
Transvaginal ultrasonography predictive model for the detection of pelvic congestion syndrome
Introduction
Pelvic congestion syndrome (PCS) is defined as the dilation and stasis in pelvic venous plexus, being one of the many reasons behind chronic pelvic pain (CPP) in female patients. CPP is defined as the non-cyclical hypogastric, lumbosacral or perineal pain lasting more than 6 months (1,2). According to the American College of Obstetrics and Gynecology (ACOG), it causes a functional disability or seeking for medical attention (3) as it causes limitations in physical, psychological, relational and work aspects of the individual’s life (4). Thus, it is a relevant health issue as it is behind up to 40% of referrals for gynecology units (1,2).
The diagnosis of PCS continues to be a concern, given the lack of universally accepted criteria for the definition of dilated pelvic venous vessels. Different nomenclatures have been used for the varied clinical presentation of stasis and backflow of interconnected vessels in the abdominal, pelvis and lower extremities, which has contributed to the skepticism around this pathology, especially regarding women suffering from CPP (5). Prevalence of CPP oscillates between 6–27%, although it is often underestimated given the lack of data from many countries and the scarce number of multidisciplinary studies (4,6).
A step into improving the understanding of this disease is the unification of all these related conditions in just one clinical term, which might help healthcare professionals to accept its importance and great impact (6). The Symptoms-Varices-Pathology (SVP) classification was recently established for assessing pelvic venous disorders (PeVD), as well as the “hemodynamic and radiological classification of ovarian veins insufficiency”. Although these tools need to be validated, they might help to obtain homogenous samples for future studies (7-9).
PeVD are caused by an insufficiency of either the ovarian or the internal iliac veins and their tributary veins, which ends up causing a dilation of venous vessels. Venous insufficiency can be primary, due to the absence or degeneration of venous valves as well as changes in their walls, such as the structural and hormonal changes related to pregnancy, shifts related to uterine malposition, or congenital absence of venous valves. In addition, insufficiency may also be secondary caused by the extrinsic compression or obstruction of pelvic venous vessels, such as the nutcracker syndrome (caused by the compression of the left renal vein between the abdominal aorta and the superior mesenteric artery) or the May Thurner syndrome (compression of the iliac vein, frequently the left, by the right common iliac artery (6,8,9).
Most authors state that venography (VG) is the gold standard for the diagnosis of PCS (2,10-12), notwithstanding that, for some years now, different groups claim that computerized axial tomography (CT), magnetic resonance imaging (MRI) or transabdominal and transvaginal ultrasonography (TVU) are valid options (10,13,14). CT and MRI have the advantage of allowing to obtain a broad view of the wide range of disorders behind pelvic venous dilation, which would allow to use the SVP classification (9,15). However, in a recent systematic review it was stated that there are not enough studies about the diagnostic value of CT for PCS. Furthermore, there is a lack of standardized criteria regarding PCS when using MRI, and along with the limited evidenced and its low availability, it is not highly recommended (11).
In contrast, TVU is a non-ionizing technique, which is easily accessible and minimally invasive. It also allows to measure pelvic veins and identify blood flow in real time (16), although it requires expertise of the examiner to properly identify the ovarian veins, which is difficult to properly do so.
Regarding the ultrasonographic assessment of PCS, there have been various suggested parameters, like the dilation and low blood flow velocity in the ovarian veins, or the dilation of the arcuate vein in the myometrium (17). Understandably, color Doppler imaging is essential for the proper assessment, as it allows to identify return or discontinued flow during Valsalva, which has been associated with backflowing or stasis in the ovarian vein in VG (18). Nonetheless, the cut-off value for the dilation of the ovarian veins is still a controverse topic, with different authors establishing it somewhere between 5 and 8 mm. A recent study was published establishing the venographic diagnostic of PCS with the identification of a pelvic vein of 8 mm or higher by TVU, as well as the disturbed flow during Valsalva and the presence of crossing veins in the myometrium (19).
The aim of this study was to design a predictive model for the venographic diagnostic of PCS using the parameters identified by TVU in patients with clinical suspicion of PCS, in order to individually assess the need to perform an invasive diagnostic and therapeutic technique such as VG. Thus, the study hypothesis was that a model based on the use of parameters identified by TVU could predict the presence of PCS in patients with clinical suspicion of PCS. We present the following article in accordance with the STROBE and STARD reporting checklists (available at https://qims.amegroups.com/article/view/10.21037/qims-22-898/rc).
Methods
This was an observational and cross-sectional prospective study, conducted between October 2020 and September 2021. It was set in the Gynecological Ultrasound Unit at the Valme University Hospital, including patients with clinical suspicion of PCS, who were referred by the Pelvic Floor, Gynecology and Vascular Surgery Units, following unpublished locally approved clinical protocol. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and received approval of Andalucia’s Board of Biomedicine Ethics Committee (No. 1314/2017). Informed consent was obtained from all participants of the study.
