
Angiography-based index of microcirculatory resistance (AccuIMR) for the assessment of microvascular dysfunction in acute coronary syndrome and chronic coronary syndrome
Introduction
Several studies have highlighted that coronary microvascular dysfunction (CMD) is one of the most important factors associated with adverse cardiovascular events in patients with coronary artery disease (CAD). Recently, CMD has become increasingly crucial in diagnosing and managing patients with chronic coronary syndrome (CCS) (1-4). The first step to the successful management of CMD is early diagnosis and identification. However, coronary microvascular diseases can result from heterogeneous pathological mechanisms (5).
A series of noninvasive physiological and imaging approaches, including myocardial contrast echocardiography, cardiac magnetic resonance, and positron emission tomography, have been suggested to assess microcirculatory dysfunction (4). However, these approaches are not readily available in the cardiac catheterization laboratory during percutaneous coronary intervention (PCI). Increasingly, invasive assessments, such as the index of microcirculatory resistance (IMR), have served as the reference standard for assessing microvasculature in the clinical setting (6).
The IMR has been validated as an optimal index for qualitative and quantitative measurement of the status of coronary microvasculature in patients with ischemic heart disease (7,8). The IMR is evaluated using a thermodilution wire during maximal hyperemia, which has been shown to be notably reproducible compared to other hemodynamic indicators of coronary microcirculation, such as hyperemic stenosis resistance (HSR), hyperemic myocardial resistance (HMR), and coronary flow reserve (CFR) (9,10). However, the adverse reactions of maximal hyperemia, the additional procedural time, and the increased procedural complexity might limit its usage in routine practice.
In recent years, angiographic derivation of fractional flow reserve (FFR), such as AccuFFRangio, has shown high diagnostic accuracy (11,12). Here, we propose a novel angiography-based IMR calculation method (AccuIMR) that does not require a pressure wire to measure IMR using the thermodilution method. Currently, the AccuIMR still lacks clinical validation. The purpose of this study was to assess the accuracy of the AccuIMR in patients with CCS, ST-segment elevation myocardial infarction (STEMI), and non-ST-segment elevation myocardial infarction (NSTEMI). Pressure wire-based IMR served as the reference standard. We present the following article in accordance with the Standards for the Reporting of Diagnostic accuracy studies (STARD) reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-961/rc).
Methods
Study design
This was a retrospective, observational study conducted at a single center with the objective of determining the diagnostic accuracy of AccuIMR in identifying clinically significant CMD by comparing the results with those obtained from wire-based IMR. The study protocol was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University with a waiver of written informed consent due to the retrospective nature of the study, in adherence to the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice Guidelines.
Study population
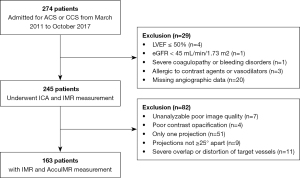
Consecutive patients aged at least 18 years who were admitted from March 2011 to October 2017 to Zhongnan Hospital of Wuhan University due to CCS, STEMI, and NSTEMI and underwent invasive coronary angiography (ICA) and IMR measurement were eligible for inclusion in this study.
The principal exclusion criteria for patients were as follows: (I) left ventricular ejection fraction (LVEF) ≤50%, (II) estimated glomerular filtration rate (eGFR) <45 mL/min/1.73 m2, (III) severe coagulopathy or bleeding disorders, and (IV) allergy to iodine contrast agents or vasodilators. The exclusion criteria for the image quality check were as follows: (I) unanalyzable poor image quality, (II) poor contrast opacification, (III) unsatisfactory projection view, and (IV) severe overlap or distortion of the target vessel.
PCI and wire-derived IMR measurement
All patients received 300 mg of aspirin and 300–600 mg of clopidogrel before PCI, and periprocedural unfractionated heparin was administered to prevent clotting. Angiography-guided PCI was performed with a second-generation drug-eluting stent. The choice of stenting technique (direct or non-direct) and other PCI techniques (e.g., atherectomy) was left to the discretion of operators. In all treated vessels, the angiographic objective was to achieve <30% residual stenosis, and it was desirable to achieve grade 3 thrombolysis in myocardial infarction (TIMI) flow.
In patients with STEMI, an invasive coronary physiology assessment of the infarct-related artery (IRA) was performed at the completion of the primary PCI. In patients with NSTEMI or CCS, an invasive coronary physiology assessment was performed after the successful PCI. IMR was also measured in some non-IRAs at the operators’ discretion.
IMR was obtained using the established thermodilution technique with a pressure wire (St. Jude Medical, St. Paul, MN, USA). Briefly, the pressure wire was first calibrated and equalized and then positioned distally to the target vessel. Before physiological measurements, intracoronary nitrate (100 µg) was administered to avoid spasms. Then, an intravenous administration of adenosine at a rate of 140 µg/kg/min was administered to induce steady-state hyperemia. Aortic pressure (Pa) and distal pressure (Pd) were recorded during sustained hyperemia. Meanwhile, the mean transit time (Tmn) was calculated as the average of transit time measurements at 3 injections of 3–4 mL of room-temperature saline. IMR was defined as the product of Pd and Tmn during hyperemia. After measurement, the pressure wire was withdrawn to the guiding catheter tip to exclude pressure drift, and a drift range ≤0.03 was acceptable.
