
Correlations between capillary density and degree of skin pigmentation in healthy children analysed by nailfold video capillaroscopy
Introduction
Nailfold videocapillaroscopy (NVC) is a simple and non-invasive method used to visualize capillaries of nailbeds. Due to skin transparency and subcutaneous location, nailfold blood vessels are easily visualized in microscopic examination, which makes NVC a convenient screening and safe tool in daily clinical practice (1). In NVC images, multiple capillary variables can be analysed, such as capillary density (i.e., number of capillaries per linear millimetre in the distal row), capillary morphology and capillary width of the apical limb and presence of haemorrhages (2). As these parameters may show abnormalities for patients with various autoimmune rheumatic diseases, NVC has become a well-established diagnostic method in clinical rheumatology (2,3). It is being used in patients with Raynaud’s phenomenon and suspected systemic autoimmune disease, mainly systemic sclerosis (SSc) but also in (juvenile) dermatomyositis (JDM) and systemic lupus erythematosus (SLE) (2,4-8). In addition, abnormalities detected in NVC images were shown to be predictive for future (visceral) organ involvement in patients with SSc (9-11), but also in childhood-onset SLE (12). Minopoulou et al. described in their systematic review and meta-analysis, that microcirculatory changes are described as a potential risk factor for pulmonary hypertension in SSc patients (13).
In children, capillary loss has been shown to be predictive for differentiating between primary and secondary Raynaud’s phenomenon (14). In this study, capillary loss was defined as <6 capillaries per linear mm (14). However, studies on normal values for NVC measurements in healthy children are scarce and Andrade et al. suggested that colour/pigmentation of skin has influence on capillary density (15) This is important, additionally because some ethnic groups are more predominant in different systemic autoimmune diseases, such as a higher prevalence of black patients in SLE. Often, in clinical studies, the white patient is assumed, even if it concerns matters that are more relevant to non-white patients.
We hypothesize that ethnic background should be taken into account in the interpretation of (lower) nailfold capillary density. This could mean that there are (different) normal values for capillary density in different ethnic backgrounds/skin pigment grades. In this work, we set out to investigate the influence of ethnic background, skin pigment and age on capillary density in NVC images of healthy children. In addition, we determined whether there is a difference in capillary density between the different fingers. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-993/rc).
Methods
Subjects
In this cross-sectional study, the subjects, children and adolescents <18 years old from the Amsterdam region in the Netherlands were asked to undergo a one-time nailfold capillaroscopy. They were recruited from families and friends of the authors and from primary and high schools in the region of the Amsterdam University Medical Centres (AUMC), by convenience sampling, between April 2016 and April 2021. To be eligible for inclusion, it was a prerequisite that subjects did not suffer from a chronic disease at the moment of the capillaroscopy. Upon inclusion, age, gender, ethnic background, Raynaud symptoms and periungual trauma were noted.
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The used capillaroscopy images from this study are part of a cohort study, and this study was approved by the Medical Ethical Committee from Amsterdam UMC (with number NL60885.018.17 from Dutch trial register). Permission from the subjects to use the capillaroscopy images for research purposes was given by a signed informed consent (for children under 12 years of age, consent from both parents/legal representatives was required to participate in the study. Children between 12 and 16 years of age give consent independently in addition to their parents/legal representatives).
Nailfold videocapillaroscopy technique and image selection
Capillaroscopic images were obtained during a one-time capillaroscopy between 2016 and 2021. The videocapillaroscope was equipped with a ×200 magnification lens from Optilia with accompanying ‘Optipix’ image analysis software. Images were collected by two investigators (DS and SB, with respectively seven and four years of experience in performing nailfold videocapillaroscopy). Prior to the examination, subjects were asked to stay in a room at 20–22 ℃ for a minimum of fifteen minutes. During the measurement, subjects were asked to sit in front of the examiner, with their hands on the table. Next, a drop of immersion oil was applied to the fingers. All fingers, excluding the thumbs, of both hands were examined. Two images per nailfold were made, resulting in a set of sixteen images per child.
