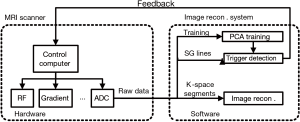
Prospective cardiac motion self-gating
Introduction
In many cardiac magnetic resonance imaging (CMR) applications, the data acquisition needs to be synchronized with the cardiac motion. Typically, electrocardiogram (ECG) is used to monitor the cardiac motion and control the timing of data acquisition. This is commonly referred as ECG gating or ECG triggering. For a normal ECG signal, the QRS complex has the highest amplitude peak and sharpest upstroke, which is often used as cardiac triggers (1). However, the ECG based cardiac gating is associated with several potential issues. First, the ECG signal is sometimes interfered by the time varying magnetic field of the MRI system. Such interferences can be severe at higher fields and eventually cause degraded image quality due to synchronization errors (2-5). Furthermore, there are applications when ECG signal is difficult to acquire or even inaccessible, such as fetal cardiac imaging (6,7). As an alternative to ECG, self-gating uses intrinsic MRI signal to detect cardiac motion and synchronize the timing of imaging events. It provides direct measurement of the mechanical motion instead of the electrical signal as is the case with ECG, and hence does not suffer from the aforementioned issues of ECG. It is potentially a valuable alternative approach for fetal cardiac motion gating in fetal cardiac MRI (8-10).
Self-gating techniques normally consist of two parts: acquisition and processing. In the acquisition part, selected k-space data is repeatedly acquired to form the time resolved cardiac motion self-gating signal. Previously reported cardiac self-gating approaches use the k-space center point in a radial (11,12) or Cartesian (13-17) sampling trajectory as the self-gating signal. A number of algorithms have been developed to process the self-gating signal, including echo peak modulation, projection-based center of mass and low-resolution region of interest correlation (11,16,17). Larson et al. (11) proposed a technique where self-gating signal is derived retrospectively from the k-space center point in a radial sampling trajectory. Cardiac triggers are generated by finding the peak of the center point signal after a low-pass filter. Previous studies by Hu et al. (18) on motion correction using multiple coil array (MOCCA) suggests that redundant data by coil arrays could provide richer information to estimate and correct motion (19,20). A MOCCA echo is formed by concatenating the k-space centerlines acquired by coil arrays into a single vector. The advantage of using a MOCCA echo in self-gating is that the motion information is greatly enriched without the need of additional acquisition time. Although the MOCCA technique is originally designed for respiratory motion gating, its principle is also applicable to cardiac motion. However, a more sophisticated and robust processing algorithm is required to fully exploit the abundant information of MOCCA echoes. In most cardiac self-gating techniques, the cardiac triggers are either generated offline after the acquisition (11,14) or online during the acquisition (13,21). Offline gating usually requires a sufficient amount of temporal oversampling and therefore suffers from longer acquisition time. Online self-gating is more efficient because the acquisition of k-space segments is controlled on the fly to make sure sufficient k-space segments are acquired within minimal time. However, it is technically more challenging because of the requirement of deriving self-gating signal and detecting self-gating triggers in real time (22). Despite a number of recent advances, cardiac motion self-gating has not been used in clinical practice, mostly due to limited reliability and reproducibility of the self-gating triggers.
The goal of this study was to develop and validate a prospective online cardiac motion self-gating technique. Several technical advances are included in our work to enable accurate and reliable trigger detection in real time while the sequence is running, including separation of self-gating acquisition from imaging acquisition and use of training based principal component analysis (PCA) algorithm on multi-coil self-gating data processing.
Methods
Prospective self-gating sequence
In a conventional self-gating approach, the self-gating signal is typically acquired concurrently with the imaging data, such as using radial sampling where the k-space center point is acquired as part of each radial projection line (11,12). For Cartesian sampling, several groups have acquired an additional echo or FID signal during the same TR as imaging but immediately before the phase-encoding gradients (14). Additionally, the self-gating data and imaging data can be acquired in an interleaved fashion on a TR to TR basis (16,17). However, these approaches could suffer from self-gating signal distortions that arise from the history of RF pulses and gradients played before the current TR, and eddy currents generated by the phase-encoding gradients that vary from TR to TR. To test this hypothesis, we run a radial-based cardiac CINE sequence on both a stationary phantom and in-vivo. The ECG signal was recorded for reference during the acquisition (simulated ECG in phantom study). The k-space center point (CP) signal from phantom study (Figure 1A) has significant drifting, which we believe is caused by the aforementioned issues. Similar artifacts can also be found in-vivo (Figure 1B), making it difficult to automatically derive reliable cardiac triggers from the CP signal in real time. To further support our hypothesis, we run a non-phase-encoded Cartesian CINE sequence again on the same phantom and human subject. The pulse sequence remains identical in every TR since there is no phase-encoding gradient. CP signal of stationary phantom (Figure 1C) is free of the aforementioned distortion and the in-vivo CP signal (Figure 1D) shows clear evidence of cardiac motion, though it is mixed with noise.