The study included 80 patients who were consecutively recruited after being referred to the Gynecological Ultrasound Unit. The inclusion criterion was the presence of non-cyclical pelvic pain for at least 6 months, whilst the exclusion criteria were: patients with a previous hysterectomy or oophorectomy, patients who were unable to undergo TVU, underage patients (under 18 years old), and patients who refused to undergo a VG. Those who accepted to participate in the study were performed a transvaginal ultrasound to take measurements as described later. After a period of time of at least 15 and no longer than 60 days since the TVU assessment, patients were submitted for a diagnostic pelvic VG to assess the presence of PCS. For the retrospective analysis of the data, patients were divided in two groups based on the results of the VG: normal group (NG) and PCS group (PCSG), depending on the absence or presence of PCS, respectively.
Data collection
The epidemiological data collected was included in a questionnaire form, which the subjects had to complete before the diagnostic tests. The epidemiological variables were: age; parity; maximum newborn birth weight; menopausal stage (considering all patients who had been amenorrheic for at least 12 months, including patients diagnosed with early menopause using hormone replacement therapy); age of the onset of symptoms; worsening of symptoms after pregnancy; presence of vulvar varicosities during pregnancy; prior medical history of endometriosis, adenomyosis, urologic or gastrointestinal disorders; presence of varicosities in lower extremities; prior pelvic surgery; presence of uterine fibroids; and the presence of varicosities in the vulva, perineum, buttocks or lower extremities. Additionally, we registered patients’ reported levels of pain in various scenarios: walking, sitting, in the supine position, dysmenorrhea, dyspareunia, postcoital pain, and lumbar pain. We considered level of pain as clinically significant if VAS score was 7 or greater.
Ultrasonography evaluation
Ultrasonography evaluation was carried out by an expert in gynecological ultrasound, with more than 15 years of experience, using a Canon Aplio 500 (Toshiba Medical systems Corp., Tokyo, Japan) with a 6.5 MHz probe. Set-up of the 2-D mode consisting in the use of 2 focal zones, with an 80–95 gain, dynamic range of 60–75. For the use of color and spectral Doppler the size of the Doppler sampling window was set at 45×45 mm, color gain between 35–45 and PRF at 5.4–7.6 cm/s. In order to carry out the examination while the bladder was empty, as is customary, patients were asked to adopt the gynecological position short after urinating. The assessment started first by completely assessing the uterus and the adnexa in a longitudinal and cross-sectional plane. We used the simplified prolate ellipsoid formula to calculate uterine and ovarian volumes. Afterwards, the uterine vein was tracked from its origin at the internal cervical os up until the internal iliac veins and collateral branches, in order to identify the largest pelvic vein as well as the venous plexus. These were measured in their anteroposterior diameter in a cross-sectional plane. For the Doppler assessment of the pelvis, we identify the flow direction with color and spectral Doppler and asked the patient to perform a Valsalva maneuver to identify changes in the flow velocity. This procedure was first performed in one side and then repeated for the contralateral side. Subsequently, we applied color Doppler in a cross-sectional plane of the uterus determine whether there were crossing veins in the myometrium, then measured its maximum anteroposterior diameter. Thus, we collected the following ultrasonographic variables: uterine volume, right and left ovarian volume, presence of polycystic ovaries (PCO), inner diameter of the largest pelvic vein (right and left side), maximum diameter of the largest venous plexus (right and left side), reverse or altered flow during Valsalva, presence of crossing veins in the myometrium, and maximum diameter of crossing veins in the myometrium.
VG assessment
After at least 15 and no longer than 60 days since the ultrasonographic assessment, patients underwent diagnostic-therapeutic invasive pelvic VG as it is the current gold standard for the diagnostic confirmation of PeVD. Said technique was performed by specialists in Angiology and Vascular Surgery who were blinded to the results of the TVU assessment. The procedure was performed under local anesthesia. Via femoral or brachial access, the gonadal and internal iliac veins were bilaterally accessed, as well as their collaterals veins and the common iliac vein. The diagnostic criteria for PCS was based on the presence of at least one of the following: enlargement of ovarian veins ≥10 mm, congestion (defined as the detention of contrast in the varicose dilations for at least 20 seconds), and valvular insufficiency (20,21). When the PCS diagnosis was confirmed according to the described criteria, an embolization of insufficient ovarian or iliac veins were performed up until the last incompetent branch, from distal to proximal until the achievement of anterograde venous axis. The procedure was performed in all 43 patients who were diagnosed with PCS. For this purpose, we used controlled release platinum coils of 12–20 mm in diameter and 20–40 mm in length. In some cases, a sandwich technique with 2–3% polidocanol was used.
