
The course and prognostic value of tumor stiffness detected by ultrasound elastography for transarterial chemoembolization of hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer, and its mortality rate ranks third worldwide (1). Transplantation and liver resection are the treatment of choice. However, many patients have advanced tumor burdens that exclude them from these treatments. In recent years, transarterial chemoembolization (TACE) has been recommended as the first-line treatment in intermediate-stage patients or a palliative treatment modality in advanced patients according to the latest Barcelona Clinic Liver Cancer (BCLC) classification system (2,3). TACE destroys malignancies via the intra-arterial injection of chemotherapeutic medicines, including lipiodol, and the subsequent embolization of the feeding artery, which results in a combination of cytotoxicity and ischemia. Patients with unresectable HCC have shown encouraging results from this treatment (4-6). However, tumor control usually requires multiple TACE interventions due to residual and recurrent lesions. Alpha-fetoprotein levels combined with tomography (CT) and magnetic resonance imaging (MRI) have exhibited unique superiority in evaluating the tumor size and intratumoral necrotic areas of residual and new lesions after TACE (7,8). However, after TACE, iodized oil may affect CT and MRI scans, resulting in underestimating tumor progression (9).
Growing evidence demonstrates that tumor stiffness (TS) might predict the nature of the tumor (10). Ultrasound elastography (US-E) is a novel, ultrasonic diagnostic technique that can be classified as either quantitative [shear wave elastography (SWE)] or qualitative (strain elastography). The strain procedures are less often used in the evaluation of liver disorders. Three primary quantitative methods are now employed in clinical practice: vibration-controlled transient elastography (VCTE), point shear wave elastography, and 2-dimensional (2D) shear wave elastography. These techniques can measure shear modulus, a surrogate of tissue stiffness and mechanical properties. The diagnostic potential of US-E is derived from the fact that healthy and diseased tissues often differ significantly in terms of their intrinsic stiffness (11). Several guidelines have recommended using US-E for the noninvasive detection of the degree of liver fibrosis (12-14). However, no studies have reported whether TS measured by US-E can be used to predict recurrence in patients with HCC selected for TACE.
Therefore, this study explored the effects of TACE on HCC elasticity using US-E and investigated whether the quantification of TS by US-E could predict HCC recurrence. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-292/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Zhengzhou University granted permission for access to the included patients’ health care follow-up data as part of a retrospective cohort study. Written informed consent was obtained from each patient for the publication of this article and any accompanying images. The data were collected between April 2019 and July 2021 in the first hospital of Zhengzhou University, Henan province, China. We manually researched for patient information using the Neusoft hospital information system (Neusoft Medical Systems, Shenyang China) and acquired the image data using the Neusoft picture archiving and communication system. The inclusion criteria were the following: (A) an age ranging from 18 to 90 years, (B) histopathological and/or radiological evidence of HCC in our clinic, (C) current therapy with TACE, and (D) lesion visible on gray-scale US. Transabdominal ultrasonography (TAU) and SWE scanning before and after TACE. The following were the exclusion criteria: (A) liver metastases of different origins, (B) inflammation in or around the liver, (C) unavailable whole US-E images, and (D) lesions deeper than 8 cm. The case selection process is presented in Figure 1.
The current study assessed data from 116 patients who satisfied the basic Inclusion and exclusion criteria, including 84 males and 32 women, with a mean age of 63.8 years and a range of 47–88 years. The patients received routine TACE (mitomycin, gemcitabine, and lipiodol) and TAU with additional US-E before and after TACE. Conventional TACE refers to lipiodol-based chemoembolization. The common chemotherapeutics used included mitomycin C and gemcitabine. US-E was performed within 3 days before treatment and on the same day after TACE therapy or on the following 2 days after the intervention. Most TAU and US-E examinations (93%) were performed immediately after TACE on the same day. After this, patients underwent TACE when tumor progression or recurrence was found, which was diagnosed when alpha-fetoprotein levels >400 ng/dL and CT or MRI examination revealed new nodules or enlargement of the original tumor. A total of 406 chemoembolization procedures were performed for 116 patients. Prior to the postoperative first US-E imaging, an average of 3.5 (range, 1–23) TACE treatments were carried out. Elastography examinations were repeated with the same principles at 1 month after discharge. At the time of the study, all patients had at least 1 year’s worth of follow-up data. After treatment, multiphasic liver CT or MRI was performed to prompt recurrence every 3 months during the first 2 years for the follow-up and then every 4 to 6 months thereafter until death or liver transplantation.