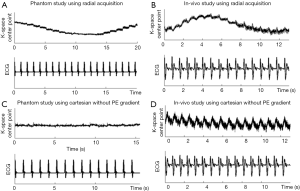
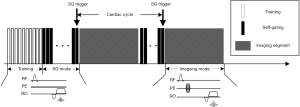
Based on data shown in Figure 1, we propose to use a two-mode sequence to solve the aforementioned self-gating signal distortion problem. Instead of acquiring self-gating and imaging data within the same or successive TRs, the self-gating signal is acquired in a dedicated self-gating acquisition mode that is separated from the image acquisition. The pulse sequence is described in Figure 2 using cardiac CINE as an example, although the same approach could be extended to other triggered cardiac MRI applications. The sequence starts with a training phase where k-space centerlines are repeatedly acquired for 300 TRs (about 1 second). These data are processed by a PCA training algorithm described in the next section. The purpose of the training is to (I) find the principal component vector that is used to process the multi-dimensional self-gating signal; (II) calculate the threshold for real-time self-gating trigger detection. The self-gating mode starts immediately after the training phase and the PCA projection algorithm is applied to the self-gating data as they are acquired. Upon detection of the self-gating trigger, the sequence immediately switches to imaging mode to acquire the k-space segments. The duration of the imaging mode is set to be shorter than the expected cardiac cycle so that the sequence can switch back to self-gating mode before the next cardiac trigger. Although the sequence switches between the two modes, the only difference in terms of pulse sequence is that the self-gating mode does not use any phase-encoding gradient. All other sequence parameters are maintained, including TR, TE and RF shape and duration. This ensures that the steady state of the magnetization is preserved even during switching, which is very important for the signal quality for both imaging and self-gating. Because the self-gating mode essentially acquires the same k-space centerline repetitively, the self-gating signal distortion problem addressed above is avoided as each new self-gating TR has the same history of RF pulse and gradients, and maintains the same steady state. The acquisitions in our preliminary study using the non-phase-encoded Cartesian CINE sequence (Figure 1C,D) are essentially the self-gating mode in the proposed sequence. The signal plot shows that the data acquired in the self-gating mode yields much improved self-gating signal quality, which is important for subsequent processing and trigger detection.
Self-gating algorithm
To maximize the available motion information, k-space centerline is acquired using multiple coils rather than k-space center point alone. A MOCCA echo (18) is formed by concatenating the centerline from all coils as shown in Figure 3. The MOCCA echo, denoted by a vector, is chosen to be the self-gating data. In a typical cardiac MRI sequence, the number of sample in a single k-space centerline ranges from 128 to 512 and up to 18 coils are used for acquisition. As a result, the size of a MOCCA vector could easily reach the order of thousands. Each of the N elements in the MOCCA vector is an independent measurement of cardiac motion because it is modulated by unique k-space positions and coil sensitivity profile (18).