Scoring system of capillary density
In this study, we followed the procedure described by Cutolo and Smith to calculate the capillary density (16). Briefly, we measured the number of capillaries along one millimetre of the distal row. A capillary density score per finger was calculated by averaging the scores of two images in the middle region of the same nailfold. Subsequently, the overall mean capillary density was calculated by averaging the capillary density scores of the eight individual fingers. If only one of the two images was of good visibility, only this image was used for calculating the ‘mean’ capillary density. The physician scored the ethnic background/skin pigment grade of the subjects.
Statistical analysis
Type of ethnic background was analysed as categorical variable (Caucasian, Asian, North-African/Middle-Eastern, African/Afro-Caribbean and ‘Mixed/other’), as well as ordinal variable graded by increasing degree of skin pigment [White as pigment ‘grade I’; Mixed (Mixed/other, Asian, North-African/Middle-Eastern) as pigment ‘grade II’; and Black (African/Afro-Caribbean) as pigment ‘grade III’].
Statistical analysis was performed using IBM SPSS statistics software, version 26 and GraphPad Prism, version 9. Descriptive statistics were used to represent demographic features of subjects. The Pearson linear correlation coefficient was calculated to quantify the linear relationship between age and capillary density. Analysis of variance (ANOVA) was performed to compare capillary density between different ethnic groups as well as different fingers. Post-hoc ANOVA analyses were performed with Sidak’s multiple comparison test with a single pooled variance for differences between ethnic groups and different fingers, respectively. A P value <0.05 was considered statistically significant.
Power analysis and sample size calculation were performed using the nQuery version 8.5.1 software. Data from Andrade et al. (15) were used for sample size calculation. In this aforementioned study, capillary density in white versus non-white subjects was compared. Based on a one-way ANOVA with variance of means of 0.2, alpha level set as 5% (0.05) and power of 95%, sample size for the present study was estimated to be 139 in total (if divided in five groups) or 115 in total (if divided in three groups). The effect size was based on the minimal statistically important difference in capillary density between different ethnicities and pigment grades. This sample size of 139 healthy subjects is likely to provide enough power for all analyses.
Results
The cohort consisted of 87 girls (60%) and 58 boys (40%), ranging from 4 to 18 years with a mean age of 11.03±3.51 years. Demographics are depicted in Table 1. A total number of 2,320 images from 145 healthy subjects were assessed. In 2,083/2,320 (89.8%) NVC images, the capillary density could be counted. Three subjects were excluded because of bad quality of NVC images. In 34 subjects it was not possible to obtain good quality images in all examined fingers, this concerned 71 fingers. For data of the missing values, see Appendix 1.
Table 1
| Baseline characteristics | Value |
|---|---|
| Female, n (%) | 87 (60.0) |
| Age at capillaroscopy in years, mean ± SD | 11.03 (±3.51) |
| Ethnicity, n (%) | |
| Caucasian | 99 (68.3) |
| African/Afro-Caribbean | 21 (14.5) |
| Mixed/other | 13 (9.0) |
| North-African/Middle-Eastern | 10 (6.9) |
| Asian | 2 (1.4) |
| Pigment grade, n (%) | |
| ‘White’, pigment grade I | 99 (68.3) |
| ‘Mixed’, pigment grade II | 25 (17.2) |
| ‘Black’, pigment grade III | 21 (14.5) |
Capillary density in different ethnicities
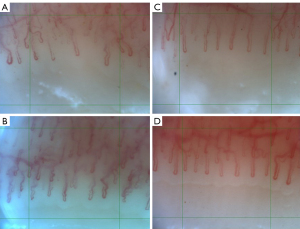
The overall mean capillary density in the subjects was 6.7±0.8 capillaries per mm. The mean capillary densities per ethnic background can be found in Table 2. Figure 1A-1D show capillaroscopic images with different capillary densities.