Statistical analysis
We carried out the statistical analysis using the statistics software IBM SPSS version 22 (IBM, Armonk, NY, USA). We used mean and standard deviations for normally distributed quantitative variables, whilst median and interquartile range were applied to non-normally distributed quantitative variables. As for qualitative variables, we used percentages to describe them. The Shapiro-Wilk test was performed to analyze the normality of the data. While Student’s t-test was used to evaluate normally distributed quantitative, Mann-Whitney U-test was carried out for non-normally distributed data. In case of qualitative variables, we used the Chi-square test. Finally, we evaluated individual predictive values with a receiver operating characteristic (ROC) curve and the area under the curve (AUC). All statistic comparisons were performed with a two-tailed test, with an alpha error of 5%.
Logistic regression models evaluation
Several binary logistic regression models were created to predict the venographic diagnosis of PCS. The parameters that showed statistical significance in the prior univariate analysis were included in the different models, creating a total of 19 binary logistic regression models. We applied a goodness-of-fit test (logarithmic probability of −2) and the Hosmer and Lemeshow test for each model. Then, we determined the Harrel’s C-index for the models who showed an adequate fit. We evaluated their discriminatory capacity with an AUC of the predicted probabilities by each model, selecting then the final model for its maximum discriminative capacity.
Results
Study population
A total of 80 patients were initially recruited for the study. Out of them, 19 patients were excluded from the study due to refusal or impossibility to perform the VG. Thus, the final sample included 61 patients, who were distributed in two groups, according to the VG results: 18 belonging to the NG and 43 to the PCSG. The recruitment summary can be seen in Figure 1. There were none adverse events amongst the 61 patients who completed the study. The results of the epidemiological data are shown in Table 1. The mean age of patients were 44.9 years in the NG and 41.3 years in the PCSG, without reaching statistical significance (P=0.09). There were statistically significative differences regarding the age of the on-set of symptoms, being lower in the PCSG (NG vs. PCSG: 37.2 vs. 32.3 years; P=0.02). No statistically significative differences were observed for the rest of the epidemiological data.
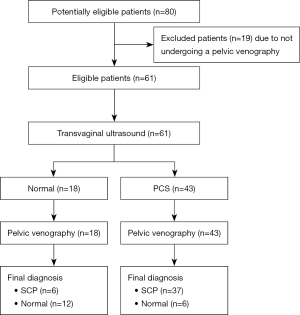
Table 1
| Variables | Normal group | PCS group | P |
|---|---|---|---|
| Age (years) | 44.9±8.4 | 41.3±6.4 | 0.09 |
| Multiparity | 16 (88.9) | 32 (74.4) | 0.31 |
| Maximum newborn birth weight (g) | 3,630.8±375.5 | 3,591±512.7 | 0.32 |
| Menopausal | 4 (22.2) | 3 (7.0) | 0.35 |
| Age of the onset of symptoms (years) | 37.2±10.5 | 32.3±8 | 0.02 |
| Worsening of symptoms during pregnancy | 8 (44.4) | 29 (67.4) | 0.09 |
| Vulvar varicosities during pregnancy | 11 (61.1) | 27 (62.8) | 0.90 |
| Medical history | 14 (77.8) | 37 (86.0) | 0.46 |
| Endometriosis | 1 (5.6) | 2 (4.7) | >0.99 |
| Adenomyosis | 3 (16.7) | 1 (2.3) | 0.07 |
| Urologic disorders | 1 (5.6) | 4 (9.3) | 0.31 |
| Gastrointestinal disorders | 1 (5.6) | 0 (0.0) | 0.30 |
| Varicosities in lower extremities | 10 (55.5) | 31 (72.1) | 0.14 |
| Prior pelvic surgery | 3 (16.7) | 4 (9.3) | 0.41 |
| Fibroids | 5 (27.8) | 5 (11.6) | 0.14 |
| Presence of varicosities (vulva, perineum, buttocks, lower extremities) | 10 (55.6) | 33 (76.7) | 0.10 |
| Pain (VAS score ≥7) | |||
| Walking | 10 (55.6) | 24 (55.8) | 0.99 |
| Sitting | 6 (33.3) | 22 (51.2) | 0.20 |
| Supine | 7 (38.9) | 17 (39.5) | 0.96 |
| Dysmenorrhea | 10 (55.6) | 26 (60.5) | 0.72 |
| Dyspareunia | 6 (33.3) | 18 (41.9) | 0.53 |
| Postcoital pain | 13 (72.2) | 25 (58.1) | 0.30 |
| Lumbar pain | 6 (33.3) | 15 (34.9) | 0.86 |
Numeric variables are expressed as mean ± SD, while qualitative variables are expressed as frequencies and percentages. PCS, pelvic congestion syndrome; VAS, visual analogue scale; SD, standard deviation.