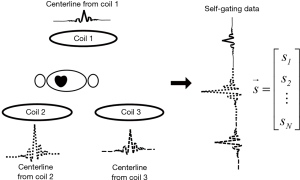
Given the abundant information provided by the MOCCA echo, it is the goal of the self-gating data processing algorithm to combine all measurements in the MOCCA echo in such a way that cardiac motion is enhanced while noise is suppressed. We assume that cardiac motion is the most significant factor in causing self-gating signal variance in a breath-held cardiac scan. Therefore, principal component analysis (PCA) algorithm was used in our algorithm because it is a useful data processing technique to represent high dimensional data by their variation significance. For simplified computation and real-time processing, PCA algorithm was implemented in a training-projection fashion as described in Figure 4. In the training phase, a total number of T=300 MOCCA echoes are collected to construct the training matrix M. Each column in the matrix represents a MOCCA echo from a single self-gating acquisition  and each row contains all the measurements of a MOCCA element X. Given the training matrix M, a covariance matrix Σ is derived by calculating the covariance of every two MOCCA element. Then, Eigen-decomposition is performed on the covariance matrix to have the eigenvectors and corresponding eigenvalues. Here, we are interested in the first eigenvector, also referred as the principal component. This is because the training dataset exhibit maximum variance in that direction, which is assumed to be the result of cardiac motion. Therefore, only the first eigenvector
and each row contains all the measurements of a MOCCA element X. Given the training matrix M, a covariance matrix Σ is derived by calculating the covariance of every two MOCCA element. Then, Eigen-decomposition is performed on the covariance matrix to have the eigenvectors and corresponding eigenvalues. Here, we are interested in the first eigenvector, also referred as the principal component. This is because the training dataset exhibit maximum variance in that direction, which is assumed to be the result of cardiac motion. Therefore, only the first eigenvector 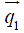 is stored for the projection phase.
is stored for the projection phase.
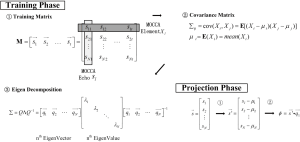
Compared with the training phase, the calculation of the projection phase is fairly simple. A new MOCCA echo 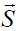 is first "centralized" by subtracting the average value of each MOCCA element. The centralized vector
is first "centralized" by subtracting the average value of each MOCCA element. The centralized vector 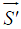 is then projected onto the principal component direction
is then projected onto the principal component direction 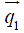 and the projected length is calculated from the dot product of vector
and the projected length is calculated from the dot product of vector 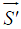 and
and 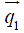 . The scalar ϕ is the desired cardiac motion measurement from which an accurate and reliable cardiac trigger can be generated.
. The scalar ϕ is the desired cardiac motion measurement from which an accurate and reliable cardiac trigger can be generated.
Self-gating trigger temporal variability
In order to validate the proposed self-gating signal acquisition and signal processing strategy, we run a breath-hold acquisition with self-gating mode only by turning off the phase-encoding gradient so that the k-space centerline is repeatedly acquired. 1.5T Avanto and 3T Trio (Siemens Healthcare, Erlangen, Germany) scanners were used with a combination of different cardiac orientations, including short axis (SA), vertical long axis (VLA), horizontal long axis (HLA), on 8 healthy volunteers. Other sequence and algorithm parameters include: TR =3.2 ms, TE =1.6 ms, FA =65 training number T =300 for balanced steady state free precession (bSSFP) sequence and TR =6.9 ms, TE =2.4 ms, FA =30, training number T =150 for gradient echo (GRE) sequence. The acquired self-gating data was exported offline and processed by a Matlab (MathWorks, Natick, MA, USA) program. Synchronous ECG signal and triggers were recorded with timestamp as the reference. We used detection rate, Eq. [1] and temporal variability, Eq. [2] to assess the reliability and reproducibility of the self-gating (SG) triggers. The temporal variability is calculated as the standard deviation of the time delay between self-gating triggers and corresponding ECG triggers. A smaller temporal variability indicates good temporal consistency between self-gating triggers and ECG triggers. Of note, the ECG monitoring system itself has an inherent systematic variation of up to ±2.5 ms because of its 400 Hz sampling rate.
In vivo prospective self-gated cine MRI
We further implemented the proposed self-gating acquisition scheme and self-gating algorithm in a prospectively self-gated cine sequence. The self-gating data processing algorithm shown in Figure 5 was developed in Siemens Image Calculation Environment (ICE) using C++ programming language. K-space measurement data from the scanner was sent to the self-gating processing module after each TR with a flag indicating the type of the acquisition (training, self-gating or imaging). The first 299 training data were stored to fill the PCA training matrix. With the arrival of the 300th training data, PCA training program was initiated to find the first principal component of the training matrix as described in Figure 4. Subsequently, the 300 training data were projected to the principal component direction, resulting in 300 (corresponds to about 1 second) scalar values representing the cardiac motion. An initial cardiac trigger was detected by finding the peak within these measurements. For the successive self-gating data, only PCA projection algorithm was used to calculate the cardiac motion from which cardiac triggers were detected by finding the signal peak that is above the threshold within a sliding window of 5 samples. The threshold was initially defined as 90% of the cardiac trigger during training phase and was updated upon each detected trigger. No filtering was applied before the peak detection due to high quality of self-gating signal. When a self-gating trigger was detected, a feedback signal was immediately sent back to the scanner to stop the current self-gating mode and start the imaging mode. Conventional Fourier based image reconstruction was applied to process the imaging data. In such a way, the sequence switches between self-gating mode and imaging mode until the entire k-space is filled. Immediately after the scan, a series of cardiac CINE image was readily available at the scanner console.