TACE interventions
All TACE procedures were performed by 2 interventional radiologists respectively with more than 10 years of experience in interventional radiology. First, routine skin sterilization and local anesthesia with lidocaine were performed. Subsequently, selective celiac angiography was conducted to evaluate the hepatic vascular architecture, structural alterations in the arteries, and portal patency. Following this, superselective catheterization of the feeding artery was performed using a 2.0 F microcatheter (Progreat, Terumo Corporation, Tokyo, Japan), and iodine oil (Jiangsu Hengrui Medicine Co. Ltd., Jiangsu, China) was used as a carrier to load mitomycin (8 mg/m2; medac, Hamburg, Germany;) and/or gemcitabine (1,000 mg/m2; Gemzar, Eli Lilly and Company, Indianapolis, IN, USA). The chemotherapeutic agents for TACE were chosen according to the administered chemotherapeutics already being used in systemic chemotherapy. Finally, 200–450 mg of starch microspheres (200 µm; Jiangsu Hengrui Medicine Co. Ltd.) was used to embolize the blood supply until complete blood flow stasis. Devascularization following embolization was verified by further hepatic artery angiography.
Ultrasonographic and SWE examination
A high-resolution ultrasonography scanner (SuperSonic Imagine, Aixen Provence, France) with a 1–6 MHz convex array probe was used to conduct B-mode ultrasonographic tests. To avoid interobserver variability, all ultrasonography was performed by a single gastroenterologist with 11 years of related experience. The size, morphology, border, echo, cystic area, calcification of tumors, and peritumor areas were recorded. The diameter of the tumor was measured at the largest part of the tumor.
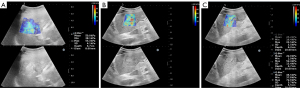
The US-E was performed after the B-mode scan. The probe was thickly coated with ultrasound transmission gel and placed perpendicularly over the abdomen’s surface with minimal pressure. The patients were instructed to hold their breath throughout the ultrasonographic examination. The probe was kept steady until a sharp B-mode picture was obtained, and the tumor was then focused on and centered on the B-mode display. The probe was then switched to US-E mode. In order to align with the solid tumor center after image stabilization, the US-E sampling frame was regulated at a size of 2–4 cm and a depth of 1–8 cm. The image was frozen and played again to produce a qualifying US-E image when the color steadily covered more than 80% of the sample frame area. The elastic modulus was then measured automatically (color bar: 0–100 kPa; Figure 2). The median of the 5 measures from the 5 elastographic pictures of the tumor was used as the valid value for data analysis. We used transverse or slightly oblique transverse sections.
Statistical analysis
The patient characteristics and outcomes are presented using descriptive statistics, categorical data are presented as percentage-based values, and continuous data are presented as the total number, percentage, mean, standard deviation, or median and range. Rank correlation analysis and simple linear regression were used to analyze the relationship between TS and progression-free survival (PFS) 5 years after TACE. Overall survival (OS), which was measured as the amount of time that passed between the time that patients received TACE and their death or the study’s conclusion, served as the experiment’s primary endpoint. PFS, the secondary endpoint, was the period of time from the start of TACE until the time of tumor progression or patient death. Kaplan-Meier analysis with log-rank testing was used to determine survival. All patients were split into 2 groups based on the TS values (high or low). According to the best Youden index on the receiver operating curve (ROC) of HCC development within 2 years, a binary cutoff value for TS was established. Univariate and multivariate Cox regression analyses were performed to identify risk factors associated with tumor progression or poor survival. All variables in the Cox regression analyses satisfied the assumption of proportional hazards (the Schoenfeld test of residuals, P=0.07). Patients who were lost to follow-up were excluded from our study. All statistical tests were performed using SPSS 22.0 (IBM Corp, Armonk, NY, USA).