Ultrasonography parameters
We can see in Table 2 the results of the ultrasonography variables. We found that patients in the PCSG had higher diameters for the larger pelvic vessel (NG vs. PCSG: 4.8 vs. 6.3 mm; P=0.04) and pelvic plexus (NG vs. PCSG: 9.7 vs. 18.2 mm; P=0.001). Moreover, the PCSG showed higher rates of reverse or altered flow during Valsalva (NG vs. PCSG: 16.7% vs. 44.2%; P=0.04), and crossing veins in the myometrium (NG vs. PCSG: 33.3% vs. 74.4%; P=0.003). No statistical significance was reached for the rest of the variables.
Table 2
| Variables | Normal group | PCS group | P |
|---|---|---|---|
| Uterine volume (mm) | 78.7±33.2 | 84.4±34.3 | 0.3 |
| Right ovarian volume (mm) | 13.1±9.5 | 10.1±5.4 | 0.33 |
| Left ovarian volume (mm) | 15.34±10.6 | 12.7±7.7 | 0.45 |
| PCO | 5 (27.8) | 7 (16.3) | 0.31 |
| Largest pelvic vein (mm) | 4.8±1.8 | 6.3±2.7 | 0.04 |
| Largest venous plexus (mm) | 9.7±4 | 18.2±11.9 | 0.001 |
| Reverse of altered flow during Valsalva | 3 (16.7) | 19 (44.2) | 0.04 |
| Crossing veins in the myometrium | 6 (33.3) | 32 (74.4) | 0.003 |
| Crossing veins in the myometrium (mm) | 6±3 | 4±1.8 | 0.18 |
Numeric variables are expressed as mean ± SD, while qualitative variables are expressed as frequencies and percentages. PCS, pelvic congestion syndrome; PCO, polycystic ovaries; SD, standard deviation.
Predictive models for PCS
Several binary logistic regression models were evaluated to predict the venographic diagnosis of PCS using TVU. The models were created using different combinations parameters that reached statistical significance in the univariate analysis: crossing veins in the myometrium, reverse or altered flow during Valsalva, and diameter of the largest pelvic vein or venous plexus, for which we used several cut-off points. A total of 19 models were created.
Models 1–4 were based on the different cut-off points of the largest pelvic vein or venous plexus, with Harrell’s C-index values oscillating between 0.64 and 0.79, determined as the AUC of the predicted probabilities (Table 3). When adding the presence of crossing veins in the myometrium, Harrell’s C-index values ranged between 0.66 and 0.70, while these oscillated between 0.56 and 0.58 when adding the presence of a reverse or altered flow during Valsalva. Lastly, the models based only on the latter two variables had a Harrell’s C-index value between 0.59 and 0.68.
Table 3
| Model | Parameters | OR | P (95% CI) | Discrimination (Harrell’s C-index 95% CI) |
|---|---|---|---|---|
| 1 | • Largest pelvic vein or venous plexusØ ≥6 mm | 5.31 | 0.03 (1.16–24.38) | 0.64 (0.45–0.83) |
| 2 | • Largest pelvic vein or venous plexusØ ≥7 mm | 9.92 | 0.003 (2.20–44.63) | 0.72 (0.64–0.90) |
| 3 | • Largest pelvic vein or venous plexusØ ≥8 mm | 19.13 | <0.001 (3.98–91.80) | 0.79 (0.63–0.96) |
| 4 | • Largest pelvic vein or venous plexusØ ≥9 mm | 14.85 | <0.001 (3.29–67.04) | 0.78 (0.62–0.94) |
| 5 | • Largest pelvic vein or venous plexusØ ≥6 mm | 3.93 | 0.04 (1.05–14.69) | 0.66 (0.49–0.84) |
| • Crossing veins in the myometrium | ||||
| 6 | • Largest pelvic vein or venous plexusØ ≥7 mm | 3.93 | 0.04 (1.05–14.69) | 0.66 (0.49–0.84) |
| • Crossing veins in the myometrium | ||||
| 7 | • Largest pelvic vein or venous plexusØ ≥8 mm | 0.18 | 0.02 (0.05–0.71) | 0.70 (0.53–0.87) |
| • Crossing veins in the myometrium | ||||
| 8 | • Largest pelvic vein or venous plexusØ ≥9 mm | 4.87 | 0.02 (1.25–19.03) | 0.69 (0.52–0.86) |
| • Crossing veins in the myometrium | ||||
| 9 | • Largest pelvic vein or venous plexusØ ≥6 mm | 2.54 | 0.27 (0.49–13.28) | 0.58 (0.41–0.75) |
| • Reverse of altered flow during Valsalva | ||||
| 10 | • Largest pelvic vein or venous plexusØ ≥7 mm | 2.54 | 0.27 (0.49–13.28) | 0.58 (0.41–0.75) |
| • Reverse of altered flow during Valsalva | ||||
| 11 | • Largest pelvic vein or venous plexusØ ≥8 mm | 2.54 | 0.27 (0.49–13.28) | 0.58 (0.41–0.75) |
