Bone marrow edema-like lesions (BMELs) are associated with higher T1ρ and T2 values of cartilage in anterior cruciate ligament (ACL)-reconstructed knees: a longitudinal study
Introduction
Anterior cruciate ligament (ACL) injuries are very common traumatic knee injuries, especially in young and physically active individuals (1). Reconstruction of ACL is often required to improve the stability and to return sport ability (2). Regardless of conservative or surgical management, an ACL tear significantly increases the risk of knee osteoarthritis (OA) (3,4). It is estimated that 50–90% of patients with ACL tears would develop post-traumatic OA in 10 to 20 years after the injury, while they are still young (4-6). There is an unmet clinical need to identify early degeneration of the joint for allowing potential early interventions, and to predict post-traumatic OA development after ACL reconstruction for optimizing patients’ management. Bone marrow edema-like lesions (BMELs), which appear as patchy high signal abnormalities on fat-suppressed T2 weighted MR images, are observed in on average 70% of patients with acute ACL injuries (7). During ACL rupture, the anterior lateral femur impacts on the posterior lateral tibia which leaves a “footprint” on the femur and tibia, also known as “kissing lesions” (8). The presence of BMELs was reported to correlate with meniscal tears, collateral ligament tears and large volumes of BMEL were associated with cortical fractures (7,9,10), suggesting BMEL as a potential indicator of injury severity. However, the longitudinal clinical significance of BMEL is not clear. Some studies reported that there was no significant association between BMEL and patient outcomes measured by International Knee Documentation Committee questionnaire (IKDC) at 1-year (10) or 12-year (11) after ACL reconstruction; while in a recent study the BMEL area was significantly negatively correlated with the return to the previous sports level (measured by Tegner score) at mid/long-term follow-up (12). BMEL was also reported as a predictor for OA after knee trauma (13). In patients with knee OA, enlargement of BMELs during follow-up was associated with development of knee pain (14). Unlike BMELs in OA, post-traumatic BMELs are more likely to resolve fast (15) with a median time interval ranging between 4 months and 8 months after acute knee trauma (16).
BMELs in ACL-injured knees have been associated with articular cartilage injuries using immunohistologic analyses (17,18) and arthroscopic evaluation (19). Loss of proteoglycan, degeneration of chondrocytes and increase of cartilage oligomeric matrix protein (COMP) were observed in cartilage overlying BMELs (17,18). Cartilage biopsies of ACL-injured knees are normally difficult to obtain. Thus, non-invasive evaluation and monitoring of cartilage degeneration associated with BMELs are desirable. Using standard MRI, BMELs were associated with high prevalence of cartilage lesions immediately and longitudinally several years after injury (20,21). However, conventional structural MR imaging lacks the ability for detecting biochemical changes within cartilage matrix before the occurrence of morphological changes.
Quantitative MR cartilage imaging, such as T1ρ and T2 mapping, has shown its ability to identify early cartilage degeneration by probing biochemical changes of cartilage collagen-proteoglycan matrix (22-25). These imaging markers of cartilage matrix lead to detect OA in its early stages and to monitor the process of the disease. Significantly elevated T1ρ and T2 of cartilage are reported extensively in knees with ACL injures (26,27). However, studies focused on the effect of BMELs on cartilage matrix changes are very limited. Significantly elevated T1ρ were observed in cartilage overlying BMELs (26,28), with lesions confirmed using arthroscopic evaluation (29). One longitudinal study with a small cohort reported persistent elevated T1ρ in cartilage overlying original BMELs despite resolution of these BMELs at 1 year after ACL reconstruction (30). No studies have evaluated the potential effect of residual BMELs on longitudinal cartilage degeneration in ACL-injured knees.
The goal of this study was to examine the effect of baseline BMELs on cartilage matrix changes over 2 years after ACL reconstruction using T1ρ and T2 mapping. The relationship between baseline BMELs and longitudinal patient outcomes as measured by the Knee Injury and Osteoarthritis Outcome Scores (KOOS) was also be explored. We hypothesized that the baseline BMEL is associated with higher T1ρ and T2 values in cartilage and inferior patient outcomes over 2 years after ACL reconstruction.