AccuIMR calculation
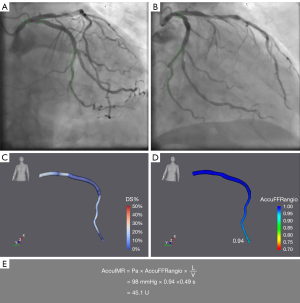
AccuIMR was computed in a blinded fashion using a dedicated software (AccuIMR, V1.0; ArteryFlow Technology, Hangzhou, China) by 2 experienced investigators. The methodology for AccuIMR has been described previously (13). Briefly, 2 angiographic projections ≥25° apart with optimal imaging quality were selected, and 3-dimensional (3D) reconstruction of the target vessel was performed, then TIMI frame count (14) analysis was performed to derive blood flow velocity for the computing of FFR value. AccuIMR was calculated as follows:
where Pa is the mean aortic pressure, AccuFFRangio is the computed FFR value, L is the length of the target vessel, and V is the mean flow velocity.
AccuIMR was derived at the same point where IMR was measured. Figure 1 summarizes the study methods.
Statistical analysis
The study was powered to achieve a diagnostic accuracy of AccuIMR that is significantly greater than 70%. It was calculated that a total of 219 vessels would provide 85% power with a 1-sided hypothesis. Quantitative variables were presented as the mean ± standard deviation (SD) or as the median (interquartile range) as appropriate. Categorical variables were presented as percentage (number). Correlation were assessed using the Pearson correlation coefficient. Bland–Altman analysis was applied to assess the agreement between AccuIMR and IMR and variability in AccuIMR computation. Diagnostic measures were calculated, including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of AccuIMR with IMR as the reference standard on a per-vessel basis. The Clopper–Pearson exact method was used to add 2-sided 95% confidence intervals (CIs) to these parameters. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated for AccuIMR in different cohorts. Comparison of AUC was performed with the DeLong method. In CCS and NSTEMI, CMD was defined by a cutoff of 25. The cutoff value of 40 was applied to IMR in STEMI. The same cutoffs were used in IRAs or non-IRAs. ROC curves were used to assess the diagnostic performance of AccuIMR in detecting abnormal IMR. A P value of <0.05 was considered statistically significant.
Results
Clinical characteristics
Clinical characteristics are presented in Table 1. A total of 163 patients with 232 vessels were included in the study, of which 61 patients had CCS, 43 had STEMI, and 59 had NSTEMI (Figure 2). AccuIMR was successfully performed in the whole population. The mean values of IMR and AccuIMR in all patients were 19.9±9.6 and 20.3±8.4 U, respectively.
Table 1
| Parameter | Value (n=163) |
|---|---|
| Demographics | |
| Age (years) | 64±11 |
| Sex, male | 58% [95] |
| Weight (kg) | 64±9 |
| Height (cm) | 163±7 |
| Systolic blood pressure (mmHg) | 134±20 |
| Diastolic blood pressure (mmHg) | 76±11 |
| LVEF (%) | 59±10 |
| Cardiovascular risk factors | |
| Diabetes | 26% [42] |
| Hypertension | 59% [96] |
| Hyperlipidemia | 32% [52] |
| Current smoker | 33% [54] |
| Previous PCI | 6% [10] |
| Previous myocardial infarction | 8% [13] |
| Clinical presentation | |
| STEMI | 26% [43] |
| NSTEMI | 36% [59] |
| CCS | 37% [61] |
| Target vessel, % (n) | |
| LAD | 55% [127] |
| LCX | 23% [54] |
| RCA | 21% [49] |
| OM | 1% [2] |
| Physiological characteristics | |
| MAP | 84±16 |
| Pre-PCI TIMI flow <3 | |
| STEMI | 24% [39] |
| NSTEMI | 15% [25] |
| CCS | 12% [20] |
| Post-PCI TIMI flow <3 | |
| STEMI | 3% [5] |
| NSTEMI | 2% [3] |
| CCS | 0% [0] |
| IMR >25 U | 21% [48] |
| IMR >40 U | 5% [12] |
Values are mean ± SD or % [n]. LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; CCS, chronic coronary syndrome; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; OM, obtuse marginal branch; MAP, mean aortic pressure; TIMI, thrombolysis in myocardial infarction; IMR, index of microcirculatory resistance.
Correlation and agreement
IMR was higher in patients with STEMI (20.3±10.3 U) compared to those with NSTEMI (19.6±10.0 U) and CCS (20.2±9.3 U). Similar results were observed in AccuIMR, which showed a higher value in patients with STEMI (20.5±9.4 U) compared with those with NSTEMI (20.2±8.8 U) and CCS (19.6±7.5 U). Although no significant differences in IMR or AccuIMR were detected among the 3 subgroups.