| • Reverse of altered flow during Valsalva | ||||
| 12 | • Largest pelvic vein or venous plexusØ ≥9 mm | 2.54 | 0.27 (0.49–13.28) | 0.58 (0.41–0.75) |
| • Reverse of altered flow during Valsalva | ||||
| 13 | • Largest pelvic vein or venous plexusØ ≥6 mm | 1.96 | 0.43 (0.37–10.44) | 0.56 (0.38–0.73) |
| • Crossing veins in the myometrium | ||||
| • Reverse of altered flow during Valsalva | ||||
| 14 | • Largest pelvic vein or venous plexusØ ≥7 mm | 1.96 | 0.423 (0.37–10.44) | 0.56 (0.38–0.73) |
| • Crossing veins in the myometrium | ||||
| • Reverse of altered flow during Valsalva | ||||
| 15 | • Largest pelvic vein or venous plexusØ ≥8 mm | 1.96 | 0.423 (0.37–10.44) | 0.56 (0.38–0.73) |
| • Crossing veins in the myometrium | ||||
| • Reverse of altered flow during Valsalva | ||||
| 16 | • Largest pelvic vein or venous plexusØ ≥9 mm | 1.96 | 0.423 (0.37–10.44) | 0.56 (0.38–0.73) |
| • Crossing veins in the myometrium | ||||
| • Reverse of altered flow during Valsalva | ||||
| 17 | • Crossing veins in the myometrium | 1.96 | 0.423 (0.37–10.44) | 0.56 (0.38–0.73) |
| • Reverse of altered flow during Valsalva | ||||
| 18 | • Crossing veins in the myometrium | 4.48 | 0.03 (1.18–16.94) | 0.68 (0.50–0.85) |
| 19 | • Reverse of altered flow during Valsalva | 2.86 | 0.21 (0.55–14.88) | 0.59 (0.42–0.77) |
Ø, diameter (mm). OR, odds, ratio; CI, confidence interval.
The selected model was Model 3, which identified the presence of a pelvic vein or venous plexus of 8 mm or greater, as predictor of the venographic diagnosis of PCS. Model 3’s Harrell’s C-index obtained from the AUC of the predicted probabilities was 0.79 (95% CI: 0.63–0.96; P<0.001), with a sensitivity of 0.90 and specificity of 0.69 (Figure 2). Table 4 displays the contrast between Model 3 and the gold standard, which shows a sensitivity of with a sensitivity of 86.05%, a specificity of 66.67% and a positive predictive value of 86.05% (Table 5). Ultrasonography captions of the measurement of pelvic vein and venous plexus are displayed in Figures 3,4.
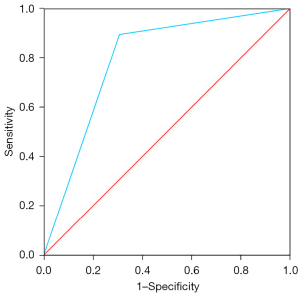
Table 4
| Category | Venography (gold standard) | P | ||
|---|---|---|---|---|
| Normal | PCS | Total | ||
| “Model 3” TVU | <0.001 | |||
| Normal | 12 | 6 | 18 | |
| PCS | 6 | 37 | 3 | |
| Total | 18 | 43 | 61 | |
PCS, pelvic congestion syndrome; TVU, transvaginal ultrasound.
Table 5
| Parameter | Value | 95% CI |
|---|---|---|
| Sensitivity | 86.05% | 72.07–94.7% |
| Specificity | 66.67% | 40.99–86.66% |
| Positive predictive value | 86.05% | 76.04–92.30% |
| Negative predictive value | 66.67% | 47.06–81.82% |
| Positive likelihood ratio | 2.58 | 1.33–5.02 |
| Negative likelihood ratio | 0.21 | 0.09–0.47 |
CI, confidence interval.
Discussion
The main finding of our study was that a model based in the presence of a pelvic vein or venous plexus of 8 mm or larger, identified with TVU, can predict 79% of patients with PCS, with good sensitivity (86.05%) and specificity (66.67%) values.
Our main goal was to design a predictive model to identify PCS using TVU. In the first place, a univariate analysis was carried out, which showed that the PCSG had higher diameters for the larger pelvic vessel and pelvic plexus, and higher rates of reverse or altered flow during Valsalva and crossing veins in the myometrium. These parameters have been associated before with PCS (17,19) and were all included in the following binary logistic regression models, using several possible combinations.
Different cut-off points for pelvic vein and venous plexus, or the addition of the crossing veins in the myometrium and the reverse or altered flow during Valsalva did not increase the predictive capacity of TVU. Given its simplicity, which includes only one easily achieved parameter by TVU, this model appears to be a feasible alternative, in contrast with the predictive models previously published (2,13,14,16,18).
