
Diagnostic performance and safety of percutaneous fine-needle aspiration immediately before microwave ablation for pulmonary ground-glass nodules
Introduction
Due to rapid advances in and the widespread use of computed tomography (CT), the detection of pulmonary ground-glass nodules (GGNs) is increasing. Pulmonary GGNs comprise pure GGNs and part-solid nodules (which comprise both ground-glass and solid components). GGNs should not be ignored, as some may be malignant or develop into cancer. Approximately 75% of persistent GGNs are malignant (1). Size progression and/or the appearance of solid components usually indicate malignancy. When there is a high suspicion of malignancy, surgical resection is recommended. Additionally, due to the low risk of metastasis, minimally percutaneous invasive therapies, such as image-guided microwave ablation (MWA) and radiofrequency ablation (RFA), have been widely applied (2-4), improving the survival of patients, particularly those unsuited for surgery.
However, unlike surgical resection, thermal ablation cannot provide a histopathologic diagnosis (5). This limitation could be addressed and unnecessary interventions could be avoided by using percutaneous CT-guided fine-needle aspiration (FNA) or core-needle biopsy (CNB) before MWA to acquire a pathological diagnosis. However, in clinical practice, it may be suboptimal to perform a percutaneous biopsy in a separate session before the thermal ablation of GGNs. Patients who choose to undergo thermal ablation are usually nonsurgical candidates due to poor respiratory function or a severe comorbidity and thus may not tolerate CT-guided percutaneous biopsy and MWA over 2 sessions. Performing the biopsy and MWA separately also increases the risk of complications, including pneumothorax, hemorrhage, gas embolism, and tumor seeding.
To address this limitation, some physicians have tried to perform percutaneous CNB and thermal ablation of lung malignancies synchronously. The performance of CNB either before or after ablation is effective and relatively safe (6,7). However, GGNs preserve normal pulmonary vessels and are highly susceptible to bleeding when biopsied. In such cases, the performance of CNB immediately before the ablation may affect the accuracy of GGN targeting and the ablation effect, as postbiopsy hemorrhages may blur the tumor. Some physicians have tried to perform percutaneous CNB immediately after thermal ablation to address this issue. Several studies have demonstrated that a malignancy diagnosis is feasible for the postablation pathological examinations of GGNs. Wang et al. (8) and Kong et al. (9) reported positive CNB diagnosis rates for GGNs post-MWA of 74.3% and 69.7%, respectively. One limitation of performing a biopsy immediately after the ablation of the GGNs includes the risk that a diagnosis will be infeasible if the samples are inadequate (e.g., if they have been severely damaged or carbonized).
Previous studies have mainly focused on percutaneous CNB (6-9). Percutaneous FNA is another useful diagnostic technique for GGNs with a low complication rate (10). However, no reports have assessed the diagnostic ability of synchronous CT-guided FNA and MWA for GGNs. In September 2021, FNA immediately before MWA became the standard procedure for obtaining specimens for the diagnosis of GGNs at Department of Minimally Invasive Tumor Therapies Center of Beijing Hospital. Our initial experience provided us with an excellent opportunity to evaluate the efficacy of synchronous FNA and WMA. This study sought to evaluate the diagnostic yield and safety of FNA pre-MWA for pulmonary GGNs. We hypothesized that performing FNA immediately before MWA would yield good diagnostic performance and safety. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1001/rc).
Methods
The study was approved by the Institutional Review Board of Beijing Hospital. Due to the retrospective nature of this analysis, the requirement for written informed consent was waived. Written informed consent was obtained from those patients who underwent biopsy and MWA. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patients
Searches of the radiology database and electronic medical records were performed to identify patients who underwent simultaneous CT-guided needle biopsy and MWA for GGNs at Beijing Hospital from September 2021 (after FNA had been adopted as a routine protocol) to March 2022. The indication for lung MWA was determined by a multidisciplinary team comprising medical oncologists, thoracic surgeons, interventional radiologists, and radiation therapists. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) be a poor candidate for surgery due to comorbid disease or have refused surgery; (II) have an Eastern Cooperative Oncology Group performance status of 0–1; and (III) have undergone FNA, CNB, or both synchronously with MWA. Ultimately, 92 consecutive patients were included in the study.
