Design of catheter radio frequency coils using coaxial transmission line resonators for interventional neurovascular MR imaging
Introduction
The use of catheter-based radio frequency (RF) coils for interventional MRI application, while invasive, has significant advantages over using conventional external RF coils as the signal detector (1-12). An endovascular RF device offers improved detection sensitivity as a result of its proximity to the imaging target, providing high efficiency of the RF magnetic fields (i.e., B1 fields) generated by the coil and improved filling factors. In endovascular interventional imaging procedures, catheter mounted RF coils must be physically small in order to meet the spatial constraints imposed by the catheter host to navigate the vessels. This poses technical challenges in designing efficient catheter coils. Conventional catheter RF coil design using lumped elements often requires a capacitor in the coil tip area to support resonance and enhance the coil’s B1 fields and thus detection sensitivity. The requirement for lumped capacitors, which are usually large in size, makes it challenging to incorporate such bulky structures into miniature endovascular devices. Additionally, the long, conductive leads of the coil are part of the coil resonant circuit and can dissipate MR signals, introduce noise, and also create a safety hazard by heating surrounding tissues (12). The long, unshielded leads can also make the coil resonance unstable and consequently degrade imaging performance.
In this work, we propose a new design method of miniature catheter RF coils for endovascular interventional MR imaging. The design is based on short-circuit transmission line resonator (TLR) technology. Unlike the conventional lumped element LC resonant circuit, the transmission line is a distributed circuit, capable of providing efficient signal detection, compact coil structure, and no need to have lumped capacitors to support resonance (13-18). Due to the unique two-conductor structure, TLR RF coils have reduced radiation losses, increased quality factors, and is an unbalanced circuit allowing much more stable resonance. Furthermore, TLRs, particularly coaxial transmission lines, can shield RF fields from surrounding tissues, ultimately reducing RF field deposition, tissue heating and noise contributions from adjacent tissues along the coil structure (10,11). To investigate this new design method, a prototype catheter coil using a short-end TLR was designed and constructed for endovascular interventional MR imaging at 1.5 T. Coil parameters. Including resonance frequency, impedance matching, resonance detuning, and signal-to-noise ratio (SNR), were assessed in vitro using standard RF measurement technology. A prototype design was also validated through MR imaging experiments on a clinical 1.5 T MR scanner.
Methods
In principle, a coaxial transmission line with finite length is a resonator. When one end of the coaxial transmission line is terminated by a short-circuit, it forms a short-end or short circuit TLR. Practically the short circuit is realized by a piece of copper wire.
If the length of the copper wire is short enough, the inductance and the resistance of the wire can be ignored and the wire can be modeled as a short circuit. In this case, the coaxial transmission line structure becomes a short-ended TLR. Its equivalent circuit is shown in Figure 1. According to the equivalent circuit, the resonant frequency of a short-end coaxial transmission resonator can be expressed as (19).
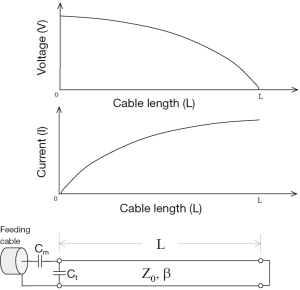
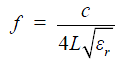
where c is the speed of light in free space; L is the physical length of the TLR; and εr is the relative permittivity of the dielectric material of the coaxial transmission line.
In this work, a shorted circuit coaxial TLR was constructed by shorting one end of a piece of 50-Ohm low loss coaxial transmission line (G01130HT, Huber + Suhner, Switzerland) which has a diameter of approximately 2.8 mm. The short circuit was implemented by using a small copper loop with a diameter of ~3–4 mm. The resistance and inductance of the copper loop at 64 MHz is negligible and thus it was modeled as a short circuit. The resonator’s other end, which is open, was connected to the feeding circuit, including tuning and matching components and also a detuning circuit containing PIN diodes. With the detuning function, the catheter coil is able to be used in a platform of body coil transmit and catheter coil receive. At the shorted end of the coaxial TLR, the current reaches a maximum and the voltage goes to zero, as illustrated in Figure 1. This feature is desired in MR imaging, providing high B1 sensitivity and low tissue heating at this critical location. Figure 2 shows the schematic of the short-circuit TLR and the prototype catheter coil operating at 64 MHz for proton imaging at 1.5 T. To understand the behavior of this coaxial transmission line catheter coil, the B1 intensity and distribution of the coaxial TLR catheter coil was compared to a conventional lumped-element catheter coil with two parallel leads. Each circuit was modeled with the finite difference time domain (FDTD) (20) numerical calculation algorithm (21-26). The calculations were performed in the loaded case. The resonance frequency, impedance matching, and detuning of the prototype catheter coil were measured in vitro with a network analyzer.