To the present day, VG is the gold standard for the diagnosis of PCS. It is an invasive and ionizing technique, with limited accessibility, as it is only performed in specialized centers, which increases the delay to the diagnosis and treatment of patients suffering from this condition. For this reason, research in pursuit of new non-invasive diagnostic techniques has risen in recent years (10,13,22,23). Steenbeek et al. published a meta-analysis comparing TVU with VG. The results showed good sensitivity and specificity for the cut-off point of 5 mm or higher for the ovarian vein, although it concludes that there is need for more studies considering the flawed methodology and the absence of diversity in the parameters used (11).
These discrepancies in the definition of PCS, along with the presence of anatomical variations of the pelvic venous system, hamper the standardization needed for research on this field. Typically, the upper limit for the diameter of the ovarian vein has been established between 5 and 8 mm (22,24). The right and left ovarian veins originate within the broad ligament and drain into the inferior vena cava and the left renal vein, respectively. Although imaging of the ovarian veins is feasibly achievable by VG, there is an increased difficulty when using TVU, given their small size and variable location far from the probe. To prevent this difficulty, we have used the methodology used in our previous study (19), which showed good results identifying patients with PCS.
Several etiologies may be behind CPP, being PCS one of them. Thus, even though 16–30% of patients with CPP present PCS, and up to 12% present PCS associated with other causes of CPP (25-27), no substantial changes have been introduced to usual gynecological practice (6). In our study there was a PCS rate of 70.5%, with a 78.9% rate of isolated PCS and a 56.5% rate of PCS associated with other causes of CPP. These differences might be due to the patients being referred after a directed anamnesis and examination by specialists in angiology and vascular surgery.
Mean age of patients at the time of the diagnosis of PCS was around 41 years in our sample, slightly above the 30–40 years described in the literature (2,18,25,28), without statistically significant differences shown in comparison with normal patients. However, there were differences regarding the age of the on-set of symptoms, with the PCSG showing symptoms around 5 years earlier (NG vs. PCSG: 37.2 vs. 32.3 years; P=0.022), which might be due to the fact that other causes of CPP usually appear in older patients. Nonetheless, it is worth noticing the existing gap between the age of the on-set of symptoms and the age at the time of the diagnosis. International Pelvic Pain Society (IPPS) has developed history and physical examination forms to facilitate CPP assessment, although it does not include questions about signs of PCS or the examination of varicosities in vulva, perineum or buttocks (6,29). Even more, although TVU is used in the assessment of CPP, there is no current recommendations to look for pelvic varicosities (6). Disinformation about CPP, by healthcare specialists as well as patients, may contribute to the normalization of pai and delay its diagnosis and treatment, which could contribute to persistence of pain due to its centralization, despite adequate treatment of primary pain (6,9).
During pregnancy, pelvic venous vessels capacity increases up to 50–60%, causing venous insufficiency and backflow after pregnancy (2,18,25,30). In addition, hormonal changes such as estrogen and progesterone increase also contribute to venous dilation (22,31,32). Thus, PCS has been defined as a condition typical of multiparous women of fertile age. The decrease of estrogen and its vasodilatory effect during menopause might explain the low rates of PCS in menopausal women, as well as the improvement experienced by patients after pharmacological or surgical induction of a hypo-estrogenic state (22). In our study, just like in Beard et al. (2), we did not observe this association with multiparity, as well as the presence of vulvar varicosities or worsening of symptoms during pregnancy, although there were higher rates of the latter in the PCSG (NG vs. PCSG: 44.4% vs. 67.4%) (33). Additionally, it is worth mentioning that menopause was not an exclusion criterion, as we considered etiology and physiopathology of PCS to be multifactorial, which is shown in the 7% rate of menopausal women in the PCSG.
PCS has been linked to dysmenorrhea (82–84%), postcoital pain (40–75%), dyspareunia (76%) and the presence of varicosities in the vulva or lower extremities, in at least a 50% of patients (25,32,34). Our data on clinical and medical history showed similar results in PCS patients, although there were no differences found with the NG. As mentioned above, CPP is caused by several concurrent conditions that have shared symptoms. For instance, pain worsened by certain activities or body positions might be related both to pelvic venous and musculoskeletal disease, while deep dyspareunia might be caused by pelvic venous conditions as well as by endometriosis (6). All patients in our sample had CPP, which might explain why there were no significant differences between groups in terms of clinical and medical history.
One of the main problems we find in relation to skepticism about this disease is the fact the dilated pelvic veins is a quite common finding. It is estimated that they exist in approximately 15% of women between the age of 20 and 50 years, although not all of them manifest symptoms. Even more, the reason why these findings are associated with CPP in some women but not others are unknown (18,35,36).
