Ultrasound exposure during pregnancy affects rabbit foetal parathyroid hormone (PTH) level
Introduction
Ultrasound is widely accepted as a safe imaging technique and it has become a crucial procedure in obstetrics and gynaecology setting, particularly for the assessment of gestational stages and diagnosis of many foetal abnormalities. Due to the non-usage of ionising radiation (1), this imaging modality has been extensively applied in various fields like cardiology, vascular studies, ophthalmology and gastroenterology. On the other hand, several previous studies revealed that ultrasound could also be incorporated into clinical interventions such as in ultrasound heat therapy, distraction osteogenesis, and sonothrombolysis procedures (1-3).
Although ultrasound offers several clinical advantages, there is still a possibility on its adverse effects especially for highly proliferative/sensitive immature tissues, which then may speculate that this imaging modality cannot be completely regarded as harmless (2). These drawbacks are mainly due to the absorption of ultrasound energy, or commonly termed as ultrasound heating. Correspondingly, maternal hyperthermia is proven to be teratogenic (2-4) and the heat produced by diagnostic ultrasound examination has been found to be one of the common causes for thermally-induced teratogenesis (5).
Hormonal activities are extremely sensitive to any changes in heat especially during foetal stage (6). The amount of transport protein and total plasma hormone content are known to change under certain physiological conditions, while altered hormone-receptor interactions may lead to endocrine abnormalities (6). Parathyroid hormone (PTH) is responsible to raise the extracellular calcium concentration, promote the absorption of calcium by the intestine, mobilize calcium salts from the bones, and increase the tendency of kidney to recover calcium from urine, thus to enhance both the excretion of phosphate by kidneys and its uptake by cells (7). Hyperparathyroidism (HPT) is a condition due to unregulated overproduction of PTH resulting in abnormal calcium homeostasis, while prenatal HPT is categorized as one of the true emergency cases and capable of causing serious problems to the mother as well as the unborn baby.
A recent in-vivo study has discovered that ultrasound heating during pregnancy could significantly increase the bone mineral density (BMD) value of newborn rabbits (8). Generally, in human gestational period, the first eight weeks after conception is known to be a very critical moment (9) since all tissues are massively proliferated and highly sensitive. In foetal physiological development, the formation of parathyroid glands begins at the middle till the end stage of gestation (7). Hence, any external disruption like ultrasound heating could possibly affect the development of the glands, thus the secretion of parathyroid hormone (PTH). To the best of our knowledge, the effect of ultrasound heating on foetal PTH level has not been previously documented and this is believed to be the first report that aims to evaluate the changes on PTH level of the rabbit foetuses.
Materials and methods
The ethical clearance was firstly obtained from the Universiti Teknologi MARA Committee on Animal Research and Ethics (UiTM CARE) and this in-vivo experimental study was carried out at the Medical Imaging Laboratory, Faculty of Health Sciences, Universiti Teknologi MARA (UiTM), Malaysia.
Animal preparation
A total of 16 pregnant New Zealand White Rabbits (NZWR) (Oryctolagus cuniculus) had been utilised in this preliminary study (Table 1). These pregnant does were then divided into 4 different groups, namely ‘1st stage’, ‘2nd stage’, ‘3rd stage’ of gestation and ‘O’ as the negative control group. All does were housed into separate cages. Facilities and healthcare services were provided in accordance to the minimum requirements of the USA Animal Welfare Act for adult rabbit (10). Full term of gestation for NZWR normally takes 31 to 33 days (10,11).
Ultrasound exposures
Based on the recommended maximum exposure time for embryo by the British Medical Ultrasound Society (9), the pregnant does of the first group were insonated for 60 minutes at the middle of 1st stage of gestation. The same length of exposure time was given to the second and third groups at the 2nd and 3rd stages, respectively. No insonation was given to the control group (group O). Prior to the insonation, doe’s fur on the abdominal area was removed by applying hair removal cream in order to facilitate the transducer application. The dimension of the area was about 10 cm × 10 cm, which located on the left side of its abdominal wall. Parker Aquasonic 100, ultrasound coupling gel was used during insonation to sustain a close contact between the abdominal skin and the transducer.
For technical accuracy, this closeness was monitored on the display screen throughout the procedure. The direction of ultrasound beam was manipulated as typically performed during the ultrasound examination commonly perform on human. By this, the ultrasound energy could fall onto as many foetuses as possible. Upon completion of the insonation at the stipulated stage, each doe was moved back to her cage until its full term delivery.