Methods
Subjects
This study was approved by the Committee for Human Research of UCSF. Patients with traumatic ACL tear and scheduled to undergo ACL reconstructions were enrolled in a prospective study. The inclusion criteria were: patients with acute ACL tear, which was diagnosed by clinicians and confirmed by MR imaging; the MR imaging was performed within 6 months from injury; decision to receive ACL reconstruction and willing to participate in long-term follow-up using MR imaging. The exclusion criteria included MR imaging contraindications, previous injury or surgery to either knee, history of rheumatoid arthritis or other inflammatory joint diseases, diagnosis of osteoarthritis, and multiligamentous injury requiring surgical treatment in addition to ACL reconstruction.
MR imaging
MR imaging was performed using a 3.0 Tesla MR scanner (General Electric, Milwaukee, WI, USA) and an 8-channel phased-array knee coil (Invivo, Orlando, FL, USA) with the patient in the supine position. The imaging protocol included high-resolution 3D fast spin-echo (CUBE) images [TR/TE, 1,500/25 ms; echo train length, 32; matrix, 384 × 384; field of view (FOV), 16 cm; slice thickness, 1 mm (interpolated into 0.5 mm)] for evaluating BMELs, and 3D T1ρ/T2 quantification sequence developed previously in our lab (31) for assessing cartilage. The parameters were as follows: for T1ρ mapping: TR/TE, 8/3 ms; TSL, 0/10/40/80 ms; spin-lock frequency, 500 Hz; FOV, 14 cm; matrix, 256 × 128; slice thickness, 4 mm; for T2 mapping: preparation TE =0/13.7/27.3/54.7 ms; total acquisition time ~9–10 mins.
Cartilage T1ρ and T2 quantification
All MR image post processing was done using in-house developed software with Matlab (Mathworks, Natick, MA, USA) integrated with Elastix library for image registration (32,33).
All the six cartilage compartments of the baseline scan were segmented semi-automatically on multiple high-resolution CUBE images using an algorithm based on edge detection and Bezier splines (34). The CUBE images and the first echo of T2 images were rigidly registered to the first T1ρ-weighted images (TSL =0). Piecewise rigid registration was applied along T1ρ-weighted and T2-weighted images to account for non-rigid movement of the femur, tibia, and patella (PAT) with respect to one another. Additionally, all contralateral and all follow-up scans were registered to the first T1ρ echo of the injured knee to assure that the same anatomical regions of cartilage were compared in the analysis. The registration was accomplished using an intensity-based multi-resolution pyramidal approach previously proposed, and T1ρ and T2 maps were reconstructed by fitting the T1ρ- and T2-weighted images pixel-by-pixel (35).
To reduce artifacts caused by partial volume effects with synovial fluid, pixels with relaxation time greater than 130 ms in T1ρ or 100 ms for T2 maps were removed from the data used for quantification. For the femoral condyle (FC) and tibial plateau (TP), the relaxation times were also calculated for weight-bearing subcompartments as shown in Figure 1 using the medial compartment as illustration. Including the trochlea (TRO) and PAT, T1ρ and T2 values were calculated in 16 cartilage compartments/subcompartments, respectively.
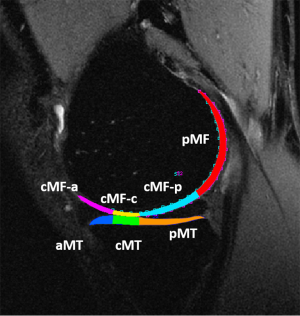
Whole-organ evaluation of knees
One radiologist (Luca Facchetti) graded knee degeneration using a modified whole-organ magnetic resonance imaging score (WORMS) of the knee on CUBE images at baseline, 6-month, 1-year and 2-year follow-up (36). The reliability assessment was not carried out in present study for WORMS grading was found to be of high inter- and intra-reader reliability in other study of our group (37). The grading criteria of BMELs are following: 0= normal bone marrow signal; 1= high signal patch with maximum width less than 5 mm; 2= high signal patch with maximum width larger than 5 mm and less than 20 mm; 3= high signal patch with maximum width larger than 20 mm. If the WORMS meniscus scores were larger than 1, it was considered that the patient had at least one meniscus tear.