Our study also has its limitations, such as the small sample size. Some studies establish that in asymptomatic patients ovarian veins may be observed, but the diameters are significantly larger in patients with CPP (18). However, there was not asymptomatic control group, which entails quite a limitation. It is also worth mentioning that the assessment was carried out by only one expert examiner in just one single act, and hence we could not analyze the inter- and intra-observer reproducibility. Therefore, there is a need for external validation of these results. Nonetheless, these will be considered in future studies.
Conclusions
The model based on the presence of pelvic veins or venous plexus of 8 mm or larger, observed by transvaginal ultrasound, can predict 79% of patients with pelvic congestive syndrome, and said assessment presents a feasible alternative that could be easily added to our usual gynecological practice.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE and STARD reporting checklists. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-898/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-898/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Andalucia’s Board of Biomedicine Ethics Committee (No. 1314-2017). All patients gave their written informed consent before starting the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taylor HC Jr. Vascular congestion and hyperemia; their effect on structure and function in the female reproductive system. Am J Obstet Gynecol 1949;57:211-30. [Crossref] [PubMed]
- Beard RW, Highman JH, Pearce S, Reginald PW. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet 1984;2:946-9. [Crossref] [PubMed]
- American College of Obstetrics and Gynecology. Revitalize Gynecology. Gynecology Data Definitions v1.0. Available online: https://www.acog.org/practice-management/health-it-and-clinical-informatics/revitalize-gynecology-data-definitions
- Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Physician 2014;17:E141-7. [Crossref] [PubMed]
- Zondervan KT, Yudkin PL, Vessey MP, Dawes MG, Barlow DH, Kennedy SH. Patterns of diagnosis and referral in women consulting for chronic pelvic pain in UK primary care. Br J Obstet Gynaecol 1999;106:1156-61. [Crossref] [PubMed]
- Khilnani NM, Winokur RS, Scherer KL, Meissner MH. Clinical Presentation and Evaluation of Pelvic Venous Disorders in Women. Tech Vasc Interv Radiol 2021;24:100730. [Crossref] [PubMed]
- Khilnani NM, Meissner MH, Learman LA, Gibson KD, Daniels JP, Winokur RS, Marvel RP, Machan L, Venbrux AC, Tu FF, Pabon-Ramos WM, Nedza SM, White SB, Rosenblatt M. Research Priorities in Pelvic Venous Disorders in Women: Recommendations from a Multidisciplinary Research Consensus Panel. J Vasc Interv Radiol 2019;30:781-9. [Crossref] [PubMed]
- Szary C, Wilczko J, Zawadzki M, Grzela T. Hemodynamic and Radiological Classification of Ovarian Veins System Insufficiency. J Clin Med 2021;10:646. [Crossref] [PubMed]
- Meissner MH, Khilnani NM, Labropoulos N, Gasparis AP, Gibson K, Greiner M, Learman LA, Atashroo D, Lurie F, Passman MA, Basile A, Lazarshvilli Z, Lohr J, Kim MD, Nicolini PH, Pabon-Ramos WM, Rosenblatt M. The Symptoms-Varices-Pathophysiology classification of pelvic venous disorders: A report of the American Vein & Lymphatic Society International Working Group on Pelvic Venous Disorders. J Vasc Surg Venous Lymphat Disord 2021;9:568-84. [Crossref] [PubMed]
- Ganeshan A, Upponi S, Hon LQ, Uthappa MC, Warakaulle DR, Uberoi R. Chronic pelvic pain due to pelvic congestion syndrome: the role of diagnostic and interventional radiology. Cardiovasc Intervent Radiol 2007;30:1105-11. [Crossref] [PubMed]
- Steenbeek MP, van der Vleuten CJM, Schultze Kool LJ, Nieboer TE. Noninvasive diagnostic tools for pelvic congestion syndrome: a systematic review. Acta Obstet Gynecol Scand 2018;97:776-86. [Crossref] [PubMed]
- Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Gloviczki ML, Lohr JM, McLafferty RB, Meissner MH, Murad MH, Padberg FT, Pappas PJ, Passman MA, Raffetto JD, Vasquez MA, Wakefield TW. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2011;53:2S-48S. [Crossref] [PubMed]
- Malgor RD, Adrahtas D, Spentzouris G, Gasparis AP, Tassiopoulos AK, Labropoulos N. The role of duplex ultrasound in the workup of pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord 2014;2:34-8. [Crossref] [PubMed]