Results
Patient characteristics
A total of 116 patients underwent TACE, including 84 men (72.4%) and 32 women (27.6%), with an average age of 63.8±10.55 years (range, 47–88 years). Table 1 shows the demographics of all included patients. The mean tumor size was 2.2±1.04 cm, and 58 patients (50%) had a single tumor; 38 patients (32.8%) were classified as Child-Pugh class A, and 96 patients (82.8%) had hepatitis B. According to the modified Union for International Cancer Control (mUICC) staging system, 56 patients (48.3%) were classified as stage I, and 60 patients (51.7%) were classified as stage II or III. The median follow-up period was 42 months (range, 12–60 months). The mean TS before TACE, after TACE, and 1 month after TACE were 40.1±14.36, 60.4±8.93, and 19.3±9.8 kPa, respectively.
Table 1
| Category | Total n=116 |
|---|---|
| Gender, n | |
| Male | 84 |
| Female | 32 |
| Age (years), mean ± SD | 63.8±10.55 |
| Etiology, n (%) | |
| Hepatitis B | 96 (82.8) |
| Hepatitis C | 7 (6.0) |
| Alcohol | 7 (6.0) |
| Unknown | 6 (5.2) |
| AFP (ng/L), mean ± SD | 135.4±124.25 |
| ECOG, n | |
| 0 | 75 |
| 1 | 41 |
| Child-Pugh, n | |
| A | 38 |
| B | 78 |
| Tumor size (cm), mean ± SD | 2.2±1.04 |
| Tumor number (1: ≥2), n | |
| Solitary nodule | 58 |
| Multiple nodules | 58 |
| Modified UICC, n | |
| I | 56 |
| II and III | 60 |
| Tumor location, n | |
| Single lobe | 78 |
| Both lobes | 38 |
| TS before TACE (kPa), mean ± SD | 40.1±14.36 |
| TS after TACE (kPa), mean ± SD | 60.4±8.93 |
| TS one month after TACE (kPa), mean ± SD | 19.3±9.80 |
SD, standard deviation; AFP, alpha-fetoprotein; ECOG, Eastern Cooperative Oncology Group; UICC, Union for International Cancer Control; TS, tumor stiffness; TACE, transarterial chemoembolization.
TS before and after TACE
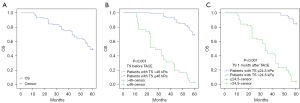
For patients with more than 1 nodule, we chose the largest nodule to evaluate its TS. Tumors before TACE had mean TS values of 40.1±4.36 kPa, according to US-E measurement data; however, after TACE, the TS increased to 60.4±8.93 kPa. One month after TACE, the TS was reduced to 19.3±9.8 kPa. There were significant differences among the 3 measurement results (Table 1). We then used rank correlation analysis and linear regression to analyze the relationship between TS and PFS 5 years after TACE. The results revealed that a higher TS before and 1 month after TACE was negatively correlated with PFS (Figure 3A,3B). In addition, the reduction ratio in TS before and 1 month after therapy was positively associated with PFS (r=72.612; P<0.001; Figure 3C).