To calculate BMEL volume, first, contours (circles with 5 mm diameter) covering the normal bone marrow in the femur shaft were placed manually, and the standard deviation (SD) of signal intensity within normal bone marrow was calculated. Second, a masked image was generated by manually drawing approximate contours of the bone marrow containing BMELs. This procedure eliminated regions with high signal intensity outside bone marrow. Lastly, BMELs was automatically segmented with a threshold that was 5 times the SD of normal bone marrow and morphological operations were used to refine the segmentation obtaining solid 3D regions of interest (ROIs). BMEL volumes were then calculated.
Statistical analysis
The prevalence of BMELs between the injured knees and the contralateral knees and anatomic distribution of BMELs in injured knees were compared using chi square tests. BMELs’ volumes and WORMS were correlated with age, gender, body mass index (BMI), interval between injury and baseline MR imaging, using Spearman or Pearson’s correlation. The correlation between BMEL (volumes and WORMS) and other lesions including effusion (WORMS) and meniscal tears were also evaluated. T1ρ and T2 values of cartilage from compartment overlying the BMELs of injured knees at baseline were compared with that of the anatomically matched cartilage of the contralateral knees using t tests at baseline and each follow-up time-point in 39 patients who had completed 2-year follow-up. The longitudinal change of baseline BMELs in the injured knees of these 39 patients during follow-up was also compared using a chi square test. Baseline WORMS BMEL scores were dichotomized into bone compartments with BMEL (WORMS >0) and without BMEL (WORMS =0). Generalized estimating equations (GEE) were used to explore influences of baseline BMEL on T1ρ and T2 values of cartilage and KOOS scores in all 54 patients. In the GEE model, follow-up time-point and cartilage compartments/subcompartments served as within-subject variables, and WORMS BMEL scores at baseline served as dependent variables, while gender, age, BMI, effusion and meniscus tear, meniscectomy and served as covariates. A P value less than 0.05 was considered significant. All statistical analyses were performed using SPSS 17.0 for Windows.
Results
Baseline characteristics of BMELs
Fifty four patients were included in present study, which consisted of 31 males and 23 females aged 29.7±8.5 years, BMI of 24.2±3.0 kg/m2. The interval between injury and baseline MR imaging was 55.5±45.3 days. One hundred and five BMELs were noted in 42 injured knees (77.8%) and 9 BMELs were observed in 7 contralateral knees (13.0%) (χ2=45.763, P<0.001) at the baseline. In the injured knees, location of the 105 BMELs were as follows: 42 (40%) in the lateral tibial plateau (LTP), 26 (24.8%) in the lateral femoral condyle (LFC), 26 (24.8%) in the medial tibial plateau (MTP), 6 (5.7%) in the medial femoral condyle (MFC), 3 (2.9%) in the PAT and 2 (1.9%) in the TRO. The prevalence of BMELs at different cartilage compartments was statistically significant (χ2=122.227, P<0.001). The WORMS and volume of BMELs were 2.36±0.65 and 386.98±382.54 mm3, respectively. Volume of BMELs was significantly associated with WORMS (r=0.681, P<0.001). The WORMS effusion scores were 1.20±0.98. There were six patients with at least one meniscus tear and nine patients received meniscectomy. There was a significant correlation between volume of BMELs and effusion WORMS (r=0.315, P=0.020), while no significant correlations of BMELs (volumes and WORMS) with age, BMI, interval between injury and MR imaging, and meniscus tear.