- Freedman J, Ganeshan A, Crowe PM. Pelvic congestion syndrome: the role of interventional radiology in the treatment of chronic pelvic pain. Postgrad Med J 2010;86:704-10. [Crossref] [PubMed]
- Smith M. Sonographic View of Pelvic Congestion Syndrome. J Diagn Med Sonogr 2017;33:193-8. [Crossref]
- Whiteley MS, Dos Santos SJ, Harrison CC, Holdstock JM, Lopez AJ. Transvaginal duplex ultrasonography appears to be the gold standard investigation for the haemodynamic evaluation of pelvic venous reflux in the ovarian and internal iliac veins in women. Phlebology 2015;30:706-13. [Crossref] [PubMed]
- Knuttinen MG, Xie K, Jani A, Palumbo A, Carrillo T, Mar W. Pelvic venous insufficiency: imaging diagnosis, treatment approaches, and therapeutic issues. AJR Am J Roentgenol 2015;204:448-58. [Crossref] [PubMed]
- Park SJ, Lim JW, Ko YT, Lee DH, Yoon Y, Oh JH, Lee HK, Huh CY. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol 2004;182:683-8. [Crossref] [PubMed]
- Valero I, Garcia-Jimenez R, Valdevieso P, Garcia-Mejido JA, Gonzalez-Herráez JV, Pelayo-Delgado I, Fernandez-Palacin A, Sainz-Bueno JA. Identification of Pelvic Congestion Syndrome Using Transvaginal Ultrasonography. A Useful Tool. Tomography 2022;8:89-99. [Crossref] [PubMed]
- Coakley FV, Varghese SL, Hricak H CT. J Comput Assist Tomogr 1999;23:429-34. [Crossref] [PubMed]
- Geier B, Barbera L, Mumme A, Köster O, Marpea B, Kaminsky C, Asciutto G. Reflux patterns in the ovarian and hypogastric veins in patients with varicose veins and signs of pelvic venous incompetence. Chir Ital 2007;59:481-8. [PubMed]
- Díaz-Reyes CG. Pelvic varicocele and pelvic congestion syndrome in woman. CES Med 2012;26:57-69.
- Sharma K, Bora MK, Varghese J, Malik G, Kuruvilla R. Role of trans vaginal ultrasound and Doppler in diagnosis of pelvic congestion syndrome. J Clin Diagn Res 2014;8:OD05-7. [PubMed]
- Amin TN, Wong M, Foo X, Pointer SL, Goodhart V, Jurkovic D. The effect of pelvic pathology on uterine vein diameters. Ultrasound J 2021;13:7. [Crossref] [PubMed]
- Corrêa MP, Bianchini L, Saleh JN, Noel RS, Bajerski JC. Pelvic congestion syndrome and embolization of pelvic varicose veins. J Vasc Bras 2019;18:e20190061. [PubMed]
- Soysal ME, Soysal S, Vicdan K, Ozer S. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Hum Reprod 2001;16:931-9. [Crossref] [PubMed]
- Meissner MH, Gibson K. Clinical outcome after treatment of pelvic congestion syndrome: sense and nonsense. Phlebology 2015;30:73-80. [Crossref] [PubMed]
- O'Brien MT, Gillespie DL. Diagnosis and treatment of the pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord 2015;3:96-106. [Crossref] [PubMed]
-
International Pelvic Pain Society - Ismail L, Normahani P, Standfield NJ, Jaffer U. A systematic review and meta-analysis of the risk for development of varicose veins in women with a history of pregnancy. J Vasc Surg Venous Lymphat Disord 2016;4:518-24.e1. [Crossref] [PubMed]
- Antignani PL, Lazarashvili Z, Monedero JL, Ezpeleta SZ, Whiteley MS, Khilnani NM, Meissner MH, Wittens CH, Kurstjens RL, Belova L, Bokuchava M, Elkashishi WT, Jeanneret-Gris C, Geroulakos G, Gianesini S, de Graaf R, Krzanowski M, Al Tarazi L, Tessari L, Wikkeling M. Diagnosis and treatment of pelvic congestion syndrome: UIP consensus document. Int Angiol 2019;38:265-83. [Crossref] [PubMed]
- Scotti N, Pappas K, Lakhanpal S, Gunnarsson C, Pappas PJ. Incidence and distribution of lower extremity reflux in patients with pelvic venous insufficiency. Phlebology 2020;35:10-7. [Crossref] [PubMed]
- Leiber LM, Thouveny F, Bouvier A, Labriffe M, Berthier E, Aubé C, Willoteaux S. MRI and venographic aspects of pelvic venous insufficiency. Diagn Interv Imaging 2014;95:1091-102. [Crossref] [PubMed]
- Herrera-Betancourt AL, Villegas-Echeverri JD, López-Jaramillo JD, López-Isanoa JD, Estrada-Alvarez JM. Sensitivity and specificity of clinical findings for the diagnosis of pelvic congestion syndrome in women with chronic pelvic pain. Phlebology 2018;33:303-8. [Crossref] [PubMed]
- Harris RD, Holtzman SR, Poppe AM. Clinical outcome in female patients with pelvic pain and normal pelvic US findings. Radiology 2000;216:440-3. [Crossref] [PubMed]
- Arnoldussen CW, de Wolf MA, Wittens CH. Diagnostic imaging of pelvic congestive syndrome. Phlebology 2015;30:67-72. [Crossref] [PubMed]