Table 2
| Ethnic background | Capillary density (Capillaries per mm, mean ± SD) |
|---|---|
| Ethnicity (categorical) | |
| Caucasian | 7.0±0.7 |
| African/Afro-Caribbean | 5.9±0.8 |
| Mixed/other | 6.5±0.6 |
| North-African/Middle-Eastern | 6.2±0.2 |
| Asian | 6.2±0.0 |
| Pigment grade (ordinal) | |
| ‘White’, pigment grade I | 7.0±0.7 |
| ‘Mixed’, pigment grade II | 6.4±0.5 |
| ‘Black’, pigment grade III | 5.9±0.8 |
In subjects with an African/Afro-Caribbean and North-African/Middle-Eastern ethnicity, a significantly lower mean capillary density was observed compared to subjects with the Caucasian ethnicity (P<0.001, and P=0.02, respectively, Figure 2A). There were no significant differences between other ethnicities (Figure 2A).
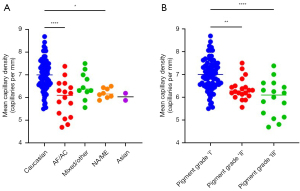
Subsequently, we analysed the capillary density as ordinal variable per degree of skin pigment-based group (Table 2 and Figure 2B). Significantly lower capillary densities were found in the grade ‘II’ and grade ‘III’ pigment groups compared to the grade ‘I’ pigment group (P<0.001 and P<0.001, respectively). There was no significant difference between grade ‘II’ and ‘III’ pigment groups.
Capillary density and age
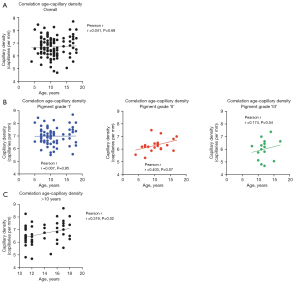
No correlation between age and capillary density could be observed (Pearson correlation r=0.041, P=0.68, Figure 3A). Also after dividing the subjects into (ordinal) ethnic subgroups, no correlation between age and capillary density could be observed (Figure 3B). However, for the ‘pigment grade II’ group a near-significant, positive correlation between age and capillary density was observed (r=0.403 P=0.07). After dividing the subjects of our cohort into two groups (≤10 years and >10 years), we found a weak significant positive correlation in the latter group (r=0.319, P=0.02) with post-hoc analysis (Figure 3C). This correlation was not significant after dividing the groups (≤10 and >10 years) into pigment grades.
Capillary density in different fingers
Next, we assessed whether there are differences in capillary density between different fingers. For both hands, both digits 5 demonstrated significantly lower capillary density compared to all other fingers, whereas none of the other pairs demonstrated significant differences in capillary density (Figure 4A,4B). For mean capillary density per finger, see Table 3.
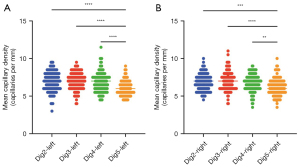
Table 3
| Digit | Capillary density (Capillaries per mm, mean ± SD) |
|---|---|
| Left hand | |
| Dig 2 | 6.9±1.3 |
| Dig 3 | 7.0±1.1 |
| Dig 4 | 6.8±1.3 |
| Dig 5 | 6.0±1.0 |
| Right hand | |
| Dig 2 | 6.8±1.1 |
| Dig 3 | 7.0±1.2 |
| Dig 4 | 6.8±1.1 |
| Dig 5 | 6.2±1.1 |
Discussion
The current study aimed to investigate the influence of ethnic background/skin pigment and age on capillary density in NVC images of healthy children. Statistically significant differences in nailfold capillary density between different ethnicities/skin pigment grades were detected but no relation between age (4–18 years) and capillary density was observed. It was also demonstrated that, compared to all other fingers, the fifth digits from both hands have a significantly lower nailfold capillary density. Future large scale and prospective studies, as are being presently conducted by the EULAR study group on microcirculation in Rheumatic Diseases, will need to evaluate the clinical significance of this density difference (17).