The abstracted data of the patients’ demographics as well as the size, location, and component of the nodule (pure or part-solid GGN) were reviewed. The pathologic results of the biopsy samples and complications of the procedures were recorded.
Biopsy and MWA procedures
The biopsy and MWA procedures were performed by 3 interventional radiologists with >5 years of experience in thermal ablation. All the procedures were conducted under local anesthesia and CT guidance. The patients were placed in a prone, supine, or lateral decubitus position to provide access to the best puncture pathway based on the location of the nodule.
All the patients underwent FNA biopsy first. All biopsies were performed with a 15-gauge coaxial introducer needle (Argon Medical Devices). After advancement the coaxial needle to the edge of the nodule, the inner stylet was replaced with a 22-gauge Chiba needle (BD) (Figure 1A,1B). A CT scan was performed to confirm the needle position. The aspiration specimens were then collected with negative pressure suction using an attached 10-ml syringe. The aspiration specimens were placed on sterile glass slides and immersed in 95% alcohol. All the aspiration specimens were transferred for cytologic diagnosis after staining with hematoxylin and eosin. Immunostaining with thyroid transcription factor 1 was performed as necessary. This procedure was performed only once without a rapid onsite evaluation (ROSE).
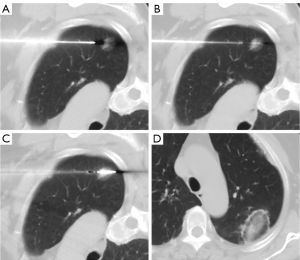
Immediately after the FNA, an 18-gauge microwave antenna (Vision-China Medical Devices R&D Center) was introduced into the GGN through the coaxial system. Ablation was then started to achieve complete ablation (Figure 1C,1D). The ablation parameters for the GGNs at our institution have been described previously. In brief, the ablation power output was set to 30–40 W, and the duration ranged from 5 to 15 minutes (9).
Post-MWA CNB was performed if the nodule location did not preclude the use of an 18-gauge core biopsy needle (Argon Medical Devices) with a 1.3- or 2.3-cm needle through the coaxial cannula. For nodules adjacent to the hilum or with a traversing segmental pulmonary artery, CNB was not routinely performed. The obtained specimen was placed in 10% formalin and transferred for histopathological diagnosis.
Chest CT was obtained immediately and 24 hours after the procedure to rule out complications, especially pulmonary hemorrhage post-FNA. Complications were assessed on the basis of the Common Terminology Criteria for Adverse Events (version 5.0) (11). A pulmonary hemorrhage was classified as a hemorrhage along the needle track or a perilesional hemorrhage.
Statistical analysis
The technical success for all patients was assessed. A technical success was defined as the accomplishment of both the biopsy and MWA. A technical failure was defined as an inability to obtain specimens or perform the MWA.
The biopsy results were divided into the following 2 categories: the positive group and the negative group. Both definite malignant and premalignant diagnoses were allocated to the positive group. Malignant diagnoses included diagnoses of adenocarcinoma, squamous cell carcinoma, or any other types of malignant tumors. Atypical adenomatous hyperplasia (AAH) was defined as a premalignant diagnosis. Normal lung tissues, red blood cells, and chronic inflammation were assigned to the negative group. The positive rates were calculated.
The statistical analyses were performed using SPSS 23.0 (IBM Corp.). The continuous variables are presented as the mean ± standard deviation and were compared using the Student t-test. The categorical variables are expressed as the frequency and percentage and were compared using Pearson chi-squared test or Fisher exact test. The FNA results were compared to the CNB-alone results and the combination results of the 2 procedures. Patients were allocated to 2 subgroups based on the nodule size (<1.5 and ≥1.5 cm) and GGO components (pure GGN and part-solid GGN). A comparison of the positive diagnosis rate was performed between the different groups. The diagnostical yields between different biopsy methods and subgroups were compared using Pearson chi-squared test. A P value <0.05 was considered statistically significant.