We tested the prospective self-gating sequence on 6 healthy volunteers using the 1.5T scanner in two orientations (SA and VLA). Real time sequence mode (training, self-gating and imaging) was also recorded as a flag in the raw data. Standard prospective ECG-gated CINE images were also acquired on each volunteer using matched slice orientation as a comparison of image quality. Real-time ECG signal and triggers were recorded for reference, which was used to calculate the temporal variability and detection rate of the prospective data sets according to Eqs. [1] and [2].
Results
Self-gating trigger temporal variability
Figure 6 shows the plot of 5 principal components generated by PCA algorithm from one selected self-gating data as well as their contributions to the total signal variance. The first principal component provide a clear and smooth measurement of cardiac motion while other component are distorted and mixed with noise. Meanwhile, the first component contributes to over 60% of total signal variance, suggesting that most of the motion information in the MOCCA echo is concentrated in the first principal component. Therefore, the first principal component direction was selected to represents the cardiac motion.
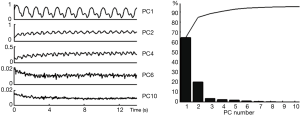
Figure 7A shows an example of the PCA processed self-gating signal and the corresponding ECG signal from a 1.5T scanner in cardiac short-axis view. The self-gating signal provided smooth cardiac motion measurement and accurate cardiac triggers that corresponded well to the ECG triggers. Figure 7B shows another result of the self-gating and ECG signal from a 3T scanner in a cardiac vertical long axis view. In this particular case, ECG signal was heavily distorted due to interference with varying magnetic field (2-5) during the scan and several ECG triggers were missed by the scanner. However, self-gating signal was capable of providing reliable gating of cardiac motion. Of note, no filter was needed on the self-gating signal.

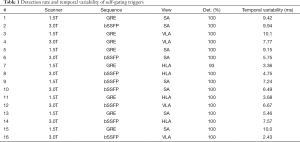
Table 1 lists the detection rate and temporal variability of the self-gating triggers from 16 experiments in different combination of scanner, sequence and slice orientation. The proposed self-gating method was able to achieve 100% detection rate in most of the experiments with only one exception (#7). In that case, the self-gating signal drifted during the last cardiac cycle so that the threshold-based trigger detection algorithm was not able to catch that cardiac trigger. We believe the drifting in this particular case was caused by respiratory motion due to non-ideal breath-hold, which was confirmed with the subject during the experiment. The temporal variability was less than 10 milliseconds, suggesting the detected self-gating triggers coincides well with the ECG triggers, though they can be shifted from the QRS complex as shown in Figure 7.
Full table
Prospective self-gated cine MRI
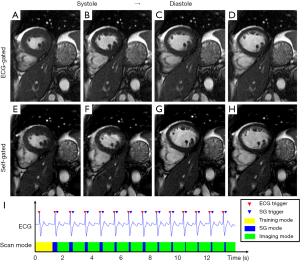
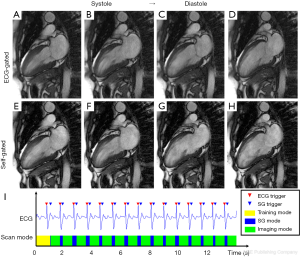
Figure 8 and Figure 9 show selected frames from example CINE images in short-axis and vertical-long-axis views, along with the self-gating signal and triggers acquired on healthy volunteers using a 1.5T scanner. There was no noticeable motion artifact in the self-gated images and the overall image quality of self-gated CINE is equivalent with that of ECG-gated. Based on the flags in the raw data, the self-gating trigger was successfully identified in both examples. There was slight variation in the heart rate during the exam and the duration of the self-gating mode for each heart beat varied accordingly as expected. Table 2 lists the statistical result of all 6 scans. The proposed prospective self-gating method was able to detect 100% of the 85 cardiac triggers over 6 subjects and switch scan mode accordingly. The average temporal variability between self-gating triggers and ECG triggers was 10.6 ms, which was similar to our findings at the temporal variation study. The mean trigger delay when compared with ECG R-wave was approximately 220–230 ms for short axis views and approximately 170–180 ms for vertical long axis views.