The current cut-off value for a normal capillary density in healthy adults is seven capillaries per mm (18). In this study, the overall mean capillary density in healthy children was found to be 6.7±0.8 capillaries per mm. This is in perfect agreement with the study by Dolezalova et al. that also reported a capillary density of 6.7 (range 5.3–9.3) (19). Moreover, fairly similar results were obtained by Ingegnoli et al. and Terreri et al., with a mean capillary density of 6.1 (range, 5–8) and 7.1±0.8, respectively (20,21). Interestingly, Piotto et al. found a higher capillary density, of 7.8±1.5 (22). In addition, Melsens et al. reported an even higher capillary density: 8.5±1.2 (17). The observed differences may be due to several factors, including the ethnic background and physiological differences of the nailfold capillary network itself (19,20). However, the ethnic background/skin pigment grade was not part of the variables in the conducted analyses in the included studies.
Our current study demonstrates that the capillary density significantly differs in children from various ethnic backgrounds (Figure 2A). In accordance, Andrade et al. also found that density of capillaries was higher among whites versus non-whites, but only slightly (15). Another study reported no statistically significant difference in capillary density between ethnic groups (21). However, these aforementioned studies divided the ethnic groups into two groups only: white versus non-white (15,21). Collectively, the results of our study also shows that more skin pigment correlates with a lower capillary density. As we hypothesized, our study suggests that degree of skin pigment should be taken into account when evaluating nailfold capillary density. It is important to know the normal values for capillary density in children (and adults) for correct interpretation of NVC images in patients.
In our study, no significant correlation between age (4–18 years) and capillary density was found, also not upon dividing the subjects into different pigment grade groups (Figure 3A,3B). This is in line with two other studies from Herrick et al. and Ingegnoli et al. (1,20). In contrast, Piotto et al. did observe a positive correlation between age and capillary density (r=0.796, P<0.001) (22). Interestingly, Melsens et al., observed a positive correlation (r=0.14, P=0.05), only after excluding subjects with 1–4 years of age (17). However, the correlation was very poor. This is similar to observations by Terreri et al. showing a significantly higher capillary density in children older than 10 years than those younger than or equal to 10 years (21). When using this same age cut-off, we also observed a significant positive correlation between age and capillary density in the >10 years group (Figure 3C). Interestingly, these findings suggest that during the pubertal phase the capillary density increases with age.
At last, our findings of a lower capillary density in the nailfolds might have a link with observed differences in pulse oximetry measurements in people with different skin pigments. Several studies hypothesized that people with a darker skin could have pigment-related errors in pulse oximeter measurements (23,24). The presence of melanin at higher concentrations, as found in patients with darker skin, might have an effect on the amount of light transmitted (mainly the red) (25,26). This could lead to an incorrect (overestimated) SpO2 value. Besides the possible effect of pigment, a lower density might also lead to suboptimal measurements.
To our knowledge, this is the first study that compared the capillary density between different fingers. The fifth digits displayed a significantly lower capillary density when compared to the other fingers. Probably this is because this finger is smaller and is less used in daily activities. We suggest that these fifth digits should not be used for interpretation of capillary density. However, mean capillary density of all the 8 fingers (thumbs always excluded) did not differ significantly compared to the density when excluding the fifth digits from the analyses.
A strength of this study is the large sample size, with 145 subjects (<18 years). Moreover, for all subjects, two investigators, using the same image analysis software, performed the same NVC procedure. Additionally, high sensitivity is achieved by standardized examination of eight fingers per child and using a high magnification videocapillaroscope. However, there were some missing data as a result of bad quality NVC images (approximately 10%). This was due to physiological differences in thickness of the nailfold of some children and this percentage of missing data is comparable to other studies (10,17).