Longitudinal changes of BMELs
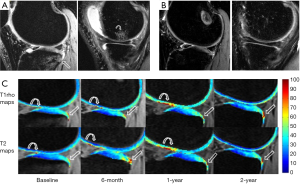
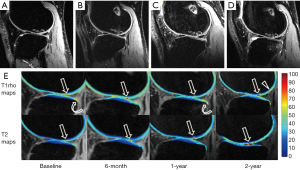
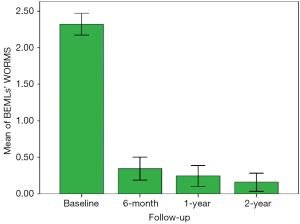
Thirty-nine patients (72.2%) had completed 2 years follow-up. In these patients, 87 BMELs (33 in LTP, 22 in LFC, 21 in MTP, 6 in MFC, 4 in PAT and 1 in TRO) were found in 34 injured knees (87.2%) at baseline. During the follow-up, 18 (20.7%) (6 in LTP, 6 in LFC, 5 in MTP and 1 in MFC) were seen at 6 months (Figure 2), 12 (13.8%) (3 in LFC, 5 in MTP, 3 in LTP and 1 in MFC) at 1 year, and 5 (5.7%) (2 in LFC, 1 in MTP and 1 in LTP) at 2 years, respectively. Regarding bone compartments, prevalence of these BMELs was 37.2% (87/234), 7.7% (18/234), 5.1% (12/234) and 2.1% (5/234), respectively. The changes over time were statistically significant (χ2=163.660, P<0.001). Three new lesions (1 in TRO, 1 in PAT and 1 in LTP) were found at 6 months follow-up MR imaging, 3 (2 in PAT and 1 in MTP) at 1 year follow-up, and 4 (1 in TRO, 1 in LFC and 2 in MTP) at 2 years follow-up (Figure 3). The nine new lesions were found in 6 knees (16.2%) with 3 knees having 2 new lesions. The WORMS BMEL scores of the 87 bone compartments with BMELs were 2.31±0.67, 0.32±0.69, 0.23±0.62 and 0.15±0.54 at baseline, 6-month, 1-year and 2-year follow-up, respectively (Figure 4). The volumes of BMELs at each time-point were 370.77±358.07, 12.87±34.85, 15.37±74.63 and 8.38±48.70 mm3, respectively.
Longitudinal changes of T1ρ and T2 values of cartilage overlying BMELs and association between baseline BMELs and cartilage changes and clinical outcomes
T1ρ and T2 values of cartilage of compartments/subcompartments overlying baseline BMELs in the injured knees were higher than those of anatomically matched cartilage in the contralateral knees at all-time points from baseline to 2-year follow-up regardless of resolution of BMELs (Table 1, Figures 2,3). The difference was statistically significant except for T2 value at 6 months follow-up.
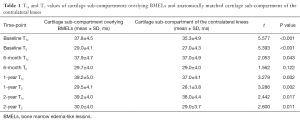
Full table
In the injured knees, GEE analysis showed that baseline BMELs were significant factors associated with higher T1ρ and T2 values of cartilage after adjustment of follow-up time-point, cartilage compartments/subcompartments, age, gender, BMI, effusion and meniscus tear (Table 2). The association between baseline BMELs and KOOS scores showed no statistical significance (data not shown).
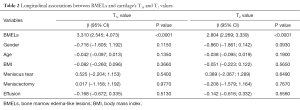
Full table
Discussion
To the best of our knowledge, this is the first study correlating longitudinal changes of BMEL with cartilage T1ρ and T2 in knees after ACL-reconstruction. The main findings of this study included: BMEL was often associated with cartilage damage, which manifests as increased T1ρ and T2 values of cartilage overlying the BMELs compared with that of contralateral cartilage; BMEL at baseline was associated with higher T1ρ and T2 values of cartilage after ACL reconstruction during follow-up. The present study also confirmed previous research showing that BMEL is a common finding in patients with acute ACL injury and resolves rapidly over time after ACL reconstruction.
