radiol Clin North Am 2010;48:561-75. [Crossref] [PubMed]
- Chou SH, Vokes T. Vertebral Morphometry J Clin Densitom 2016;19:48-53. [Crossref] [PubMed]
- Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone 2017;104:54-65. [Crossref] [PubMed]
- Gong B, Mandair GS, Wehrli FW, Morris MD. Novel assessment tools for osteoporosis diagnosis and treatment. Curr Osteoporos Rep 2014;12:357-65. [Crossref] [PubMed]
- Pisani P, Renna MD, Conversano F, Casciaro E, Muratore M, Quarta E, Paola MD, Casciaro S. Screening and early diagnosis of osteoporosis through X-ray and ultrasound based techniques. World J Radiol 2013;5:398-410. [Crossref] [PubMed]
- Edelmann-Schäfer B, Berthold LD, Stracke H, Lührmann PM, Neuhäuser-Berthold M. Identifying elderly women with osteoporosis by spinal dual X-ray absorptiometry, calcaneal quantitative ultrasound and spinal quantitative computed tomography: a comparative study. Ultrasound Med Biol 2011;37:29-36. [Crossref] [PubMed]
- Guglielmi G, Adams J, Link TM. Quantitative ultrasound in the assessment of skeletal status. Eur Radiol 2009;19:1837-48. [Crossref] [PubMed]
- McLeod KM, Johnson S, Rasali D, Verma A. Discriminatory Performance of the Calcaneal Quantitative Ultrasound and Osteoporosis Self-Assessment Tool to Select Older Women for Dual-Energy X-ray Absorptiometry. J Clin Densitom 2015;18:157-64. [Crossref] [PubMed]
- Minniti D, Davini O, Gualano MR, Gianino MM. Techniques for diagnosing osteoporosis: a systematic review of cost-effectiveness studies. Int J Technol Assess Health Care 2014;30:273-81. [Crossref] [PubMed]
- Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med 2013;158:588-95. [Crossref] [PubMed]
- Majumdar SR, Leslie WD. Conventional computed tomography imaging and bone mineral density: opportunistic screening or “incidentaloporosis”? Ann Intern Med 2013;158:630-1. [Crossref] [PubMed]
- Pickhardt PJ, Lee LJ, del Rio AM, Lauder T, Bruce RJ, Summers RM, Pooler BD, Binkley N. Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res 2011;26:2194-203. [Crossref] [PubMed]
- Inoue K, Hamano T, Nango N, Matsui I, Tomida K, Mikami S, Fujii N, Nakano C, Obi Y, Shimomura A, Kusunoki Y, Rakugi H, Isaka Y, Tsubakihara Y. Multidetector-row computed tomography is useful to evaluate the therapeutic effects of bisphosphonates in glucocorticoid-induced osteoporosis. J Bone Miner Metab 2014;32:271-80. [Crossref] [PubMed]
- Takasu M, Yamagami T, Nakamura Y, Komoto D, Kaichi Y, Tani C, Date S, Kiguchi M, Awai K. Multidetector computed tomography-based microstructural analysis reveals reduced bone mineral content and trabecular bone changes in the lumbar spine after transarterial chemoembolization therapy for hepatocellular carcinoma. PLoS One 2014;9:e110106. [Crossref] [PubMed]
- Issever AS, Kentenich M, Köhlitz T, Diederichs G, Zimmermann E. Osteoporosis and atherosclerosis: a post-mortem MDCT study of an elderly cohort. Eur Radiol 2013;23:2823-9. [Crossref] [PubMed]
- Deng M, Wang YX, Griffith JF, Lu G, Ahuja AT, Poon WS. Characteristics of rat lumbar vertebral body bone mineral density and differential segmental responses to sex hormone deficiency: a clinical multidetector computed tomography study. Biomed Environ Sci 2012;25:607-13. [PubMed]
- Cohen A, Dempster DW, Müller R, Guo XE, Nickolas TL, Liu XS, Zhang XH, Wirth AJ, van Lenthe GH, Kohler T, McMahon DJ, Zhou H, Rubin MR, Bilezikian JP, Lappe JM, Recker RR, Shane E. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int 2010;21:263-73. [Crossref] [PubMed]