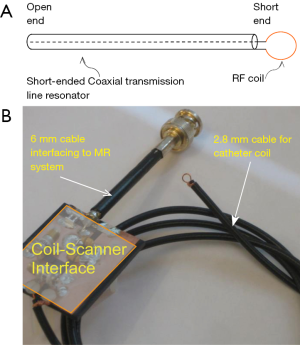
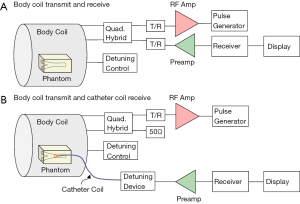
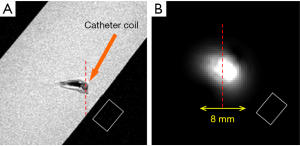
The system setup for MR imaging experiment to further test the imaging abilities of the proposed design is indicated in Figure 3. A conventional whole body MR scanner (Philips Achieva, Best, the Netherlands) was used with body coil transmission. In the system configuration of body coil transmit and receive, the catheter RF coil was in the magnet but not connected to the scanner. The system body coil was connected in quadrature to generate circularly polarized B1 fields for transmit and also receive. In the configuration of body coil transmit and the TLR catheter coil receive, during the transmission phase, the body coil is active and the TLR catheter coil is detuned. During the signal reception phase, the body coil is detuned by the scanner detuning device and the TLR catheter coil is tuned and active. All MR imaging experiments were performed using the prototype catheter coil with a vascular phantom (the details are shown in Figure 4). A simple gradient echo sequence was used in all imaging acquisitions. As a comparison, imaging with body coil transmit and receive was conducted using the same phantom and same acquisition parameters with the presence of the catheter coil.
Results
A prototype catheter coil was successfully constructed and tuned. Reflection coefficient S11 measurements taken on the network analyzer show a well-defined resonance of the prototype catheter coil. Its resonance frequency was successfully tuned to 64 MHz, the Larmor frequency of proton at 1.5 T, and the input impedance of the coil was matched to the system 50 Ohm with a reflection coefficient of better than −30 dB. The unbalanced circuit of the coaxial transmission resonator sufficiently suppresses the common mode currents along the catheter coil structure, yielding a stabilized resonance and impedance. B1 field calculations using a FDTD algorithm indicated an expected field distribution of the circular loop current as shown in Figure 5. The B1 field intensity of the proposed TLR catheter coil was 6 times stronger than that of the conventional lumped element catheter coil with the same coil size. This gain in B1 results mainly from the coaxial structure that confines a large portion of the magnetic fields within the outer conduction of the coaxial transmission line.
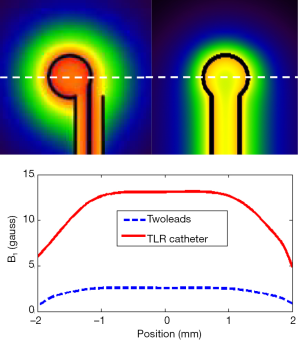
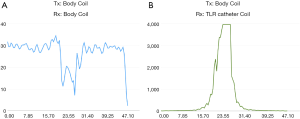
Figure 6 shows the preliminary MR imaging results acquired using the MR system body coil transmit and receive, and system body coil transmit and the TLR catheter coil for receive. As expected, the TLR catheter coil locally achieves much higher SNR than that of the body coil. In the aspect of B1 distribution, the image acquired using the TLR catheter coil shows a typical B1 pattern of a local coil or surface coil. These imaging results demonstrate that the proposed TLR coil technique is feasible in designing catheter RF coils. Profiles taken through the center of the catheter coil demonstrate the relative performance of the catheter coil to the body coil for signal reception (Figure 7). The SNR along the red dashed line was calculated by using the signal intensity along the red dash line on each image divided by the standard deviation of the noise measured in the rectangular box shown in the images (Figure 6). The noise standard deviations of the images (Figure 6A,B) were 57.45 and 1.73, respectively. With the same acquisition parameter, catheter coil reception has an approximately 200- to 300-fold SNR gain over imaging with body coil reception. For this result, a better illustration is shown in Figure 8. In endovascular interventional imaging using the miniature catheter RF coils, imaging coverage is a critical parameter in coil design, requiring a reasonably large imaging coverage to cover the area of interest. Based on the imaging results shown in Figures 6,7, imaging coverage of this 3-mm diameter catheter coil may reach the 8–10 mm level, which is sufficient to image vessels with inner diameters of approximately 5–8 mm and the perivascular environment.