Although BMEL can be seen in other musculoskeletal disorders, it is very common in ACL injury with a prevalence of 55–98% (7,12,16,38). The specific prevalence of BMEL depends on the time between injury and BMEL, cohorts and MR machine (low field vs. high field). In the present study, the prevalence of BMELs was 77.8%, which was significantly higher than 13.0% of the contralateral knees and they were mostly seen at LFC (40%), followed by LT (24.8%) and MT (24.8%). Quantitative measurement showed that the volume of BMELs was correlated with WORMS effusion scores at baseline. Therefore, BMELs in patients with ACL injury were related to trauma and more likely to be bone bruise. Due to the characteristics of bruise or contusion, BMELs in patients with ACL injury were also more like to be resolved sooner than those in patients with OA (15). Filardo and colleagues reported that only 25% of BMELs could be detected at more than 3 months follow-up (12). Our present study also showed that most BMELs had resolved at 6 months after ACL reconstruction and only 22.2% remained at that time-point, and even a smaller portion (6.5%) could be seen at 2 years follow-up MR imaging. The rapid resolution of BMELs over time in this study was similar to what other studies have reported (39,40). Nine new BMELs in six injured knees (16.2%) were detected in the course of the 2-year follow-up (3 at 6-month, 4 at 1-year and 2 at 2-year). Frobell et al. reported that 21 knees (34%) developed new BMELs in 63 patients during a 2-year period, which was contributed to repetitive microtrauma (41). The prevalence of new lesions this study is lower than what Frobell reported. Due to the limited sample size, analysis of these new lesions was not carried out in present study. The cause and influence of new BMELs in patients after ACL reconstruction need further investigation.
During ACL injury, the impacts of bones also often result in artilage and meniscus damages (42). The compression can lead to chondrocyte death and matrix injury (43). At biopsies in patients with acute ACL rupture, histological examinations have shown chondrocyte and matrix degeneration, and biochemical variations in the cartilage overlying BMEL (17). Cartilage damage can be evaluated based on morphological changes and/or increased T2 signal intensity, and increased T1ρ or T2 values (44-47). The latter may result from increased water content, decreased macromolecular proteoglycan content, and disruption of collagen matrix ultrastructure. In lower grade cartilage injury, signal intensity alternations and morphological changes are often too subtle to be assessed visually. Quantitative measurement of T1ρ or T2 values provides an objective modality to assess cartilage injury. The damage of cartilage overlying BMELs might be irreversible or partially reversible (48,49). Our previous studies indicated that T1ρ values of cartilage overlying BMELs were higher than that of surrounding cartilage in patients with OA and ACL injury (27,50). In the present study, we found that both T1ρ and T2 values of cartilage overlying BMELs were higher that of anatomically matched cartilage in the contralateral knees at baseline. Even at 6-month to 2-year follow-up after most BMELs were resolved, the difference was still statistically significant except for T2 values at 6 months. Due to lack of blood supply, cartilage damage can not heal spontaneously. Impaction of cartilage and bone at the time of ACL injury may result in a cascade of biologic events that result in knee OA (41). Therefore, the damage of cartilage at baseline may remain for a long time.
Another finding of this study was that BMELs (WORMS >0) at baseline were significantly associated with higher T1ρ and T2 values of cartilage during the 2-year follow-up. Even after BMELs have resolved, changes of cartilage are still present and cartilage degeneration might be accelerated in the cartilage overlying BMELs. These findings also support the idea that the cartilage damage overlying BMELs is irreversible and may be a trigger to start the cascade of OA through accelerating articular degeneration (41). Therefore, BMEL at baseline might be a risk factor for OA in patients with ACL injury.
This study has several limitations. First, the sample size was moderate with 39 patients who had completed 2-year follow-up. Further studies with more patients and longer follow-up are recommended. Second, cartilage injuries during trauma may be very focal. We measured T1ρ and T2 values of the sub-compartments of FC and TP, and global PAT and TRO, overlying BMEL. Therefore, T1ρ and T2 values of the damaged cartilage could be underestimated. Third, cause and influence of the new BMELs were not analyzed due to small sample size and low occurrence rate. These new BMELs might have resulted from repetitive micro-trauma or from early OA.