Results
In total, 95 patients were treated with MWA for a lung GGN between September 2021 and March 2022. All the 95 GGNs were considered malignant based on the radiologic findings. Ultimately, 92 patients with 92 GGNs were enrolled in the study. Of the excluded patients, 3 patients refused to undergo a biopsy (Figure 2). FNA was performed in all the nodules, and CNB was performed in 62 nodules. The demographics of the patients and the characteristics of the lesions are summarized in Table 1. There were no significant differences in terms of age, sex, nodule size, location, and nodule component (P>0.05).
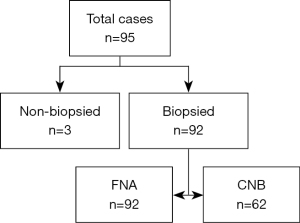
Table 1
| Characteristics | FNA | CNB | P value |
|---|---|---|---|
| Age, years (mean ± SD) | 60.4±12.5 | 62.1±12.4 | 0.401 |
| Gender, n (%) | 0.851 | ||
| Male | 37 (40.2) | 24 (38.7) | |
| Female | 55 (59.8) | 38 (61.3) | |
| Nodule size, cm (mean ± SD) | 1.4±0.6 | 1.6±0.7 | 0.109 |
| <1.5, n (%) | 60 (65.2) | 33 (53.2) | |
| ≥1.5, n (%) | 32 (34.8) | 29 (46.8) | |
| Location, n (%) | 0.901 | ||
| Right lobe | 51 (55.4) | 35 (56.5) | |
| Left lobe | 41 (44.6) | 27 (43.5) | |
| Component of nodule, n (%) | 0.463 | ||
| Pure GGN | 53 (57.6) | 32 (51.6) | |
| Part-solid GGN | 39 (42.4) | 30 (48.4) | |
| Compared with previous CT, n (%) | 0.960 | ||
| Growth | 79 (85.8) | 54 (87.1) | |
| Stable | 11 (12.0) | 7 (11.3) | |
| No previous CT | 2 (2.2) | 1 (1.6) |
FNA, fine-needle aspiration; CNB, core-needle biopsy; GGN, ground-glass nodule; CT, computed tomography.
Both a biopsy and MWA were successfully performed for 92 patients with 92 nodules. The technical success rate was 100%. FNA was completed for all patients, and an additional CNB was accomplished in 62 patients (Table 2). The FNA added only 1–2 minutes to the overall procedure time. The additional cost per patient for using the FNA technique was about USD $50–100. Thus, the FNA application did not significantly increase the procedure time and cost. The final diagnosis consisted of a summary of the FNA and CNB pathological results. Of the 92 GGNs, 75 were neoplasms, including 65 adenocarcinomas, 9 AAHs, and 1 mucosa-associated lymphoid tissue (MALT), representing a positive rate of 81.5% (75/92). Among the remaining 17 negative cases, 12 samples turned out to be lung tissue, 3 specimens had red blood cells, and 2 samples contained inflammatory cells (mainly lymphocytes). For further details, see Table 3.
Table 2
| Procedure | Samples | Positive rate, % [n] |
|---|---|---|
| FNA | 92 | 70.7 [65] |
| CNB | 62 | 72.6 [45] |
| FNA + CNB | 62 | 88.7 [55] |
FNA, fine-needle aspiration; CNB, core-needle biopsy.
Table 3
| Pathology results | Pure GGN | Part-solid GGN | Total |
|---|---|---|---|
| Adenocarcinoma | 35 | 30 | 65 |
| AAH | 5 | 4 | 9 |
| MALT | 0 | 1 | 1 |
| Lung tissue | 8 | 4 | 12 |
| Red blood cells | 3 | 0 | 3 |
| Inflammatory cells | 2 | 0 | 2 |
| Total | 53 | 39 | 92 |
AAH, atypical adenomatous hyperplasia; GGN, ground-glass nodule; MALT, mucosa-associated lymphoid tissue.