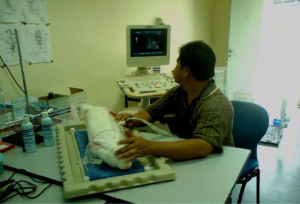
For all insonations, a Philips HD3 ultrasound system (Philips HD 3, Philips Electronics E.V., Germany) installed with 2D B-mode imaging was used. This system utilised an all-digital broadband ultrasound beam former with dynamic focal tuning, 150 dB dynamic range and consisted of a linear array broadband transducer (L9-5, Philips Electronics E.V., Germany) for superficial applications. The transducer was operated at 110 dB intensity displayed with its focal depth (~7.5 cm) that was similar to the midline of the pregnant doe (Figure 1). In every session, the maximum frequency used was 9 MHz, at 7.5 cm focal depth, at 21 frames per second, with Mechanical Index (MI) was at 0.7 and the Thermal Index (TIS) at 0.1, displayed on the monitor. These values were precisely observed and remained constant for all pregnant does throughout the intervention.
In this particular study, for the given 60 minutes exposure, the power (W) =557.3 W and the intensity (I) =147.44 mW/cm2 gave temperature rise (∆T) to 0.3 °C of 1,600 mL tap water.
Laboratory test
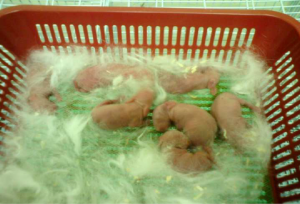
Shortly after delivery, blood samples were withdrawn from each kitten (Figure 2) and labeled accordingly in Ethylene Diamine Tetraacetic Acid (EDTA) tubes with anticoagulant. These samples were then sent to a commercial laboratory (Gnosis Laboratory, Bandar Sunway, Selangor, Malaysia) for Parathyroid Hormone-Intact (PTH-I) test. The test was performed and reported by a medical laboratory technologist (MLT), and the results in pg/mL were 13d 6.93 pg/mL, respectively (Table 1).

Full Table
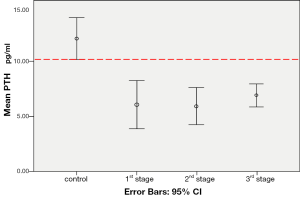
Error bars (Figure 3) of the control group did not overlap with those of the 1st, 2nd and 3rd stage groups. This illustration indicated that the mean values of PTH level of the control group were of difference from all three treated groups. Results of the independent samples t-test implied statistically significant differences (P<0.05) between the control group and the 1st stage (P=0.001), the 2nd stage (P<0.001) and the 3rd stage group (P<0.001). These results indicated that the differences were not likely due to chance but were probably caused by the given insonation.
Discussion
Since hormonal activities are extremely sensitive to any changes in heat, and known to change under certain physiological conditions, the alteration in foetal PTH level during pregnancy may possibly result in endocrine abnormalities, which may lead to physiological defects. With regards to the effect of increased body temperature (hyperthermia) on hormonal concentrations, Radomski, Cross and Buguet (12) found a significant exponential relationship between the increment in core temperature and plasma growth hormone, prolactin, and catecholamines responses, respectively. There was also a statement on the dissociation of hormonal reaction with general hyperthermia, resulting from a response of the endocrinic system to the thermal stress.
In relation to the effect of PTH on bone cell biochemical process, Centrella and his co-workers (13) concluded that some functions attributed to parathyroid hormone in bone might have resulted from alterations in TGF-β activity. In examining the feasibility, safety and efficacy of using high-intensity focused ultrasound (HIFU) to treat primary hyperparathyroidism in menopausal women, Kovatcheva and her colleagues (14) found that serum PTH levels decreased and normalised, and parathyroid tumors had decreased in size after the second session of ultrasound heating. Consequently, a non-invasive technique has been established as an option for a non-surgical treatment of menopausal women with primary HPT (14). The technique has become an alternative option for elderly patients with comorbidities, or in patients who declined surgery. Result from another pilot study by Kovatcheva et al. (15) also found that HIFU treatment caused a marked decrease of serum PTH after up to three sessions of ultrasound heating applied to enlarged parathyroid glands. They concluded that this might be feasible in controlling secondary HPT in patients with chronic kidney disease (15).