A limitation in this study is overrepresentation of the Caucasian ethnicity in our cohort (70% of all subjects), which is not representative for some rheumatic autoimmune diseases such as SLE. Lastly, the medical history of children that were investigated at schools was taken without their parents, meaning that some information (for symptoms of Raynaud or trauma) might be incomplete, especially for the younger children.
If the findings presented in this study prove to be reproducible in more studies, it will be important, when evaluating capillary density from patients with Raynaud’s phenomenon and/or autoimmune rheumatic diseases, to correct for baseline differences caused by ethnic background/ skin pigment and type of finger. Possibly, a tailor-made fast-track for children, taking ethnic background/skin pigment grade into account, could be useful (18) Importantly, our observations in children cannot be directly extrapolated to the adult population. To evaluate this, nailfold capillaroscopy examinations in healthy adults are first needed. Recently, a study in healthy adults was performed in India by Bairwa et al. (27) In line with our results, they found that the density of capillaries visualized by nailfold capillaroscopy decreases as the skin pigmentation increases. Of note, they included 118 healthy adults with Type III, IV, V, and VI skin phototypes (classified by Standard Fitzpatrick classification of skin phototype).
Conclusions
In conclusion, our findings show that children with more skin pigment have a significant lower nailfold capillary density at NVC analysis. A weak positive significant correlation between age and capillary density was found, but only for children between 10–18 years old (r=0.319, P=0.02). In both hands, the fifth fingers displayed lower capillary density compared to the other fingers. These findings presented here should be taken into account for interpretation of capillary density in a NVC image of all rheumatic patients, especially in children.
Acknowledgments
We would like to thank all healthy children, their parents and the schools “Piet Hein”, “de Knotwilg” and “Burgemeester Amersfoordtschool” for participating in our study, by undergoing a one-time capillaroscopy. The authors are member of the EULAR study group on microcirculation in Rheumatic Diseases.
Funding: This work was supported by the ‘Stichting Steun Emma’, a Dutch Research foundation for the clinical researchers of the Emma children’s hospital, Amsterdam UMC [to SCB, study supervised by DSM] and by the ‘Stichting Zeldzame Ziekten Fonds’ [to SCB, study supervised by DSM].
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-993/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-993/coif). SCB was funded for her PhD trajectory by Stichting Zeldzame Ziektenfonds and the Stichting Steun Emma. VS reports that she is a Senior Clinical Investigator of the Research Foundation – Flanders (Belgium) (FWO) [1.8.029.20N]. The FWO was not involved in study design, collection, analysis and interpretation of data, writing of the report, nor in the decision to submit the manuscript for publication. VS has received consulting fees from Boehringer Ingelheim and Janssen-Cilag and speaker fees from Boehringer Ingelheim, Janssen-Cilag and Galapagos. Janssen-Cilag NV was not involved in study design, collection, analysis and interpretation of data, writing of the report, nor in the decision to submit the manuscript for publication. MC is Advisor for Education at EULAR School of Rheumatology (ESoR). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The used capillaroscopy images from this study are part of a cohort study, and this study was approved by the Medical Ethical Committee from Amsterdam UMC (with number NL60885.018.17 from Dutch trial register). Permission from the subjects to use the capillaroscopy images for research purposes was given by a signed informed consent (For children under 12 years of age, consent from both parents/legal representatives is required to participate in the study. Children between 12 and 16 years of age give consent independently in addition to their parents/legal representatives).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herrick AL, Moore T, Hollis S, Jayson MI. The influence of age on nailfold capillary dimensions in childhood. J Rheumatol 2000;27:797-800. [PubMed]