The positive diagnosis rate of FNA was 70.7% (65/92). Of the 65 positive results, there were 53 adenocarcinomas, 11 AAHs, and 1 non-small cell lung cancer (NSCLC). The remaining 27 FNA results were negative. The CNB identified 45 positive results and 17 negative results, representing a positive rate of 72.6%. Of the 45 nodules that had positive results, there were 41 were adenocarcinomas, 3 AAHs, and 1 MALT. The positive rate of the nodules that had samples obtained by sequential FNA and CNB was 88.7% (55/62). Of these 55 nodules, the CNB was diagnostic while the FNA was not diagnostic in 10 patients. In 5 cases, the FNA identified 4 AAHs and 1 NSCLC, while the CNB identified adenocarcinomas. Interestingly, the FNA made a positive diagnosis in 10 nodules not diagnosed by the CNB. For further details, see Table 4.
Table 4
| FNA/CNB results | No. of cases |
|---|---|
| Adenocarcinoma/adenocarcinoma | 29 |
| Adenocarcinoma/negative | 8 |
| AAH/ adenocarcinoma | 4 |
| AAH/AAH | 1 |
| AAH/negative | 2 |
| NSCLC/adenocarcinoma | 1 |
| Negative/adenocarcinoma | 7 |
| Negative/AAH | 2 |
| Negative/MALT | 1 |
| Negative/negative | 7 |
Negative = negative for malignancy and pre-malignancy. FNA, fine-needle aspiration; CNB, core-needle biopsy; AAH, atypical adenomatous hyperplasia; NSCLC, non-small cell lung cancer; MALT, mucosa-associated lymphoid tissue.
There was no significant difference between the FNA and CNB in terms of their abilities to achieve a positive diagnosis (P=0.8). The sequential FNA and CNB had a significantly better diagnostic performance than did either method alone (P=0.008 and P=0.023, respectively). The diagnostic performance values are presented in Table 5. In total, the diagnostic yield for nodules <1.5 cm was 78.3% (47/60), which did not differ significantly to that for nodules ≥1.5 cm (87.5%, 28/32) (P=0.28). The positive rate for pure GGNs was 75.5% (40/53), which did not differ significantly from that for part-solid nodules (89.7%, 35/39) (P=0.08). As for the pure GGNs and part-solid GGNs, only the CNB showed a significant difference in terms of its diagnostic performance. The diagnostic yield of the CNB for pure GGNs was significantly lower than that for part-solid GGNs (P=0.016; Table 6). The differences in the diagnostic performance of the FNA, CNB, and both between the <1.5 cm lesions and ≥1.5 cm lesions were not significant (Table 7).
Table 5
| Modality | Positive rate (%) | Significance |
|---|---|---|
| FNA vs. CNB | 70.7 vs. 72.6 | P=0.8 |
| FNA+CNB vs. FNA | 88.7 vs. 70.7 | P=0.008 |
| FNA+CNB vs. CNB | 88.7 vs. 72.6 | P=0.023 |
FNA, fine needle aspiration; CNB, core-needle biopsy.
Table 6
| Variables | GGN | P value | |
|---|---|---|---|
| Pure | Part-solid | ||
| FNA | 66.0% (35/53) | 76.9% (30/39) | 0.26 |
| CNB | 59.4% (19/32) | 86.7% (26/30) | 0.016 |
| Both FNA and CNB | 84.4% (27/32) | 93.3% (28/30) | 0.43 |
| Total | 75.5% (40/53) | 89.7% (35/39) | 0.08 |
FNA, fine needle aspiration; CNB, core-needle biopsy; GGN, ground glass nodule.
Table 7
| Variables | Lesion size | P value | |
|---|---|---|---|
| <1.5 cm | ≥1.5 cm | ||
| FNA | 71.6% (43/60) | 68.8% (22/32) | 0.77 |
| CNB | 66.7% (22/33) | 79.3% (23/29) | 0.27 |
| Both FNA and CNB | 87.9% (29/33) | 89.7% (26/29) | 0.83 |
| Total | 78.3% (47/60) | 87.5% (28/32) | 0.28 |
FNA, fine needle aspiration; CNB, core-needle biopsy.