Heating effect is said to trigger biological interruptions, modify plasma membrane permeability and transport properties (16). In this study, newborns of all treated groups have significantly lower PTH levels as compared to the control group. The results show that the effect of ultrasound heating on PTH level of immature subject is an indication of hormonal interruption. The changes in hormonal environment due to ultrasound heating will reduce PTH secretion in both mature and immature subjects (8). Hence, any evidence of differences in PTH level during pregnancy could possibly result in alteration in physiological developments of the baby.
Nevertheless, this study has some limitations. Instead of negative control, sham control (without switching-on the ultrasound system) should be employed to mimic similar possible external disturbance experienced by each pregnant rabbit during insonation. Although the study’s data cannot be extrapolated directly to human exposures, a comprehensive clinical trial would provide the background information for such possibilities.
Conclusions
The findings of this experimental study support that there are effects on foetal PTH level from diagnostic ultrasound exposure. This study observed significantly low PTH level for all treated groups insonated at the 1st, 2nd and 3rd stages. Therefore, ultrasound heating has caused a significant decrease in PTH level at all stages of gestation. This heating effect could trigger further studies to look for some clinical effects in human during foetal stage.
Acknowledgements
The author would like to acknowledge the Research Management Institute (RMI) of Universiti Teknologi MARA, Shah Alam, Malaysia for the Research Intensive Fund (RIF) grant awarded (600-RMI/DANA 5/3/RIF(229/2012).
Disclosure: The authors declare no conflict of interest.
References
- Abramowicz JS, Lewin PA, Goldberg BB. Ultrasound bioeffects for the perinatologist. Glob libr women’s med 2008. DOI 10.3843/GLOWM.10204.
- Barnett SB. Key issues in the analysis of safety of diagnostic ultrasound. ASUM Ultrasound Bull 2003;6:41-3.
- Deanne C. Safety of diagnostic ultrasound in fetal imaging: Diploma in fetal medicine & ISUOG educational series. Doppler in Obstetrics 2002.
- Edwards MJ, Walsh DA, Li Z. Hyperthermia, teratogenesis and the heat shock response in mammalian embryos in culture. Int J Dev Biol 1997;41:345-58.
- Miller MW, Nyborg WL, Dewey WC, et al. Hyperthermic teratogenicity, thermal dose and diagnostic ultrasound during pregnancy: implications of new standards on tissue heating. Int J Hyperthermia 2002;18:361-84.
- Peavy DE. Medical Physiology, Part IX, Endocrine Physiology Chapter 31; Endocrine Control Mechanisms 2009.
- Terranova PF. Medical Physiology, Part X, Reproductive Physiology Chapter 39; Fertilization, Pregnancy, and Fetal Development 2009:684.
- Dom SM, Hassan HF, Salikin MS, et al. The effect of B-mode diagnostic ultrasound exposure on rabbit foetal bone mineral density (BMD). Radiography 2012;18:197-200.
- BMUS (British Medical Ultrasound Society). Guidelines for the safe use of diagnostic ultrasound equipment. Policies, statements and guidelines 2007.
- HSUS (Humane Society of the United States). Animals in research - Species used in research. Rabbit 2009.
- Lebas F, Coudert P, Rouvier R, et al. eds. The rabbit - Husbandry, health and Production. 1986.
- Radomski MW, Cross M, Buguet A. Exercise-induced hyperthermia and hormonal responses to exercise. Can J Physiol Pharmacol 1998;76:547-52.
- Centrella M, McCarthy TL, Canalis E. Parathyroid hormone modulates transforming growth factor β activity and binding in osteoblast-enriched cell cultures from fetal rat parietal bone. Proceedings of the National Academy of Sciences of the USA 1988.
- Kovatcheva RD, Vlahov JD, Shinkov AD, et al. High-intensity focused ultrasound to treat primary hyperparathyroidism: a feasibility study in four patients. AJR Am J Roentgenol 2010;195:830-5.
- Kovatcheva RD, Vlahov JD, Stoinov JI, et al. High-intensity focussed ultrasound (HIFU) treatment in uraemic secondary hyperparathyroidism. Nephrol Dial Transplant 2012;27:76-80.
- NCRP (National Council of Radiation Protection and Measurements). Diagnostic ultrasound safety: Exposure criteria for medical diagnostic ultrasound II - Criteria based on all known mechanisms. Summary of NCRP Report 2002.