PFS analysis
After a mean follow-up period of 39.1±20.5 months, 72 patients (62.1%) developed recurrence. The 1-, 3-, and 5-year PFS rates were 81.0%, 56.9%, and 37.9%, respectively (Figure 4A). The patients were further divided into 2 groups by TS levels. When the Youden index was set at 0.708, the sensitivity and specificity were 0.87 and 0.839, respectively, for the ROC curve of HCC recurrence within 1 year. The cutoff of TS before TACE was determined to be 46 kPa. In the Kaplan-Meier analysis (Figure 4B), patients with high TS (>46 kPa) had poor PFS compared to patients with low TS (≤46 kPa). The mean PFS was 17.943 months (95% CI: 13.238–22.647) in the TS >46 kPa group, and the estimated 1-, 3-, and 5-year PFS rates were 42.9%, 17.7%, and 2.9%, respectively. The mean PFS was 47.58 months (95% CI: 44.086–51.075) in the TS ≤46 kPa group, and the estimated 1-, 3-, and 5-year PFS rates were 96.3%, 72.8%, and 51.9%, respectively. The cutoff of TS 1 month after treatment was set at 24.5 kPa under the above-described method, while the Youden index was 0.645, and the sensitivity and specificity were 0.957 and 0.688, respectively. Similarly, patients with high TS (>24.5 kPa) had poor PFS compared to patients with low TS (≤24.5 kPa; Figure 4C). The mean PFS was 18.78 months (95% CI: 15.751–21.817) and 55.09 months (95% CI: 53.012–57.173) in the 2 groups. The 1-, 3-, and 5-year PFS with TS >24.5 kPa were 56.9%, 7.8%, and 2.0%, respectively, while the 1-, 3-, and 5-year PFS with TS ≤24.5 kPa were 100.0%, 95.4%, and 67.7%, respectively.

OS analysis
A total of 59 patients (50.9%) died after a mean follow-up time of 48.6±15.8 months. The survival rates for the first, third, and fifth years were 95.7%, 75.0%, and 49.1%, respectively (Figure 5A). The patients were further divided into 2 groups by TS levels as described in the statistical analysis section. In the comparisons of TS before TACE between TS >46 kPa and TS ≤46 kPa, the mean OS was 30.743 months (95% CI: 25.740–35.746) and. 56.247 months (95% CI: 54.563–57.930; Figure 5B), respectively. The 1-, 3-, and 5-year OS rates with TS ≤46 kPa were 100.0%, 93.8%, and 69.1.0%, respectively, while the 1-, 3-, and 5-year OS rates with residual TS >46 kPa were 85.7%, 31.4%, and 2.9%, respectively. In the comparisons of TS 1 month after TACE between TS >24.5 kPa and TS ≤24.5 kPa, the mean OS was 35.059 months (95% CI: 30.900–39.218) vs. 59.138 months (95% CI: 58.509–59.768; Figure 5C). The 1-, 3-, and 5-year OS rates with TS >24.5 kPa were 90.2%, 43.1%, and 3.9%, respectively, while the 1-, 3-, and 5-year OS rates with TS ≤24.5 kPa were 100.0%, 100.0%, and 84.6%, respectively.
Factors affecting PFS and OS
Univariate Cox regression analyses indicated that tumor size, tumor number (≥2), mUICC (II or III), tumor location (both lobes), TS before TACE, and TS 1 month after TACE were significantly associated with a shorter OS (Table 2). Considering the results of univariate analysis and clinical significance, multivariate Cox regression was performed and revealed that high TS before TACE and high TS 1 month after TACE were significantly associated with a poor OS [>46 kPa: hazard ratio (HR) =1.097, 95% CI: 1.048–1.148; 24.5 kPa: 1.233; 95% CI: 1.140–1.334]. Additionally, a poor OS was substantially correlated with the number and location of tumors.