In conclusion, BMEL is a very common finding in patients with ACL injury at MR imaging and resolves rapidly over time after ACL reconstruction. It is often accompanied by cartilage damage that is not fully recovered. During follow-up, BMELs at baseline are associated with higher T1ρ and T2 values of cartilage. BMEL might be an independent predictor for faster cartilage degeneration in patients with ACL injury and a risk factor for OA. Clinicians and radiologists should pay more attention to BMEL and take it into account for patients’ management decision.
Acknowledgements
Funding: This work was supported in part by NIH P50 AR060752 and Shenzhen Science & Technology Program (JCYJ2014041622811967).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Committee for Human Research of UCSF and written informed consent was obtained from all patients.
References
- Brophy RH, Wright RW, Matava MJ. Cost analysis of converting from single-bundle to double-bundle anterior cruciate ligament reconstruction. Am J Sports Med 2009;37:683-7. [Crossref] [PubMed]
- Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med 2008;359:2135-42. [Crossref] [PubMed]
- Barenius B, Ponzer S, Shalabi A, Bujak R, Norlén L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med 2014;42:1049-57. [Crossref] [PubMed]
- Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med 2007;35:1756-69. [Crossref] [PubMed]
- Neyret P, Donell ST, DeJour D, DeJour H. Partial meniscectomy and anterior cruciate ligament rupture in soccer players. A study with a minimum 20-year followup. Am J Sports Med 1993;21:455-60. [Crossref] [PubMed]
- von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis 2004;63:269-73. [Crossref] [PubMed]
- Papalia R, Torre G, Vasta S, Zampogna B, Pedersen DR, Denaro V, Amendola A. Bone bruises in anterior cruciate ligament injured knee and long-term outcomes. A review of the evidence. Open Access J Sports Med 2015;6:37-48. [PubMed]
- Murphy BJ, Smith RL, Uribe JW, Janecki CJ, Hechtman KS, Mangasarian RA. Bone signal abnormalities in the posterolateral tibia and lateral femoral condyle in complete tears of the anterior cruciate ligament: a specific sign? Radiology 1992;182:221-4. [Crossref] [PubMed]
- Frobell RB, Roos HP, Roos EM, Hellio Le Graverand MP, Buck R, Tamez-Pena J, Totterman S, Boegard T, Lohmander LS. The acutely ACL injured knee assessed by MRI: are large volume traumatic bone marrow lesions a sign of severe compression injury? Osteoarthritis Cartilage 2008;16:829-36. [Crossref] [PubMed]
- Kijowski R, Sanogo ML, Lee KS, Muñoz Del Río A, McGuine TA, Baer GS, Graf BK, De Smet AA. Short-term clinical importance of osseous injuries diagnosed at MR imaging in patients with anterior cruciate ligament tear. Radiology 2012;264:531-41. [Crossref] [PubMed]
- Hanypsiak BT, Spindler KP, Rothrock CR, Calabrese GJ, Richmond B, Herrenbruck TM, Parker RD. Twelve-year follow-up on anterior cruciate ligament reconstruction: long-term outcomes of prospectively studied osseous and articular injuries. Am J Sports Med 2008;36:671-7. [Crossref] [PubMed]
- Filardo G, Kon E, Tentoni F, Andriolo L, Di Martino A, Busacca M, Di Matteo B, Marcacci M. Anterior cruciate ligament injury: post-traumatic bone marrow oedema correlates with long-term prognosis. Int Orthop 2016;40:183-90. [Crossref] [PubMed]
- Koster IM, Oei EH, Hensen JH, Boks SS, Koes BW, Vroegindeweij D, Hunink MG, Bierma-Zeinstra SM. Predictive factors for new onset or progression of knee osteoarthritis one year after trauma: MRI follow-up in general practice. Eur Radiol 2011;21:1509-16. [Crossref] [PubMed]
- Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, Torner J, Lewis CE, Nevitt MC. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum 2007;56:2986-92. [Crossref] [PubMed]
- Frobell RB, Le Graverand MP, Buck R, Roos EM, Roos HP, Tamez-Pena J, Totterman S, Lohmander LS. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage 2009;17:161-7. [Crossref] [PubMed]