- Jayakar RY, Cabal A, Szumiloski J, Sardesai S, Phillips EA, Laib A, Scott BB, Pickarski M, Duong LT, Winkelmann CT, McCracken PJ, Hargreaves R, Hangartner TN, Williams DS. Evaluation of high-resolution peripheral quantitative computed tomography, finite element analysis and biomechanical testing in a pre-clinical model of osteoporosis: a study with odanacatib treatment in the ovariectomized adult rhesus monkey. Bone 2012;50:1379-88. [Crossref] [PubMed]
- Schneider R. Imaging of Osteoporosis. Rheum Dis Clin North Am 2013;39:609-31. [Crossref] [PubMed]
- Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal a BMD: an in vivo HR-pQCT study. J Bone Miner Res 2010;25:882-90. [PubMed]
- Shen W, Gong X, Weiss J, Jin Y. Comparison among T1-weighted magnetic resonance imaging, modified dixon method, and magnetic resonance spectroscopy in measuring bone marrow fat. J Obes 2013;2013:298675. [Crossref] [PubMed]
- Li GW, Xu Z, Chen QW, Tian YN, Wang XY, Zhou L, Chang SX. Quantitative evaluation of vertebral marrow adipose tissue in postmenopausal female using MRI chemical shift-based water-fat separation. Clin Radiol 2014;69:254-62. [Crossref] [PubMed]
- Tang GY, Lv ZW, Tang RB, Liu Y, Peng YF, Li W, Cheng YS. Evaluation of MR spectroscopy and diffusion-weighted MRI in detecting bone marrow changes in postmenopausal women with osteoporosis. Clin Radiol 2010;65:377-81. [Crossref] [PubMed]
- Biffar A, Dietrich O, Sourbron S, Duerr HR, Reiser MF, Baur-Melnyk A. Diffusion and perfusion imaging of bone marrow. Eur J Radiol 2010;76:323-8. [Crossref] [PubMed]
- Tokgöz N, Akdeniz M, Uçar M, Kılıç K, Celik A. Is quantitative magnetic resonance imaging valuable in the assessment of trabecular bone structure in osteoporosis? Eklem Hastalik Cerrahisi 2013;24:2-6. [Crossref] [PubMed]
- Griffith JF, Wang YX, Zhou H, Kwong WH, Wong WT, Sun YL, Huang Y, Yeung DK, Qin L, Ahuja AT. Reduced bone perfusion in osteoporosis: likely causes in an ovariectomy rat model. Radiology 2010;254:739-46. [Crossref] [PubMed]
- Shen Y, Zhang YH, Shen L. Postmenopausal women with osteoporosis and osteoarthritis show different microstructural characteristics of trabecular bone in proximal tibia using high-resolution magnetic resonance imaging at 3 tesla. BMC Musculoskeletal Disorders 2013;14:136. [Crossref] [PubMed]
- Rebuzzi M, Vinicola V, Taggi F, Sabatini U, Wehrli FW, Capuani S. Potential diagnostic role of the MRI-derived internal magnetic field gradient in calcaneus cancellous bone for evaluating postmenopausal osteoporosis at 3 T. Bone 2013;57:155-63. [Crossref] [PubMed]
- Manenti G, Capuani S, Fanucci E, Assako EP, Masala S, Sorge R, Iundusi R, Tarantino U, Simonetti G. Diffusion tensor imaging and magnetic resonance spectroscopy assessment of cancellous bone quality in femoral neck of healthy, osteopenic and osteoporotic subjects at 3T: Preliminary experience. Bone 2013;55:7-15. [Crossref] [PubMed]
- Quek ST, Peh WC. Radiology of osteoporosis. Semin Musculoskelet Radiol 2002;6:197-206. [Crossref] [PubMed]
- Freedman BA, Heller JG. Kummel disease: a not-so-rare complication of osteoporotic vertebral compression fractures. J Am Board Fam Med 2009;22:75-8. [Crossref] [PubMed]
- Baur A, Stäbler A, Arbogast S, Duerr HR. Acute osteoporotic and neoplastic vertebral compression fractures: fluid sign at MR imaging. Radiology 2002;225:730-5. [Crossref] [PubMed]
- Thomsen K, Jepsen DB, Matzen L, Hermann AP, Masud T, Ryg J. Is calcaneal quantitative ultrasound useful as a prescreen stratification tool for osteoporosis? Osteoporos Int 2015;26:1459-75. [Crossref] [PubMed]