- Smith V, Herrick AL, Ingegnoli F, Damjanov N, De Angelis R, Denton CP, et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud's phenomenon and systemic sclerosis. Autoimmun Rev 2020;19:102458. [Crossref] [PubMed]
- Gerhold K, Becker MO. Nailfold capillaroscopy in juvenile rheumatic diseases: known measures, patterns and indications. Clin Exp Rheumatol 2014;32:S-183-8. [PubMed]
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747-55. [Crossref] [PubMed]
- Schonenberg-Meinema D, Bergkamp SC, Nassar-Sheikh Rashid A, van der Aa LB, de Bree GJ, Ten Cate R, Cutolo M, Hak AE, Hissink Muller PC, van Onna M, Kuijpers TW, Smith V, van den Berg JM. Nailfold capillary abnormalities in childhood-onset systemic lupus erythematosus: a cross-sectional study compared with healthy controls. Lupus 2021;30:818-27. [Crossref] [PubMed]
- Bergkamp SC, Schonenberg-Meinema D, Nassar-Sheikh Rashid A, Melsens K, Vanhaecke A, Boumans MJH, Hissink Muller PCE, Cutolo M, Kuijpers TW, van den Berg JM, Smith V. Reliable detection of subtypes of nailfold capillary haemorrhages in childhood-onset systemic lupus erythematosus. Clin Exp Rheumatol 2021;39:1126-31. [Crossref] [PubMed]
- Johnson D, van Eeden C, Moazab N, Redmond D, Phan C, Keeling S, Gniadecki R, Cohen Tervaert JW, Osman M. Nailfold Capillaroscopy Abnormalities Correlate With Disease Activity in Adult Dermatomyositis. Front Med (Lausanne) 2021;8:708432. [Crossref] [PubMed]
- Herrick AL, Cutolo M. Clinical implications from capillaroscopic analysis in patients with Raynaud's phenomenon and systemic sclerosis. Arthritis Rheum 2010;62:2595-604. [Crossref] [PubMed]
- Vanhaecke A, Cutolo M, Distler O, Riccieri V, Allanore Y, Denton CP, Hachulla E, Ingegnoli F, Deschepper E, Avouac J, Jordan S, Launay D, Melsens K, Pizzorni C, Sulli A, Vasile M, Herrick AL, Smith V. Nailfold capillaroscopy in SSc: innocent bystander or promising biomarker for novel severe organ involvement/progression? Rheumatology (Oxford) 2022;61:4384-96. [Crossref] [PubMed]
- Cutolo M, Herrick AL, Distler O, Becker MO, Beltran E, Carpentier P, Ferri C, Inanç M, Vlachoyiannopoulos P, Chadha-Boreham H, Cottreel E, Pfister T, Rosenberg D, Torres JV, Smith VCAP Study Investigators. Nailfold Videocapillaroscopic Features and Other Clinical Risk Factors for Digital Ulcers in Systemic Sclerosis: A Multicenter, Prospective Cohort Study. Arthritis Rheumatol 2016;68:2527-39. [Crossref] [PubMed]
- Soulaidopoulos S, Triantafyllidou E, Garyfallos A, Kitas GD, Dimitroulas T. The role of nailfold capillaroscopy in the assessment of internal organ involvement in systemic sclerosis: A critical review. Autoimmun Rev 2017;16:787-95. [Crossref] [PubMed]
- Schonenberg-Meinema D, Bergkamp SC, Nassar-Sheikh Rashid A, Gruppen MP, Middelkamp-Hup MA, Armbrust W, Dolman K, Hak AE, Hissink Muller PCE, van Onna M, Swart JF, Kuijpers TW, Kamphuis SSM, Smith V, van den Berg JM. Nailfold capillary scleroderma pattern may be associated with disease damage in childhood-onset systemic lupus erythematosus: important lessons from longitudinal follow-up. Lupus Sci Med 2022; [Crossref] [PubMed]
- Minopoulou I, Theodorakopoulou M, Boutou A, Arvanitaki A, Pitsiou G, Doumas M, Sarafidis P, Dimitroulas T. Nailfold Capillaroscopy in Systemic Sclerosis Patients with and without Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. J Clin Med 2021; [Crossref] [PubMed]
- Farenhorst CA, Roon AM, Gessel AI, Stel AJ, Bootsma H, Armbrust W, Mulder DJ. Capillary microscopy is a potential screening method for connective tissue disease in children with Raynaud's phenomenon. Pediatr Rheumatol Online J 2022;20:11. [Crossref] [PubMed]
- Andrade LE, Gabriel Júnior A, Assad RL, Ferrari AJ, Atra E. Panoramic nailfold capillaroscopy: a new reading method and normal range. Semin Arthritis Rheum 1990;20:21-31. [Crossref] [PubMed]
- Cutolo M, Smith V. Nailfold Capillaroscopy. In: Varga J, Denton CP, Wigley FM, editors. Scleroderma: From Pathogenesis to Comprehensive Management. Boston, MA: Springer US; 2012. p. 331-46.