Full table
Discussion
In this paper, we introduce a prospective cardiac self-gating technique and demonstrate it in a self-gated cardiac cine sequence that is capable of detecting 100% of the cardiac trigger in real time. Our technique is different from other existing self-gating methods in three aspects. First, MOCCA echo (k-space centerline with coil arrays) is used as self-gating data that could provide abundant motion information. Second, the self-gating data is processed by PCA algorithm in a training-projection scheme. Third, a two-mode sequence structure is adopted in which dedicated self-gating acquisitions are separated from the normal imaging acquisition. We evaluated the proposed technique by comparing the self-gating triggers with ECG triggers and the results indicate good temporal consistency between the two. We further tested the self-gating technique in a prospectively self-gated cardiac CINE sequence and showed excellent correspondence of our self-gating triggers to the ECG triggers. Our data suggests that this sequence is very reliable in trigger detection and can provide excellent cardiac image quality. Our solution uses the clinically available image reconstruction computer to process the self-gating data and send feedback signal to the MRI scanner. Such an implementation is feasible on MRI systems from most major manufacturers without any hardware modification. In this work, we demonstrate the feasibility of the proposed self-gating technique using a self-gated cardiac CINE sequence. Other applications using this self-gating technique have yet to be developed. Some of the examples include, but not limited to self-gated coronary angiography (MRA), cardiac imaging at high magnetic field (7T and up), and fetal cardiac imaging.
The MOCCA echo used in the proposed self-gating method could better capture cardiac motion than other self-gating data sampling strategy. While k-space center point is only capable to capture the variance of the image DC component and the k-space centerline can further detect the non-DC variance in the k-space readout direction, the MOCCA echo has the intrinsic capability to detect motion in all directions. This is because up to 16 coils are placed in almost every direction around the heart in a conventional cardiac MRI setup. As a result, motion information in any direction can be modulated by individual coil's sensitivity map and reflected in the MOCCA echo. Although a systematic evaluation of the potential of MOCCA echo is beyond the scope of this paper, we believe the signal quality improvement of Figure 7 over Figure 1 result from the use of MOCCA echo instead of k-space center point.
PCA algorithm can better exploit cardiac motion information provided by the MOCCA echo. To address the theory behind the proposed PCA-based algorithm, we can interpret the task as a signal-processing problem in which we want to enhance the desired signal component (i.e., cardiac motion) and suppress the unwanted component (i.e., other motion, noise etc.) In such a task, a precise definition of the signal is needed to differentiate it from the noise. Most existing processing algorithms use an explicit definition in image domain to characterize the cardiac motion signal. For example, the method of using the k-space center point defines the cardiac motion as the change of overall image intensity. This is based on the assumption that the variation of blood pool volume is the major contributor of the overall image intensity, which is why some of the existing techniques typically works better at short-axis view because this view is associated with most significant change in blood volume (11). However, our approach appears to work equally well in both short axis and long axis views because the PCA algorithm is not dependent on in-plane blood volume Other algorithms define the cardiac motion by looking for certain features from the Fourier transformed k-space line, including sharp edges, center of mass (COM) etc. Despite the fact that these methods highly depend on specific imaging parameters (e.g., contrast, slice orientation) and the anatomy of individual subjects, they are unable to take advantage of the motion information provided by multiple coils because the processing is done in image domain after combining the signals. On the other hand, the proposed PCA-based algorithm defines the cardiac motion in an implicit way: the cardiac motion is the most significant factors in causing the variance of self-gating signal in a breath-hold cardiac scan. First, this definition is independent of imaging parameters or individual subjects. Second, the processing is performed in k-space signal domain, before combining information from multiple coils and thereby has the potential to take advantage of the MOCCA echo. Third, abundant information in MOCCA echo is better used as all MOCCA channels are combined together in a way to maximize the signal variance. In addition, the proposed PCA algorithm shows good performance in suppressing noise, as shown by the clarity and smoothness of the signal plot in Figure 7 and Figure 6 even in the absence of any filtering of the signal.