No procedure-related deaths occurred. The mortality rate was 0.0% within 30 and 90 days of the MWA. Grade 1 pulmonary hemorrhages occurred in 10 of the 92 (10.9%) procedures immediately after FNA and included 8 cases of hemorrhage along the needle track and 2 cases of perilesional hemorrhage; however, these did not hamper the accuracy of the initial MW antenna placement. Small pneumothoraxes were observed in 2 (2.2%) sessions on post-FNA CT, but chest tube insertion was not required. The overall pulmonary hemorrhage rate after biopsy and MWA was 22.8% (21/92), but no further interventions were required. The overall pneumothorax rate was 16.3% (15/92). Of these patients, 9 (9.8%) required chest tube insertion. The median length of the tube drainage after MWA was 1 day (range: 1–3 days). Additionally, 2 patients developed small pleural effusion, but drainage was not required.
Discussion
The results of the present study showed that the sequential use of FNA and MWA for GGNs had a high rate of technical success and acceptable complication rates. The findings suggest that FNA and CNB are comparable at achieving positive diagnoses, and no significant differences were observed. The results also demonstrated that the sequential FNA and CNB improved the diagnosis of GGNs.
Treatment options need not be limited to surgery but could also include minimally invasive strategies, such as thermal ablation and radiotherapy, if the malignant GGN is an early-phase cancer that has not yet metastasized (12). One of the challenges of MWA lies in arriving at a pathological diagnosis. As a general rule, a malignant diagnosis of GGN by biopsy should be performed before the MWA. A correct preablation diagnosis can prevent unnecessary interventions for benign GGNs. However, the performance of the biopsy and MWA in 2 separate sessions could increase patients’ discomfort and the risk of complications. Choi et al. (13) found a significantly higher risk of pulmonary hemorrhage in CT-guided biopsies of GGNs compared to solid nodules.
Separate biopsies for GGNs before the MWA are not performed at our clinic. If malignancy is highly suspected, the biopsy and MWA are performed synchronously. There is no consensus as to the biopsy modality of choice. FNA is preferred by some interventionalists and pathologists, while CNB is preferred by others. Previously, we preferred to perform the CNB after the MWA of the GGNs for the following 2 reasons. First, several studies have demonstrated that malignancy can be identified on postablation pathological examinations for lung tumors even after complete ablation (5,7,8). Clasen et al. reported that tumor tissue appeared to be thermally fixed 3 days after RFA and still preserved the histological features of tumor cells in standard histological staining; however, an ultrastructural analysis and terminal deoxynucleotidyl transferase-mediated nick end-labeling confirmed that the tissue showed coagulative necrosis (14). Second, postablation biopsy prevents the accuracy of the initial MW antenna or RF probe placement being hampered due to postbiopsy hemorrhage or pneumothorax before ablation.
An earlier investigation reported an immediate post-MWA positive diagnosis rate for GGNs of 69.7% (9). This result is similar to the positive diagnosis rate reported in a study of Wang et al. (8) of 74.3%. Both of these studies showed that the characteristics of the tumor cells were preserved and the histological types could be distinguished immediately in the post-MWA biopsy samples. Postablation CNB is a useful diagnostic tool for GGNs; however, due to its diagnostic yield, it is not ideal for clinical use. Thus, we investigated the performance of FNA immediately before MWA, focusing on the diagnostic yield and safety.
Our results suggest that FNA is comparable to CNB, and sequential FNA and CNB improve the diagnostic yield of the GGNs. There is insufficient evidence to support a significant difference between FNA and CNB in determining lung malignancies in patients with lung nodules (15). Our study demonstrated that the FNA result was similar to the CNB result, with positive rates of 70.7% and 72.6%, respectively. The sequential FNA and CNB had a significantly better diagnostic yield than did either FNA or CNB alone (P=0.008 and P=0.023, respectively). This is in line with the findings of some previous reports (16,17). However, other studies have shown that FNA, CNB, and their combination are comparable in arriving at a diagnosis of malignancy (10,18). Kiranantawat et al. (10) reported that the diagnostic yield of malignancy for GGNs was 86.0% with a sensitivity of 97%. Their improved results may be related to the availability of ROSE. The process of FNA was repeated several times as needed according to the adequacy of each cytologic specimen for diagnosis. Our low positive rate could be partly explained by there being only 1 FNA without a ROSE.
