A pre-tracer approach for improving the accuracy of metabolic measurements by hyperpolarized nuclear magnetic resonance
Hyperpolarized nuclear magnetic resonance (HP-NMR) can probe the reaction kinetics of metabolic enzymes in vivo (1), and the translation of this technology into human patients based on the most commonly used tracer 13C-pyruvate has been initiated in recent years (2). Currently the metabolite signal ratios are used as indicators for biological processes in healthy and diseased status. For example, to probe the reaction of lactate dehydrogenase (LDH) with hyperpolarized 13C-pyruvate, the peak, mean, or integrated (area-under-curve) signal ratios of 13C-lactate to 13C-pyruvate are used to differentiate tumor and normal tissues, and to evaluate treatment response (3-5). However, these ratios do not have clearly defined biochemical meaning, and it is desirable to quantify the reaction rate constants.
The accurate determination of reaction rate constants remains a challenge. The first-order rate constants of LDH reaction have been quantified in tissues by modeling the signal time courses of hyperpolarized 13C-labeled pyruvate and lactate (3-7). Nevertheless, the rate constant quantification suffers from inaccuracy for the following reasons:
- Both intracellular and extracellular 13C-labeled pyruvate contributes to the detected HP-NMR signal, but it is the intracellular pyruvate that is coupled to the LDH reaction in the cell. The extracellular space (ECS) volume fraction varies with tissue and physiological state, being about 20% in brain and up to 70% in tumor (8,9). A significant increase in the lactate/pyruvate ratio when using hyperpolarized [1-13C]alanine compared to hyperpolarized [1-13C]pyruvate have been reported in rats (10). This results support the significant contribution to HP-NMR signals by extracellular pyruvate that does not involve in LDH reaction directly. The modeling of hyperpolarized pyruvate signals without differentiating signals from intracellular and ECS will underestimate the reaction rate constant for the conversion of pyruvate to lactate. This underestimation is expected to be particularly severe for some perfused organ studies using non-selective radiofrequency pulses for signal acquisition (5);
- The transport of tracer from blood to interstitial space, and then through cell membrane to intracellular space complicates the mathematical modeling of enzyme kinetics. The rate constants determined for LDH by the two-site exchange modeling are really apparent rate constants, depending on the speeds of these transport processes (11).
To determine the kinetic constants of an intracellular enzyme in vivo more accurately by HP-NMR, here I propose a simple pre-tracer approach to remove the complexities caused by extracellular metabolite signals and transport processes. Generally speaking, a hyperpolarized isotope-labeled pre-tracer (A), once it enters the space of interest (e.g., inside the cell), will be converted to the tracer of interest (B) via a local chemical reaction (e.g., by an intracellular enzyme E1) (Figure 1A). The tracer B will then be converted into additional metabolites of interest (e.g., C and D) via other reactions (e.g., by enzyme E2 and E3 respectively). Tracer B and derived metabolites may have unique NMR chemical shifts so that they can be differentiated from A. Their hyperpolarized NMR signals are mainly intracellular, assuming slow or negligible outward flux into ECS within the typical short time scale of HP-NMR experiments of tens of seconds. Therefore, the kinetics of intracellular enzyme such as E2 and E3 may be more accurately studied based on modeling of HP-NMR signals of tracer B and derived metabolites. In addition, the intracellular metabolite ratio (at least with a major intracellular contribution), rather than the metabolite ratio averaged across both extracellular and intracellular space, can be determined to provide useful information about treatment response and cellular metabolic/redox state as discussed below.
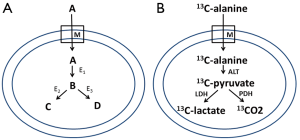
Let us again take the reaction of LDH as an example. As shown in Figure 1B, we can infuse hyperpolarized [1-13C]alanine instead of [1-13C]pyruvate to probe LDH kinetics. The [1-13C]alanine, once entering the intracellular space, will be converted into [1-13C]pyruvate by alanine transaminase (ALT). The [1-13C]pyruvate will then be metabolized by LDH and pyruvate dehydrogenase (PDH) to generate [1-13C]lactate and 13CO2. Because of the enhanced glycolysis (the Warburg effect) in cancer, the labeled pyruvate will be mainly turned into lactate. Commonly the modeling of LDH activity in cancer is done by neglecting PDH activity and assuming same T1 for [1-13C]lactate and [1-13C]pyruvate (4). Under these assumptions, we expect to improve the LDH modeling accuracy, i.e., determination of the forward (kp) and backward (kl) rate constants, by modeling intracellular signals only. We can also quantify the intracellular ratio of lactate/pyruvate, which is equal to NADH·H+/NAD+ divided by the equilibrium constant of LDH reaction (12). With a separate measurement of pH by 31P-MRS, we may be able to quantify more accurately the redox potential NAD+/NADH, which is a key metabolic state parameter mediating a number of biological processes (13,14).
The pre-tracer can be the ester form of metabolites which may be de-esterified by intracellular esterase to generate the tracer. For instance, hyperpolarized [1-13C]ethylpyruvate has been utilized for metabolic imaging of brain in animals (15). Hyperpolarized [1-13C]ethylpyruvate and its hydrate, with their 13C chemical shifts sufficiently different from those of pyruvate and lactate, allows non-interfered observation of intravascular pyruvate and lactate signals. This will result in an accurate determination of intracellular lactate/pyruvate ratio. Another example is hyperpolarized diethyl-succinate producing several metabolites of TCA cycle in vivo such as succinate and fumarate (16). Since the derived metabolites succinate and fumarate are coupled with each other via the succinate dehydrogenase (SDH), in principle, the ratio of succinate to fumarate is proportional to FADH2/FAD differing by a factor of the equilibrium constant of SDH. Thus, the intracellular succinate/fumarate ratio may be measured to reflect the mitochondrial redox potential FADH2/FAD, which also mediates the electron transport activity in mitochondria.