The proposed PCA algorithm is a training based algorithm. The first 300 self-gating samples are chosen to construct the training matrix. It is because 300 samples take about 1 second (TR =3 ms), which is approximately a complete cardiac cycle. From these training samples, the component with maximum signal variation is found, which is assumed as the cardiac motion component. Therefore, it is desirable that the training period is sufficiently long to cover a complete cardiac motion cycle, but not too long as overall imaging efficiency would decrease. The advantage of such training-based algorithm is that the signal process algorithm is individually tailored for each subject in each scan and no specific parameters is required at the users’ end. This is further supported by the data from Table 1 that the same algorithm can be used to process self-gating signals from different scans, on different subjects, using different contrasts and slice orientations.
We demonstrated the utility of our technique in online prospective self-gating. Several technical components of our approach can also be used in an offline retrospective self-gating, which might have certain benefits. For example, using the approach in Figure 2 for CINE imaging inevitably will miss a fraction of the cardiac cycle as it needs to be used as a dedicated self-gating mode. This might be undesirable for CINE imaging and related volume and ejection fraction calculations. A retrospective offline self-gating might be more desirable. Nevertheless, our current approach suits well for non-cine type cardiac applications.
The PCA-based signal processing algorithm plays a key role in enabling online self-gating. A number of processing algorithms rely on a high order band-pass filter to suppress the non-cardiac signal component. Such high-order frequency filters are inherently slow and unsuitable for real time processing because of their group delay (22). In the proposed PCA algorithm, each self-gating sample is simply projected onto the principal component direction defined in the training phase. The PCA algorithm itself is causal with no processing delay, although the peak detection algorithm introduces a delay of 2 samples. As a result, it takes less than 10ms for the sequence to detect the trigger and change mode accordingly, making the online prospective self-gating possible.
It should be noted that the self-gating triggers were delayed from the ECG triggers by an average of 228ms for short-axis and 177 ms for vertical-long-axis. This is because: (I) there is an inherent delay between the electrical signal and the actual myocardial motion in which the electrical signal always comes first; (II) current self-gating trigger detection algorithm is based on finding the signal peak and thus tends to trigger on end-systole instead of end-diastole as the ECG R-wave based algorithm. A similar shift is also reported in other self-gating methods (23,24).
Acknowledgement
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Numbers R21HL113427, R01HL127153 and the American Heart Association under the Award Number 10SDG4200076. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors of this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by UCLA IRB (#11-003519) and written informed consent was obtained from all subjects.
References
- Deklerck RaS P, Taeymans Y, Lafruit G, Cornelis J. An ECG trigger module for the acquisition of cardiac MR images. Bethesda, MD, USA. 1994:533-6.