FNA and CNB can compensate for each other’s inadequacies to provide an accurate diagnosis. In this study, 20 patients had a positive diagnosis according to either FNA or CNB but not both modalities. Additionally 5 cases had a diagnosis of AAH or NSCLC according to FNA that were finally confirmed to be adenocarcinomas by the sequential CNBs. Thus, the sequential FNA and CNB minimizes the risk of inadequate specimen sampling for diagnosis.
In relation to the tumor size, the positive rate was 78.3% for tumors <1.5 cm and 87.5% for tumors ≥1.5 cm. The diagnostic yield was lower in the smaller-size group but did not reach statistical significance (P>0.05). These findings are comparable to those reported by Kiranantawat et al. (10) and Hur et al. (19). Conversely, Shimizu et al. (20) demonstrated that the diagnostic accuracy significantly decreased in relation to the smaller nodules. The lower diagnostic value of the smaller nodules may be partly explained by the difficulty of hitting a smaller nodule. We also evaluated the difference in the diagnostic yield between the pure and part-solid GGNs. The results showed that the pure GGNs had a relatively lower diagnostic yield than did the part-solid GGNs. Only CNB showed a significant difference (P=0.016). However, there was no significant difference for FNA or both FNA and CNB (P>0.05). This supports the findings of a few related studies (10,12,21). Conversely, other studies have reported significantly lower diagnostic yields for pure GGNs than for part-solid GGNs (19,20,22). The lower diagnostic accuracy of pure GGNs is probably due to the lower cellularity of pure GGNs compared to part-solid GGNs (20).
The study confirmed that synchronous CT-guided biopsy and the MWA of GGNs is a relatively safe procedure with an acceptable rate of complications. A biopsy performed immediately before MWA carries a risk of bleeding or pneumothorax, which may alter the accuracy of the MW antenna placement for lung nodules, especially in CNBs (7,10). FNA had a pulmonary hemorrhage rate of 10.9% and a pneumothorax rate of 2.2% before MWA. Notably, none of the pulmonary hemorrhages hampered the accuracy of the subsequent MW antenna placement by blurring the lesion. The 2 cases of pneumothorax post-FNA were mild and did not induce the needle to slip away from the tumor for the MWA. Thus, FNA immediately before MWA can be performed safely with the best puncture path preserved for tumor ablation. The overall pulmonary hemorrhage and pneumothorax rates were 22.8% and 16.3%, respectively, which are comparable to those reported previously (2-4,23). Most of the complications were minor, and no interventions were required. Thus, synchronous FNA and MWA with a coaxial technique was found to be a safe and effective method for obtaining a histopathological diagnosis.
This study had several limitations. First, it was a retrospective, single-center study, which might have led to selection bias. Second, the study was limited by a lack of ROSEs, which might have resulted in a relatively reduced diagnostic yield. The overall positive rate was 81.5%, which is in line with the 64.6–87.5% rate reported for CT-guided biopsies of GGNs (20,24-26). However, some of the remaining 18.5% negative biopsy results may be false negatives, as most cases were selected for the intervention based on the persistence or growth of the nodules and malignant image morphology. Molecular imaging (MI) has recently emerged as an important tool for precision surgery and has demonstrated considerable success in identifying the pathology of lung tumors by facilitating tumor removal (27). MI-guided tissue sampling may be a future research direction to improve the diagnostic accuracy of lung nodule biopsies.
Conclusions
FNA immediately before MWA is a reliable technique for the histological diagnosis of lung GGNs and does not alter the accuracy of the MW antenna placement. Sequential FNA pre‑MWA and CNB post‑MWA can improve the diagnosis of GGNs.
Acknowledgments
We would like to thank Dr. Lan Chen, Department of Pathology, Beijing Hospital, for her support in pathological diagnosis.