- Melsens K, Cutolo M, Schonenberg-Meinema D, Foeldvari I, Leone MC, Mostmans Y, et al. Standardized nailfold capillaroscopy in children with rheumatic diseases: a worldwide study. Rheumatology (Oxford) 2023;62:1605-15. [Crossref] [PubMed]
- Smith V, Vanhaecke A, Herrick AL, Distler O, Guerra MG, Denton CP, Deschepper E, Foeldvari I, Gutierrez M, Hachulla E, Ingegnoli F, Kubo S, Müller-Ladner U, Riccieri V, Sulli A, van Laar JM, Vonk MC, Walker UA, Cutolo MEULAR Study Group on Microcirculation in Rheumatic Diseases. Fast track algorithm: How to differentiate a "scleroderma pattern" from a "non-scleroderma pattern". Autoimmun Rev 2019;18:102394. [Crossref] [PubMed]
- Dolezalova P, Young SP, Bacon PA, Southwood TR. Nailfold capillary microscopy in healthy children and in childhood rheumatic diseases: a prospective single blind observational study. Ann Rheum Dis 2003;62:444-9. [Crossref] [PubMed]
- Ingegnoli F, Zeni S, Gerloni V, Fantini F. Capillaroscopic observations in childhood rheumatic diseases and healthy controls. Clin Exp Rheumatol 2005;23:905-11. [PubMed]
- Terreri MT, Andrade LE, Puccinelli ML, Hilário MO, Goldenberg J. Nail fold capillaroscopy: normal findings in children and adolescents. Semin Arthritis Rheum 1999;29:36-42. [Crossref] [PubMed]
- Piotto DP, Sekiyama J, Kayser C, Yamada M, Len CA, Terreri MT. Nailfold videocapillaroscopy in healthy children and adolescents: description of normal patterns. Clin Exp Rheumatol 2016;34:193-9. [PubMed]
- Kyriacou PA, Charlton PH, Al-Halawani R, Shelley KH. Inaccuracy of pulse oximetry with dark skin pigmentation: clinical implications and need for improvement. Br J Anaesth 2023;130:e33-6. [Crossref] [PubMed]
- Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial Bias in Pulse Oximetry Measurement. N Engl J Med 2020;383:2477-8. [Crossref] [PubMed]
- Okunlola OE, Lipnick MS, Batchelder PB, Bernstein M, Feiner JR, Bickler PE. Pulse Oximeter Performance, Racial Inequity, and the Work Ahead. Respir Care 2022;67:252-7. [Crossref] [PubMed]
- Norton HL. Variation in pulse oximetry readings: melanin, not ethnicity, is the appropriate variable to use when investigating bias. Anaesthesia 2022;77:354-5. [Crossref] [PubMed]
- Bairwa D, Kavadichanda CG, Dunga S, Mathew A. G A, M S G, Mamatha G, Thabah MM, Negi VS. Effect of skin phototype on quantitative nailfold capillaroscopy. J Scleroderma Relat Disord 2022;7:197-203. [Crossref] [PubMed]