- Kijowski R, Roemer F, Englund M, Tiderius CJ, Swärd P, Frobell RB. Imaging following acute knee trauma. Osteoarthritis Cartilage 2014;22:1429-43. [Crossref] [PubMed]
- Johnson DL, Urban WP Jr, Caborn DN, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med 1998;26:409-14. [PubMed]
- Fang C, Johnson D, Leslie MP, Carlson CS, Robbins M, Di Cesare PE. Tissue distribution and measurement of cartilage oligomeric matrix protein in patients with magnetic resonance imaging-detected bone bruises after acute anterior cruciate ligament tears. J Orthop Res 2001;19:634-41. [Crossref] [PubMed]
- Nishimori M, Deie M, Adachi N, Kanaya A, Nakamae A, Motoyama M, Ochi M. Articular cartilage injury of the posterior lateral tibial plateau associated with acute anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc 2008;16:270-4. [Crossref] [PubMed]
- Faber KJ, Dill JR, Amendola A, Thain L, Spouge A, Fowler PJ. Occult osteochondral lesions after anterior cruciate ligament rupture. Six-year magnetic resonance imaging follow-up study. Am J Sports Med 1999;27:489-94. [PubMed]
- Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med 2012;40:276-85. [Crossref] [PubMed]
- Li X, Majumdar S. Quantitative MRI of articular cartilage and its clinical applications. J Magn Reson Imaging 2013;38:991-1008. [Crossref] [PubMed]
- Wáng YX, Zhang Q, Li X, Chen W, Ahuja A, Yuan J. T1ρ magnetic resonance: basic physics principles and applications in knee and intervertebral disc imaging. Quant Imaging Med Surg 2015;5:858-85. [PubMed]
- Matzat SJ, van Tiel J, Gold GE, Oei EH. Quantitative MRI techniques of cartilage composition. Quant Imaging Med Surg 2013;3:162-74. [PubMed]
- Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 2007;15:789-97. [Crossref] [PubMed]
- Klocke NF, Amendola A, Thedens DR, Williams GN, Luty CM, Martin JA, Pedersen DR. Comparison of T1ρ, dGEMRIC, and quantitative T2 MRI in preoperative ACL rupture patients. Acad Radiol 2013;20:99-107. [Crossref] [PubMed]
- Bolbos RI, Ma CB, Link TM, Majumdar S, Li X. In vivo T1rho quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance imaging. Invest Radiol 2008;43:782-8. [Crossref] [PubMed]
- Li X, Ma BC, Bolbos RI, Stahl R, Lozano J, Zuo J, Lin K, Link TM, Safran M, Majumdar S. Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla. J Magn Reson Imaging 2008;28:453-61. [Crossref] [PubMed]
- Gupta R, Virayavanich W, Kuo D, Su F, Link T, Ma B, Li X. MR T. (1)ρ quantification of cartilage focal lesions in acutely injured knees: correlation with arthroscopic evaluation. Magn Reson Imaging 2014;32:1290-6. [Crossref] [PubMed]
- Theologis AA, Haughom B, Liang F, Zhang Y, Majumdar S, Link TM, Ma CB, Li X. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc 2014;22:298-307. [Crossref] [PubMed]
- Li X, Wyatt C, Rivoire J, Han E, Chen W, Schooler J, Liang F, Shet K, Souza R, Majumdar S. Simultaneous acquisition of T1ρ and T2 quantification in knee cartilage: repeatability and diurnal variation. J Magn Reson Imaging 2014;39:1287-93. [Crossref] [PubMed]
- Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 2010;29:196-205. [Crossref] [PubMed]
- Shamonin DP, Bron EE, Lelieveldt BP, Smits M, Klein S, Staring M. Alzheimer's Disease Neuroimaging Initiative. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer's disease. Front Neuroinform 2014;7:50. [PubMed]
- Carballido-Gamio J, Bauer JS, Stahl R, Lee KY, Krause S, Link TM, Majumdar S. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal 2008;12:120-35. [Crossref] [PubMed]
- Pedoia V, Li X, Su F, Calixto N, Majumdar S. Fully automatic analysis of the knee articular cartilage T1ρ relaxation time using voxel-based relaxometry. J Magn Reson Imaging 2016;43:970-80. [Crossref] [PubMed]
- Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;12:177-90. [Crossref] [PubMed]
- Schwaiger BJ, Gersing AS, Mbapte Wamba J, Nevitt MC, McCulloch CE, Link TM. Can Signal Abnormalities Detected with MR Imaging in Knee Articular Cartilage Be Used to Predict Development of Morphologic Cartilage Defects? 48-Month Data from the Osteoarthritis Radiology 2016;281:158-67.