Funding: This work was supported by the Central Healthcare Research Fund (No. 2020YB10).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-1001/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1001/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of Beijing Hospital approved this study. The requirement for patient written informed consent was waived for this retrospective analysis. Written informed consent was obtained from those patients who underwent biopsy and MWA.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim HY, Shim YM, Lee KS, Han J, Yi CA, Kim YK. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 2007;245:267-75. [Crossref] [PubMed]
- Kodama H, Yamakado K, Hasegawa T, Takao M, Taguchi O, Fukai I, Sakuma H. Radiofrequency ablation for ground-glass opacity-dominant lung adenocarcinoma. J Vasc Interv Radiol 2014;25:333-9. [Crossref] [PubMed]
- Iguchi T, Hiraki T, Gobara H, Fujiwara H, Matsui Y, Soh J, Toyooka S, Kiura K, Kanazawa S. Percutaneous radiofrequency ablation of lung cancer presenting as ground-glass opacity. Cardiovasc Intervent Radiol 2015;38:409-15. [Crossref] [PubMed]
- Yang X, Ye X, Lin Z, Jin Y, Zhang K, Dong Y, Yu G, Ren H, Fan W, Chen J, Lin Q, Huang G, Wei Z, Ni Y, Li W, Han X, Meng M, Wang J, Li Y. Computed tomography-guided percutaneous microwave ablation for treatment of peripheral ground-glass opacity-Lung adenocarcinoma: A pilot study. J Cancer Res Ther 2018;14:764-71. [Crossref] [PubMed]
- Hasegawa T, Kondo C, Sato Y, Inaba Y, Yamaura H, Kato M, Murata S, Onoda Y, Kuroda H, Sakao Y, Yatabe Y. Pathologic Diagnosis and Genetic Analysis of a Lung Tumor Needle Biopsy Specimen Obtained Immediately After Radiofrequency Ablation. Cardiovasc Intervent Radiol 2018;41:594-602. [Crossref] [PubMed]
- Schneider T, Puderbach M, Kunz J, Bischof A, Giesel FL, Dienemann H, Herth FJ, Schnabel PA, Safi S, Hoffmann H, Heussel CP. Simultaneous computed tomography-guided biopsy and radiofrequency ablation of solitary pulmonary malignancy in high-risk patients. Respiration 2012;84:501-8. [Crossref] [PubMed]
- Tselikas L, de Baere T, Deschamps F, Hakimé A, Besse B, Teriitehau C, de Montpreville V, Adam J. Diagnostic yield of a biopsy performed immediately after lung radiofrequency ablation. Eur Radiol 2017;27:1211-7. [Crossref] [PubMed]
- Wang J, Ni Y, Yang X, Huang G, Wei Z, Li W, Han X, Meng M, Ye X, Lei J. Diagnostic ability of percutaneous core biopsy immediately after microwave ablation for lung ground-glass opacity. J Cancer Res Ther 2019;15:755-9. [Crossref] [PubMed]
- Kong F, Bie Z, Li Y, Li B, Guo R, Wang C, Peng J, Xu S, Li X. Synchronous microwave ablation followed by core-needle biopsy via a coaxial cannula for highly suspected malignant lung ground-glass opacities: A single-center, single-arm retrospective study. Thorac Cancer 2021;12:3216-22. [Crossref] [PubMed]
- Kiranantawat N, McDermott S, Petranovic M, Mino-Kenudson M, Muniappan A, Sharma A, Shepard JO, Digumarthy SR. Determining malignancy in CT guided fine needle aspirate biopsy of subsolid lung nodules: Is core biopsy necessary? Eur J Radiol Open 2019;6:175-81. [Crossref] [PubMed]
- National Institute of Health. National Cancer Institute. Common terminology criteria for adverse events (CTCAE version 5) (Internet). Washington, DC: US Department of Health and Human Services; 2017. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/About.html
- Yamauchi Y, Izumi Y, Nakatsuka S, Inoue M, Hayashi Y, Mukai M, Nomori H. Diagnostic performance of percutaneous core needle lung biopsy under multi-CT fluoroscopic guidance for ground-glass opacity pulmonary lesions. Eur J Radiol 2011;79:e85-9. [Crossref] [PubMed]