- Damji AA, Snyder RE, Ellinger DC, Witkowski FX, Allen PS. RF interference suppression in a cardiac synchronization system operating in a high magnetic field NMR imaging system. Magn Reson Imaging 1988;6:637-40. [Crossref] [PubMed]
- Rokey R, Wendt RE, Johnston DL. Monitoring of acutely ill patients during nuclear magnetic resonance imaging: use of a time-varying filter electrocardiographic gating device to reduce gradient artifacts. Magn Reson Med 1988;6:240-5. [Crossref] [PubMed]
- Tenforde TS. Magnetically induced electric fields and currents in the circulatory system. Prog Biophys Mol Biol 2005;87:279-88. [Crossref] [PubMed]
- Wendt RE 3rd, Rokey R, Vick GW 3rd, Johnston DL. Electrocardiographic gating and monitoring in NMR imaging. Magn Reson Imaging 1988;6:89-95. [Crossref] [PubMed]
- Wielandner A, Mlczoch E, Prayer D, Berger-Kulemann V. Potential of magnetic resonance for imaging the fetal heart. Semin Fetal Neonatal Med 2013;18:286-97. [Crossref] [PubMed]
- Peters M, Crowe J, Pieri JF, Quartero H, Hayes-Gill B, James D, Stinstra J, Shakespeare S. Monitoring the fetal heart non-invasively: a review of methods. J Perinat Med 2001;29:408-16. [Crossref] [PubMed]
- Manganaro L, Savelli S, Di Maurizio M, Perrone A, Tesei J, Francioso A, Angeletti M, Coratella F, Irimia D, Fierro F, Ventriglia F, Ballesio L. Potential role of fetal cardiac evaluation with magnetic resonance imaging: preliminary experience. Prenat Diagn 2008;28:148-56. [Crossref] [PubMed]
- Roy CW, Seed M, van Amerom JF, Al Nafisi B, Grosse-Wortmann L, Yoo SJ, Macgowan CK. Dynamic imaging of the fetal heart using metric optimized gating. Magn Reson Med 2013;70:1598-607. [Crossref] [PubMed]
- Yamamura J, Frisch M, Ecker H, Graessner J, Hecher K, Adam G, Wedegartner U. Self-gating MR imaging of the fetal heart: comparison with real cardiac triggering. Eur Radiol 2011;21:142-9. [Crossref] [PubMed]
- Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magn Reson Med 2004;51:93-102. [Crossref] [PubMed]
- Hiba B, Richard N, Janier M, Croisille P. Cardiac and respiratory double self-gated cine MRI in the mouse at 7 T. Magn Reson Med 2006;55:506-13. [Crossref] [PubMed]
- Buehrer M, Curcic J, Boesiger P, Kozerke S. Prospective self-gating for simultaneous compensation of cardiac and respiratory motion. Magn Reson Med 2008;60:683-90. [Crossref] [PubMed]
- Crowe ME, Larson AC, Zhang Q, Carr J, White RD, Li D, Simonetti OP. Automated rectilinear self-gated cardiac cine imaging. Magn Reson Med 2004;52:782-8. [Crossref] [PubMed]
- Hiba B, Richard N, Thibault H, Janier M. Cardiac and respiratory self-gated cine MRI in the mouse: comparison between radial and rectilinear techniques at 7T. Magn Reson Med 2007;58:745-53. [Crossref] [PubMed]
- Spraggins TA. Wireless retrospective gating: application to cine cardiac imaging. Magn Reson Imaging 1990;8:675-81. [Crossref] [PubMed]
- White RD, Paschal CB, Clampitt ME, Spraggins TA, Lenz GW. Electrocardiograph-independent, "wireless" cardiovascular cine MR imaging. J Magn Reson Imaging 1991;1:347-55. [Crossref] [PubMed]
- Hu P, Hong S, Moghari MH, Goddu B, Goepfert L, Kissinger KV, Hauser TH, Manning WJ, Nezafat R. Motion correction using coil arrays (MOCCA) for free-breathing cardiac cine MRI. Magn Reson Med 2011;66:467-75. [Crossref] [PubMed]
- Bammer R, Aksoy M, Liu C. Augmented generalized SENSE reconstruction to correct for rigid body motion. Magn Reson Med 2007;57:90-102. [Crossref] [PubMed]
- Bydder M, Atkinson D, Larkman DJ, Hill DL, Hajnal JV. SMASH navigators. Magn Reson Med 2003;49:493-500. [Crossref] [PubMed]
- Offerman EJ, Koktzoglou I, Glielmi C, Sen A, Edelman RR. Prospective self-gated nonenhanced magnetic resonance angiography of the peripheral arteries. Magn Reson Med 2013;69:158-62. [Crossref] [PubMed]
- Spincemaille P, Nguyen TD, Prince MR, Wang Y. Kalman filtering for real-time navigator processing. Magn Reson Med 2008;60:158-68. [Crossref] [PubMed]
- Liu J, Spincemaille P, Codella NC, Nguyen TD, Prince MR, Wang Y. Respiratory and cardiac self-gated free-breathing cardiac CINE imaging with multiecho 3D hybrid radial SSFP acquisition. Magn Reson Med 2010;63:1230-7. [Crossref] [PubMed]
- Kolbitsch C, Prieto C, Schaeffter T. Cardiac functional assessment without electrocardiogram using physiological self-navigation. Magn Reson Med 2014;71:942-54. [Crossref] [PubMed]