Table 2
| Factors | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender, male | 0.931 (0.524–1.653) | 0.81 | 0.656 (0.305–1.408) | 0.28 | |
| Age, years | 1.012 (0.988–1.036) | 0.33 | 1.009 (0.977–1.042) | 0.58 | |
| Etiology, viral | 0.857 (0.445–1.650) | 0.64 | 0.580 (0.250–1.344) | 0.20 | |
| AFP, ≥200 ng/mL | 1.035 (0.599–1.787) | 0.90 | 1.226 (0.640–2.349) | 0.54 | |
| ECOG (0 vs. 1) | 1.649 (0.983–2.766) | 0.06 | 0.784 (0.385–1.596) | 0.50 | |
| Child-Pugh (A vs. B) | 1.156 (0.674–1.983) | 0.60 | 1.693 (0.886–3.234) | 0.11 | |
| Tumor size | 3.240 (2.458–4.272) | <0.001 | 0.806 (0.529–1.228) | 0.32 | |
| Tumor number (single vs. ≥2) | 0.059 (0.027–0.127) | <0.001 | 0.243 (0.076–0.777) | 0.02 | |
| Modified UICC, II or III | 14.219 (6.638–30.457) | <0.001 | 0.513 (0.186–1.411) | 0.20 | |
| Tumor location (single lobe vs. Both lobes) | 5.157 (3.023–8.798) | <0.001 | 2.156 (1.083–4.291) | 0.03 | |
| TS before TACE | 1.124 (1.099–1.150) | <0.001 | 1.097 (1.048–1.148) | <0.001 | |
| TS after TACE | 1.029 (0.997–1.062) | 0.08 | 0.981 (0.950–1.012) | 0.22 | |
| TS one month after TACE | 1.283 (1.213–1.356) | <0.001 | 1.233 (1.140–1.334) | <0.001 | |
P<0.05 was considered statistically significant. SD, standard deviation; AFP, alpha-fetoprotein; ECOG, Eastern Cooperative Oncology Group; UICC, Union for International Cancer Control; TS, tumor stiffness; TACE, transarterial chemoembolization; HR, hazard ratio.
Univariate Cox regression analyses indicated that tumor size, tumor number (≥2), mUICC (II or III), tumor location (both lobes), TS before TACE, and TS 1 month after TACE were significantly associated with a poor PFS (Table 3). Furthermore, multivariate Cox regression revealed that a high TS 1 month after TACE was significantly associated with a poor PFS (>24.5 kPa; HR: 1.426; 95% CI: 1.315–1.545). In addition, mUICC (II or III) and tumor location (both lobes) were independent risk factors for early recurrence in patients who underwent TACE.
Table 3
| Factors | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender, male | 0.234 (0.451–1.25) | 0.81 | 0.534 (0.284–1.005) | 0.05 | |
| Age, years | 1.006 (0.985–1.027) | 0.61 | 1.011 (0.985–1.037) | 0.43 | |
| Etiology, viral | 1.140 (0.600–2.166) | 0.69 | 0.880 (0.418–1.854) | 0.74 | |
| AFP, ≥200 ng/mL | 1.073 (0.653–1.761) | 0.78 | 1.286 (0.684–2.418) | 0.44 | |
| ECOG (0 vs. 1) | 1.331 (0.825–2.145) | 0.24 | 0.858 (0.468–1.571) | 0.62 | |
| Child-Pugh (A vs. B) | 1.291 (0.798–2.089) | 0.30 | 1.262 (0.694–2.296) | 0.45 | |
| Tumor size | 3.017 (2.348–3.877) | <0.001 | 1.057 (0.741–1.507) | 0.76 | |
| Tumor number (single vs. ≥2) | 0.121 (0.069–0.211) | <0.001 | 0.882 (0.308–2.524) | 0.81 | |
| Modified UICC, II or III | 7.209 (3.842–11.432) | <0.001 | 0.353 (0.141–0.887) | 0.03 | |
| Tumor location (single lobe vs. both lobes) | 3.231 (1.143–3.373) | <0.001 | 2.208 (1.034–4.712) | 0.04 | |
| TS before TACE | 1.093 (1.074–1.112) | <0.001 | 1.033 (0.996–1.072) | 0.08 | |
| TS after TACE | 1.023 (0.995–1.053) | 0.11 | 0.981 (0.951–1.012) | 0.24 | |
| TS one month after TACE | 1.386 (1.305–1.471) | <0.001 | 1.426 (1.315–1.545) | <0.001 | |
P<0.05 was considered statistically significant. SD, standard deviation; AFP, alpha-fetoprotein; ECOG, Eastern Cooperative Oncology Group; UICC, Union for International Cancer Control; TS, tumor stiffness; TACE, transarterial chemoembolization; HR, hazard ratio.