- Choi JW, Park CM, Goo JM, Park YK, Sung W, Lee HJ, Lee SM, Ko JY, Shim MS. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of small (≤ 20 mm) lung nodules: diagnostic accuracy and complications in 161 patients. AJR Am J Roentgenol 2012;199:W322-30. [Crossref] [PubMed]
- Clasen S, Krober SM, Kosan B, Aebert H, Fend F, Bomches A, Claussen CD, Pereira PL. Pathomorphologic evaluation of pulmonary radiofrequency ablation: proof of cell death is characterized by DNA fragmentation and apoptotic bodies. Cancer 2008;113:3121-9. [Crossref] [PubMed]
- Yao X, Gomes MM, Tsao MS, Allen CJ, Geddie W, Sekhon H. Fine-needle aspiration biopsy versus core-needle biopsy in diagnosing lung cancer: a systematic review. Curr Oncol 2012;19:e16-27. [Crossref] [PubMed]
- Yamagami T, Iida S, Kato T, Tanaka O, Nishimura T. Combining fine-needle aspiration and core biopsy under CT fluoroscopy guidance: a better way to treat patients with lung nodules? AJR Am J Roentgenol 2003;180:811-5. [Crossref] [PubMed]
- Aviram G, Greif J, Man A, Schwarz Y, Marmor S, Graif M, Blachar A. Diagnosis of intrathoracic lesions: are sequential fine-needle aspiration (FNA) and core needle biopsy (CNB) combined better than either investigation alone? Clin Radiol 2007;62:221-6. [Crossref] [PubMed]
- Coley SM, Crapanzano JP, Saqi A. FNA, core biopsy, or both for the diagnosis of lung carcinoma: Obtaining sufficient tissue for a specific diagnosis and molecular testing. Cancer Cytopathol 2015;123:318-326. [Crossref] [PubMed]
- Hur J, Lee HJ, Nam JE, Kim YJ, Kim TH, Choe KO, Choi BW. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2009;192:629-34. [Crossref] [PubMed]
- Shimizu K, Ikeda N, Tsuboi M, Hirano T, Kato H. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006;51:173-9. [Crossref] [PubMed]
- Kim TJ, Lee JH, Lee CT, Jheon SH, Sung SW, Chung JH, Lee KW. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2008;190:234-9. [Crossref] [PubMed]
- De Filippo M, Saba L, Concari G, Nizzoli R, Ferrari L, Tiseo M, Ardizzoni A, Sverzellati N, Paladini I, Ganazzoli C, Sconfienza LM, Carrafiello G, Brunese L, Genovese EA, Ampollini L, Carbognani P, Rusca M, Zompatori M, Rossi C. Predictive factors of diagnostic accuracy of CT-guided transthoracic fine-needle aspiration for solid noncalcified, subsolid and mixed pulmonary nodules. Radiol Med 2013;118:1071-81. [Crossref] [PubMed]
- Zhou D, Zhang Y, Chen W, Jiang J, Chen Y, Zhou X, Tang Q. Enhanced ultrasound-guided versus non-enhanced ultrasound-guided percutaneous needle biopsy in tissue cellularity of lung malignancies: a propensity score matched study. Quant Imaging Med Surg 2022;12:5056-67. [Crossref] [PubMed]
- Maxwell AW, Klein JS, Dantey K, Mount SL, Butnor KJ, Leiman G. CT-guided transthoracic needle aspiration biopsy of subsolid lung lesions. J Vasc Interv Radiol 2014;25:340-6, 346.e1.
- Lu CH, Hsiao CH, Chang YC, Lee JM, Shih JY, Wu LA, Yu CJ, Liu HM, Shih TT, Yang PC. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J Thorac Oncol 2012;7:143-50. [Crossref] [PubMed]
- Inoue D, Gobara H, Hiraki T, Mimura H, Kato K, Shibamoto K, Iishi T, Matsui Y, Toyooka S, Kanazawa S. CT fluoroscopy-guided cutting needle biopsy of focal pure ground-glass opacity lung lesions: diagnostic yield in 83 lesions. Eur J Radiol 2012;81:354-9. [Crossref] [PubMed]
- Azari F, Zhang K, Kennedy GT, Chang A, Nadeem B, Delikatny EJ, Singhal S. Precision Surgery Guided by Intraoperative Molecular Imaging. J Nucl Med 2022;63:1620-7. [Crossref] [PubMed]