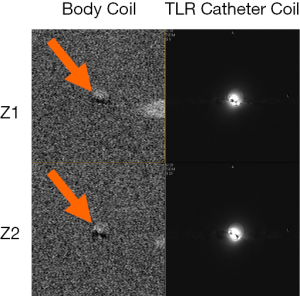
Decoupling performance of the catheter coil during body coil transmission is also critically important. Insufficient decoupling could lead to distorted MR images and degraded detection sensitivity. Figure 9 shows two images acquired at two different positions along Z-direction (i.e., B0-direction) or catheter axis direction from the body coil and the TLR catheter coil. Minimal MR signal difference can be observed in the area of the catheter loop. This is indicative of effective decoupling of the body coil and the TLR catheter coil. As shown in Figure 3, the decoupling in the coil system used in this study is achieved by the two detuning systems in the scanner body coil and the catheter coil, respectively.
Conclusions
A new design method of catheter RF coils for endovascular interventional MR imaging was successfully developed and validated at 1.5 T. Bench tests and MR imaging results demonstrated the feasibility and advantages of the coaxial TLR technology in achieving a miniature catheter RF coil for endovascular interventional imaging. In contrast to conventional lumped-element catheter coil designs, the coaxial transmission line does not require discrete capacitors to achieve resonance, making it more practical to build with a small physical profile. The unique feature of short-end TLRs is that their voltage and current distributions offer high B1 fields and low E-fields near the area of the catheter tip, which is advantageous in achieving sensitive yet safe detection in endovascular imaging procedures.
One of the challenges in endovascular interventional imaging is to gain a sufficiently large imaging area to cover the vessel wall and possibly some adjacent tissues by using the size-limited catheter-mounted coil. Usually the catheter coil is formed by a loop-type circuit of which the B1 pattern is exactly the same as that of a regular surface coil. Higher coil sensitivity or coil Q factors would help to improve the MR signals in the far end of the imaging profile, resulting in enlarged effective imaging coverage while keeping the coil loop diameter unchanged. The long leads of conventional lumped-element catheter coils are part of their resonant circuit, and have a strong interaction with the surrounding tissues along the catheter leads. In contrast, the outer conductor of the coaxial TLR serves as an RF shield and prevents electromagnetic energy loss, thus helping to achieve higher Q factors and stronger B1 fields in the targeted imaging area. Therefore, with the same coil size, the TLR catheter coils should be able to achieve a larger imaging coverage than that of the conventional lumped-element catheter coils.
This technology may also be applicable to the design of large size coils for non-interventional imaging applications. With large coil loops, however, the lengthened coil wire will likely not be able to be treated as a short circuit, and thus would have to be modeled with appropriate inductance and resistances. The design principle should still hold and may result in improved coil performance.
The TLR concept can also be a potential method to design resonant markers (27) in tracking the catheter tip during interventional procedures. When using it as a resonant marker, the catheter coil should be a stand-alone device and would not need to be electrically connected to the MR system, allowing elimination of long catheter-shaft leads. However, a passive detuning circuit would have to be applied in that case.
Acknowledgements
Funding: This work was supported in part by National Institutes of Health (NIH) grants R01EB008699, R21EB020283, R01EB012031, and P41EB013598, and a UCSF Academic Senate Award to X Zhang.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rudin S, Bednarek DR, Hoffmann KR. Endovascular image-guided interventions (EIGIs). Med Phys 2008;35:301-9. [Crossref] [PubMed]
- Koebbe CJ, Pandey A, Veznedaroglu E, Rosenwasser RH. The evolution and future directions of endovascular therapy. Clin Neurosurg 2006;53:191-5. [PubMed]
- Kandarpa K, Jakab P, Patz S, Schoen FJ, Jolesz FA. Prototype miniature endoluminal MR imaging catheter. J Vasc Interv Radiol 1993;4:419-27. [Crossref] [PubMed]
- Rudin S, Wang Z, Kyprianou I, Hoffmann KR, Wu Y, Meng H, Guterman LR, Nemes B, Bednarek DR, Dmochowski J, Hopkins LN. Measurement of flow modification in phantom aneurysm model: comparison of coils and a longitudinally and axially asymmetric stent--initial findings. Radiology 2004;231:272-6. [Crossref] [PubMed]
- Martin AJ, Baek B, Acevedo-Bolton G, Higashida RT, Comstock J, Saloner DA. MR imaging during endovascular procedures: an evaluation of the potential for catheter heating. Magn Reson Med 2009;61:45-53. [Crossref] [PubMed]
- Ladd ME, Quick HH, Debatin JF. Interventional MRA and intravascular imaging. J Magn Reson Imaging 2000;12:534-46. [Crossref] [PubMed]
- Zhang Q, Wendt M, Aschoff AJ, Lewin JS, Duerk JL. A multielement RF coil for MRI guidance of interventional devices. J Magn Reson Imaging 2001;14:56-62. [Crossref] [PubMed]
- Shellock FG. Radiofrequency energy-induced heating during MR procedures: a review. J Magn Reson Imaging 2000;12:30-6. [Crossref] [PubMed]
- Atalar E. Radiofrequency safety for interventional MRI procedures. Acad Radiol 2005;12:1149-57. [Crossref] [PubMed]
- Zhang X, Martin AB, Lillaney P, Losey A, Pang Y, Cooke D, Hetts S. Catheter coil design using transmission line resonators for endovascular MR imaging. Proceedings of ISMRM. Milan, Italy, 2014:3701.