Discussion
Diagnostic palpation, a clinical technique to assess the stiffness and consistency of an organ or a lesion, served as a model for developing elastography methods. After undergoing deforming stress, tissues may restore their original shape and size. Shear waves were created in the tissue as a result of these displacements (15). US-E and magnetic resonance elastography (MRE) are the most notable elastographic techniques, and the latter has several advantages compared to ultrasound techniques. However, because of its high price and restricted availability, MRE has a considerably reduced value in liver examination compared to ultrasound techniques (16). Several studies have shown that elastography is an objective assessment method for hepatic fibrosis, which is a prognostic factor of chronic liver disease (17-20). Additionally, in patients with HCC, elastography could provide additional information regarding focal liver lesion stiffness and might predict their nature (21). However, whether TS, measured by the US, helps to predict tumor progression or survival in patients after TACE treatments remains unclear.
This study demonstrated that tumor location, number, and mUICC were independent prognostic factors in univariable and multivariable analyses, similar to the findings of previous reports (22,23). More interestingly, we found that TS was closely correlated with predictable patient outcomes before and 1 month after TACE. Rank correlation analysis and linear regression revealed that a higher TS before or 1 month after TACE was negatively correlated with PFS and that the reduction ratio in TS before and 1 month after therapy was positively associated with PFS. The results indicate that TACE can soften ischemic tumor tissue and lesions. In addition, a significant increase in the stiffness of the liver lesions was observed 2 days after TACE. The good therapeutic benefits of TACE treatment and the focused accumulation of the medicines in the injured liver site may be the cause of this tendency. The tumor response of HCC to TACE treatment may thus be assessed using US-E, which has the added benefit of improving disease progression prediction.
Elevated alpha-fetoprotein level (a cutoff of 400 ng/mL) was a diagnostic indicator of HCC. Furthermore, in previous studies, total bilirubin, γ-glutamyl transpeptidase, and serum albumin have been reported as important factors affecting the prognosis (24-26). However, our study had only a small number of patients with an alpha-fetoprotein level significantly greater than 400 ng/mL. This difference most likely occurred because these TACE-treated individuals were chosen because they had a liver function that was comparatively well preserved. Consequently, liver function measurements were not acknowledged as independent prognostic variables.
Our study set the optimal cutoff TS value at 46 and 24.5 kPa before and 1 month after TACE, respectively, based on the optimal Youden index. The patients were further divided into a high-TS-level group and a low-TS-level group. Kaplan-Meier survival analyses demonstrated that the 2 groups had significant differences in OS and PFS and that a higher TS was positively related to OS and PFS. These results indicated that US-E-assessed TS could potentially predict an early recurrence of HCC after TACE treatment. Previous studies by Park et al. (27) reported that whole TS was inversely correlated with PFS, which is an opposite result to ours. However, in their study, the average tumor size was 4.73 cm, which was considerably larger than the 2.2 cm in our research. Necrotic regions often have lower stiffness values than do solid tumor components, and large tumors typically exhibit greater necrosis (28). The diagnostic and prognostic values of hepatic stiffness have been demonstrated. Even so, few studies have examined the use of US-E to assess localized liver lesions following TACE therapy (29,30).