The shortcoming of this pre-tracer approach is the time delay for tracer generation in situ via chemical reactions and significant reduction of HP tracer signals compared to non-pretracer approach. At low SNR, it may be a challenge to do modeling of the signal time courses to extract reaction rate constants under certain circumstances. The weakness of low SNR may be surmountable along with the future development of HP technologies for tracers with longer T1. Nevertheless, using the pretracer approach, it may be easier to determine intracellular metabolite ratios than reaction kinetics. Whether the reaction kinetic constants or intracellular metabolite signals and ratios obtained by the pretracer approach, will provide certain advantages over the metabolite signals/ratios not differentiating between intracellular and extracellular contributions, for studying disease progression and treatment response, remains an open question in the field.
Acknowledgements
The author thanks Drs. Kevin Brindle, Rahim Rizi, Stephen Kadlececk, and He N. Xu for valuable discussions.
Funding: This work is supported by US NIH Grant R01-CA155348.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, Deberardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia 2011;13:81-97. [Crossref] [PubMed]
- Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Science Translational Medicine 2013;5:198ra108. [Crossref] [PubMed]
- Park JM, Josan S, Jang T, Merchant M, Yen YF, Hurd RE, Recht L, Spielman DM, Mayer D. Metabolite kinetics in C6 rat glioma model using magnetic resonance spectroscopic imaging of hyperpolarized [1-(13)C]pyruvate. Magn Reson Med 2012;68:1886-93. [Crossref] [PubMed]
- Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med 2007;13:1382-7. [Crossref] [PubMed]
- Xu HN, Kadlececk S, Shaghaghi H, Zhao H, Profka H, Pourfathi M, Rizi R, Li LZ. Differentiating inflamed and normal lungs by the apparent reaction rate constants of lactate dehydrogenase probed by hyperpolarized (13)C labeled pyruvate. Quant Imaging Med Surg 2016;6:57-66. [PubMed]
- Zierhut ML, Yen YF, Chen AP, Bok R, Albers MJ, Zhang V, Tropp J, Park I, Vigneron DB, Kurhanewicz J, Hurd RE, Nelson SJ. Kinetic modeling of hyperpolarized 13C1-pyruvate metabolism in normal rats and TRAMP mice. J Magn Reson 2010;202:85-92. [Crossref] [PubMed]
- Li LZ, Kadlececk S, Xu HN, Daye D, Pullinger B, Profka H, Chodosh L, Rizi R. Ratiometric analysis in hyperpolarized NMR (I): test of the two-site exchange model and the quantification of reaction rate constants. NMR Biomed 2013;26:1308-20. [Crossref] [PubMed]
- Jakobsen I, Lyng H, Kaalhus O, Rofstad EK. MRI of human tumor xenografts in vivo: proton relaxation times and extracellular tumor volume. Magn Reson Imaging 1995;13:693-700. [Crossref] [PubMed]
- Zhang H, Verkman AS. Microfiberoptic measurement of extracellular space volume in brain and tumor slices based on fluorescent dye partitioning. Biophys J 2010;99:1284-91. [Crossref] [PubMed]
- Hu S, Zhu M, Yoshihara HA, Wilson DM, Keshari KR, Shin P, Reed G, von Morze C, Bok R, Larson PE, Kurhanewicz J, Vigneron DB. In vivo measurement of normal rat intracellular pyruvate and lactate levels after injection of hyperpolarized [1-(13)C]alanine. Magn Reson Imaging 2011;29:1035-40. [Crossref] [PubMed]
- Witney TH, Kettunen MI, Brindle KM. Kinetic modeling of hyperpolarized 13C label exchange between pyruvate and lactate in tumor cells. J Biol Chem 2011;286:24572-80. [Crossref] [PubMed]
- Li LZ, Xu HN, Kadlececk S, Nath K, Cai K, Hariharan H, Glickson JD, Rizi R. Non-invasive quantification of intracellular redox state in tissue by hyperpolarized 13C-NMR. Proc Intl Soc Mag Reson Med 2012;20:4308.
- Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 2008;10:179-206. [Crossref] [PubMed]
- Li LZ. Imaging mitochondrial redox potential and its possible link to tumor metastatic potential. J Bioenerg Biomembr 2012;44:645-53. [Crossref] [PubMed]
- Hurd RE, Yen YF, Mayer D, Chen A, Wilson D, Kohler S, Bok R, Vigneron D, Kurhanewicz J, Tropp J, Spielman D, Pfefferbaum A. Metabolic imaging in the anesthetized rat brain using hyperpolarized [1-13C] pyruvate and [1-13C] ethyl pyruvate. Magn Reson Med 2010;63:1137-43. [Crossref] [PubMed]
- Zacharias NM, Chan HR, Sailasuta N, Ross BD, Bhattacharya P. Real-time molecular imaging of tricarboxylic acid cycle metabolism in vivo by hyperpolarized 1-(13)C diethyl succinate. J Am Chem Soc 2012;134:934-43. [Crossref] [PubMed]