- Zhang X, Martin AB, Lillaney P, Losey A, Cooke D, Hetts S. Performance evaluation of catheter imaging coils for endovascular MR imaging. ENC. Pacific Grove, CA, 2015:465.
- Bartels LW, Bakker CJ. Endovascular interventional magnetic resonance imaging. Phys Med Biol 2003;48:R37-64. [Crossref] [PubMed]
- Zhang X, Ugurbil K, Chen W. Microstrip RF surface coil design for extremely high-field MRI and spectroscopy. Magn Reson Med 2001;46:443-50. [Crossref] [PubMed]
- Zhang X, Ugurbil K, Chen W. A microstrip transmission line volume coil for human head MR imaging at 4T. J Magn Reson 2003;161:242-51. [Crossref] [PubMed]
- Zhang X, Zhu XH, Chen W. Higher-order harmonic transmission-line RF coil design for MR applications. Magn Reson Med 2005;53:1234-9. [Crossref] [PubMed]
- Adriany G, Van de Moortele PF, Wiesinger F, Moeller S, Strupp JP, Andersen P, Snyder C, Zhang X, Chen W, Pruessmann KP, Boesiger P, Vaughan T, Uğurbil K. Transmit and receive transmission line arrays for 7 Tesla parallel imaging. Magn Reson Med 2005;53:434-45. [Crossref] [PubMed]
- Weiss S, Vernickel P, Schaeffter T, Schulz V, Gleich B. Transmission line for improved RF safety of interventional devices. Magn Reson Med 2005;54:182-9. [Crossref] [PubMed]
- Yan X, Wei L, Xue R, Zhang X. Hybrid monopole/loop coil array for human head MR imaging at 7T. Appl Magn Reson 2015;46:541-50. [Crossref] [PubMed]
- Zhang X, Ugurbil K, Sainati R, Chen W. An inverted-microstrip resonator for human head proton MR imaging at 7 tesla. IEEE Trans Biomed Eng 2005;52:495-504. [Crossref] [PubMed]
- Yee KS. Numerical solution of initial boundary value problems involving Maxwell equations in isotropic media. IEEE Trans Ant Propag 1966;14:302-7. [Crossref]
- Collins CM, Yang QX, Wang JH, Zhang X, Liu H, Michaeli S, Zhu XH, Adriany G, Vaughan JT, Anderson P, Merkle H, Ugurbil K, Smith MB, Chen W. Different excitation and reception distributions with a single-loop transmit-receive surface coil near a head-sized spherical phantom at 300 MHz. Magn Reson Med 2002;47:1026-8. [Crossref] [PubMed]
- Yang QX, Wang J, Zhang X, Collins CM, Smith MB, Liu H, Zhu XH, Vaughan JT, Ugurbil K, Chen W. Analysis of wave behavior in lossy dielectric samples at high field. Magn Reson Med 2002;47:982-9. [Crossref] [PubMed]
- Wang J, Yang QX, Zhang X, Collins CM, Smith MB, Zhu XH, Adriany G, Ugurbil K, Chen W. Polarization of the RF field in a human head at high field: a study with a quadrature surface coil at 7.0 T. Magn Reson Med 2002;48:362-9. [Crossref] [PubMed]
- Pang Y, Xie Z, Li Y, Xu D, Vigneron D, Zhang X. Resonant Mode Reduction in Radiofrequency Volume Coils for Ultrahigh Field Magnetic Resonance Imaging. Materials (Basel) 2011;4:1333-44. [Crossref] [PubMed]
- Pang Y, Vigneron DB, Zhang X. Parallel traveling-wave MRI: a feasibility study. Magn Reson Med 2012;67:965-78. [Crossref] [PubMed]
- Pang Y, Wu B, Wang C, Vigneron DB, Zhang X. Numerical Analysis of Human Sample Effect on RF Penetration and Liver MR Imaging at Ultrahigh Field. Concepts Magn Reson Part B Magn Reson Eng 2011;39B:206-16. [Crossref] [PubMed]
- Ladd ME, Zimmermann GG, McKinnon GC, von Schulthess GK, Dumoulin CL, Darrow RD, Hofmann E, Debatin JF. Visualization of vascular guidewires using MR tracking. J Magn Reson Imaging 1998;8:251-3. [Crossref] [PubMed]