Limitations
Our study had certain limitations. First, this study was a retrospective and single-center study; therefore, selection bias might have been introduced. Second, although US-E has a higher plane resolution than MR, the depth is relatively limited, especially in obese patients. Thus, adequate quality in images could not be obtained, leading to some patients being excluded. Third, in this study, patients with various tumor stages were enrolled. The effects of TACE vary depending on the stage of the tumor and the assessed stiffness. Moreover, this is a time-consuming procedure that sometimes takes up to 20–30 min. Despite these limitations, our study had a sizable sample; therefore, we believe that the measurement of TS can be viably used to help predict the outcomes of patients with HCC.
Conclusions
Our results confirmed that US-E provides additional information for HCC characterization regarding TS, is a useful tool to evaluate the tumor response after TACE therapy in patients, and can be an independent prognostic factor. Patients with a high TS had a higher risk of recurrence and a worse survival time. Prospective studies in the future are necessary to confirm the efficacy of US-E in controlling HCC both before and after therapy.
Acknowledgments
Funding: This work was supported by the Young and Middle-Aged Health Science and Technology Innovation Talent Project of Henan Province in 2020 (No. yxkc2020037).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-292/rc).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-292/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of Zhengzhou University approved this study. Written informed consent was obtained from each patient for the publication of this article and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Bruix J, Sherman MAmerican Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021;10:181-223. [Crossref] [PubMed]
- Peisen F, Maurer M, Grosse U, Nikolaou K, Syha R, Ketelsen D, Artzner C, Bitzer M, Horger M, Grözinger G. Predictive performance of the mHAP-II score in a real-life western cohort with hepatocellular carcinoma following trans-arterial chemoembolisation with drug-eluting beads (DEB-TACE). Eur Radiol 2020;30:3782-92. [Crossref] [PubMed]
- Wang Q, Xia D, Bai W, Wang E, Sun J, Huang M, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J Hepatol 2019;70:893-903. [Crossref] [PubMed]
- Zhao P, Zhao J, Deng Y, Zeng G, Jiang Y, Liao L, Zhang S, Tao Q, Liu Z, Tang X, Tu X, Jiang L, Zhang H, Zheng Y. Application of iron/barium ferrite/carbon-coated iron nanocrystal composites in transcatheter arterial chemoembolization of hepatocellular carcinoma. J Colloid Interface Sci 2021;601:30-41. [Crossref] [PubMed]
- Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015;261:947-55. [Crossref] [PubMed]
- Crocetti L, Della Pina C, Cioni D, Lencioni R. Peri-intraprocedural imaging: US, CT, and MRI. Abdom Imaging 2011;36:648-60. [Crossref] [PubMed]
- Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology 2014;273:241-60. [Crossref] [PubMed]
- Li B, Zhao X, Wang Q, Jing H, Shao H, Zhang L, Cheng W. Prediction of high nodal burden in invasive breast cancer by quantitative shear wave elastography. Quant Imaging Med Surg 2022;12:1336-47. [Crossref] [PubMed]
- Lupsor-Platon M, Serban T, Silion AI, Tirpe A, Florea M. Hepatocellular Carcinoma and Non-Alcoholic Fatty Liver Disease: A Step Forward for Better Evaluation Using Ultrasound Elastography. Cancers (Basel) 2020.
- Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med 2017;38:e48. [Crossref] [PubMed]
- Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol 2015;41:1161-79. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Governing Board. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol 2021;75:659-89. [Crossref] [PubMed]
- Srinivasa Babu A, Wells ML, Teytelboym OM, Mackey JE, Miller FH, Yeh BM, Ehman RL, Venkatesh SK. Elastography in Chronic Liver Disease: Modalities, Techniques, Limitations, and Future Directions. Radiographics 2016;36:1987-2006. [Crossref] [PubMed]
- Sarvazyan A, Hall TJ, Urban MW, Fatemi M, Aglyamov SR, Garra BS AN. OVERVIEW OF ELASTOGRAPHY - AN EMERGING BRANCH OF MEDICAL IMAGING. Curr Med Imaging Rev 2011;7:255-82. [Crossref] [PubMed]
- Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017;7:1303-29. [Crossref] [PubMed]
- Wang XP, Wang Y, Ma H, Wang H, Yang DW, Zhao XY, Jin EH, Yang ZH. Assessment of liver fibrosis with liver and spleen magnetic resonance elastography, serum markers in chronic liver disease. Quant Imaging Med Surg 2020;10:1208-22. [Crossref] [PubMed]
- Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan(®)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease - Where do we stand? World J Gastroenterol 2016;22:7236-51. [Crossref] [PubMed]
- Ferraioli G, Wong VW, Castera L, Berzigotti A, Sporea I, Dietrich CF, Choi BI, Wilson SR, Kudo M, Barr RG. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol 2018;44:2419-40. [Crossref] [PubMed]
- Grgurevic I, Bokun T, Salkic NN, Brkljacic B, Vukelić-Markovic M, Stoos-Veic T, Aralica G, Rakic M, Filipec-Kanizaj T, Berzigotti A. Liver elastography malignancy prediction score for noninvasive characterization of focal liver lesions. Liver Int 2018;38:1055-63. [Crossref] [PubMed]
- Wu S, Zeng N, Sun F, Zhou J, Wu X, Sun Y, Wang B, Zhan S, Kong Y, Jia J, You H, Yang HI. Hepatocellular Carcinoma Prediction Models in Chronic Hepatitis B: A Systematic Review of 14 Models and External Validation. Clin Gastroenterol Hepatol 2021;19:2499-513. [Crossref] [PubMed]
- Dawood RM, Salum GM, El-Meguid MA, Elsayed A, Yosry A, Abdelaziz A, Shousha HI, Nabeel MM, El Awady MK. Development of a gene signature for predicting cirrhosis risk score of chronic liver disease associated with HCV infection in Egyptians. Microb Pathog 2021;153:104805. [Crossref] [PubMed]
- Park Y, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, Park YE, Park JH, Lee YI, Yun HR, Han KH. Addition of tumor multiplicity improves the prognostic performance of the hepatoma arterial-embolization prognostic score. Liver Int 2016;36:100-7. [Crossref] [PubMed]
- Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018;154:1706-1718.e1. [Crossref] [PubMed]
- Kadalayil L, Benini R, Pallan L, O'Beirne J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK, Palmer DH, Meyer T. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol 2013;24:2565-70. [Crossref] [PubMed]
- Park SJ, Yoon JH, Lee DH, Lim WH, Lee JM. Tumor Stiffness Measurements on MR Elastography for Single Nodular Hepatocellular Carcinomas Can Predict Tumor Recurrence After Hepatic Resection. J Magn Reson Imaging 2021;53:587-96. [Crossref] [PubMed]
- Vogl TJ, Mack MG, Balzer JO, Engelmann K, Straub R, Eichler K, Woitaschek D, Zangos S. Liver metastases: neoadjuvant downsizing with transarterial chemoembolization before laser-induced thermotherapy. Radiology 2003;229:457-64. [Crossref] [PubMed]
- Gerber L, Fitting D, Srikantharajah K, Weiler N, Kyriakidou G, Bojunga J, Schulze F, Bon D, Zeuzem S, Friedrich-Rust M. Evaluation of 2D- Shear Wave Elastography for Characterisation of Focal Liver Lesions. J Gastrointestin Liver Dis 2017;26:283-90. [Crossref] [PubMed]
- Guibal A, Boularan C, Bruce M, Vallin M, Pilleul F, Walter T, Scoazec JY, Boublay N, Dumortier J, Lefort T. Evaluation of shearwave elastography for the characterisation of focal liver lesions on ultrasound. Eur Radiol 2013;23:1138-49. [Crossref] [PubMed]