- Dunn WR, Spindler KP, Amendola A, Andrish JT, Kaeding CC, Marx RG, McCarty EC, Parker RD, Harrell FE Jr, An AQ, Wright RW, Brophy RH, Matava MJ, Flanigan DC, Huston LJ, Jones MH, Wolcott ML, Vidal AF, Wolf BR. MOON ACL Investigation. Which preoperative factors, including bone bruise, are associated with knee pain/symptoms at index anterior cruciate ligament reconstruction (ACLR)? A Multicenter Orthopaedic Outcomes Network (MOON) ACLR Cohort Study. Am J Sports Med 2010;38:1778-87. [Crossref] [PubMed]
- Bretlau T, Tuxøe J, Larsen L, Jørgensen U, Thomsen HS, Lausten GS. Bone bruise in the acutely injured knee. Knee Surg Sports Traumatol Arthrosc 2002;10:96-101. [Crossref] [PubMed]
- Miller MD, Osborne JR, Gordon WT, Hinkin DT, Brinker MR. The natural history of bone bruises. A prospective study of magnetic resonance imaging-detected trabecular microfractures in patients with isolated medial collateral ligament injuries. Am J Sports Med 1998;26:15-9. [PubMed]
- Frobell RB. Change in cartilage thickness, posttraumatic bone marrow lesions, and joint fluid volumes after acute ACL disruption: a two-year prospective MRI study of sixty-one subjects. J Bone Joint Surg Am 2011;93:1096-103. [Crossref] [PubMed]
- Naraghi A, White LM. MR imaging of cruciate ligaments. Magn Reson Imaging Clin N Am 2014;22:557-80. [Crossref] [PubMed]
- Pathria MN, Chung CB, Resnick DL. Acute and Stress-related Injuries of Bone and Cartilage: Pertinent Anatomy, Basic Biomechanics, and Imaging Perspective. Radiology 2016;280:21-38. [Crossref] [PubMed]
- Mlynárik V, Trattnig S, Huber M, Zembsch A, Imhof H. The role of relaxation times in monitoring proteoglycan depletion in articular cartilage. J Magn Reson Imaging 1999;10:497-502. [Crossref] [PubMed]
- Nieminen MT, Rieppo J, Töyräs J, Hakumäki JM, Silvennoinen J, Hyttinen MM, Helminen HJ, Jurvelin JS. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med 2001;46:487-93. [Crossref] [PubMed]
- Watrin A, Ruaud JP, Olivier PT, Guingamp NC, Gonord PD, Netter PA, Blum AG, Guillot GM, Gillet PM, Loeuille DH. T2 mapping of rat patellar cartilage. Radiology 2001;219:395-402. [Crossref] [PubMed]
- Liess C, Lüsse S, Karger N, Heller M, Glüer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage 2002;10:907-13. [Crossref] [PubMed]
- Rosen MA, Jackson DW, Berger PE. Occult osseous lesions documented by magnetic resonance imaging associated with anterior cruciate ligament ruptures. Arthroscopy 1991;7:45-51. [Crossref] [PubMed]
- Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ, Smith MB. Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology 2005;234:245-9. [Crossref] [PubMed]
- Zhao J, Li X, Bolbos RI, Link TM, Majumdar S. Longitudinal assessment of bone marrow edema-like lesions and cartilage degeneration in osteoarthritis using 3 T MR T1rho quantification. Skeletal Radiol 2010;39:523-31. [Crossref] [PubMed]