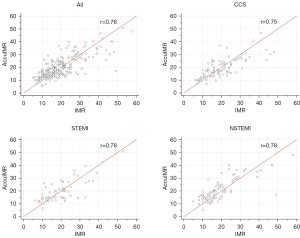
Overall, AccuIMR was significantly correlated with wire-based IMR (Pearson correlation coefficient r=0.76, P<0.001). A good correlation was maintained when focusing on different coronary syndromes (STEMI: r=0.78, P<0.001; NSTEMI: r=0.78, P<0.001; CCS: r=0.75, P<0.001), as shown in Figure 3.
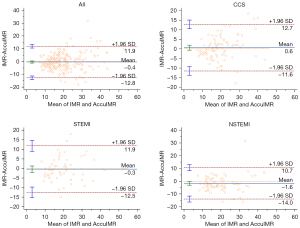
Good agreements were also found between AccuIMR and IMR, with a mean difference of –0.4±6.3 U in all patients, −0.3±6.2 U in patients with STEMI, −1.6±6.3 U in patients with NSTEMI, and 0.6±6.2 U in patients with CCS (Figure 4).
Diagnostic performance
Using a cutoff value of IMR >40 U for AccuIMR in patients with STEMI and IMR >25 U in patients with NSTEMI and CCS, the AUC of AccuIMR for predicting abnormal IMR value was 0.917 (95% CI: 0.874 to 0.949) in all patients, 1.000 (95% CI: 0.937 to 1.000) in patients with STEMI, 0.941 (95% CI: 0.867 to 0.980) in NSTEMI, and 0.918 (95% CI: 0.841 to 0.966) in CCS (Figure 5). The overall diagnostic accuracy, sensitivity, specificity, PPV, and NPV of AccuIMR were 94.83% (95% CI: 91.14% to 97.30%), 92.11% (95% CI: 78.62% to 98.34%), 95.36% (95% CI: 91.38% to 97.86%), 79.55% (95% CI: 67.11% to 88.11%), and 98.40% (95% CI: 95.41% to 99.46%), respectively. Of note, AccuIMR showed numerically higher diagnostic accuracy in patients with CCS [93.33% (95% CI: 86.05% to 97.51%)] than in patients with NSTEMI [92.94% (95% CI: 85.27% to 97.37%)], and a lower sensitivity in patients with CCS [88.89% (95% CI: 65.29% to 98.62%)] than in those with NSTEMI [94.44% (95% CI: 72.71% to 99.86%)]. As for patients with STEMI, AccuIMR showed an accuracy of 100.00% (95% CI: 93.73% to 100.00%), a sensitivity of 100.00% (95% CI: 15.81% to 100.00%), and a specificity of 100.00% (95% CI: 93.51% to 100.00%) (Table 2).
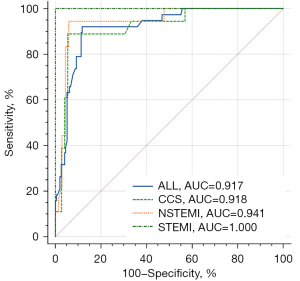
Table 2
| Diagnostic characteristic | AccuIMR, % (95% CI) | |||
|---|---|---|---|---|
| All | CCS | NSTEMI | STEMI | |
| Sensitivity | 92.11 (78.62 to 98.34) | 88.89 (65.29 to 98.62) | 94.44 (72.71 to 99.86) | 100.00 (15.81 to 100.00) |
| Specificity | 95.36 (91.38 to 97.86) | 94.44 (86.38 to 98.47) | 92.54 (83.44 to 97.53) | 100.00 (93.51 to 100.00) |
| +LR | 19.85 (10.42 to 37.83) | 16.00 (6.09 to 42.05) | 12.66 (5.41 to 29.63) | – |
| –LR | 0.08 (0.03 to 0.25) | 0.12 (0.03 to 0.44) | 0.06 (0.01 to 0.40) | 0.00 |
| PPV | 79.55 (67.11 to 88.11) | 80.00 (60.35 to 91.31) | 77.27 (59.22 to 88.84) | 100.00 |
| NPV | 98.40 (95.41 to 99.46) | 97.14 (90.19 to 99.21) | 98.41 (90.21 to 99.76) | 100.00 |
| Accuracy | 94.83 (91.14 to 97.30) | 93.33 (86.05 to 97.51) | 92.94 (85.27 to 97.37) | 100.00 (93.73 to 100.00) |
CI, confidence interval; CCS, chronic coronary syndrome; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; +LR, positive likelihood ratio; –LR, negative likelihood ratio; PPV, positive predictive value; NPV, negative predictive value; AccuIMR, angiography-based IMR.
Reproducibility and computational performance
Intraobserver and interobserver variability in AccuIMR analysis were 0.3±0.5 and 0.5±0.4, respectively. The median time for AccuIMR computation was approximately 5 minutes, including automatic lumen delineation, 3D reconstruction, and computational fluid dynamics (CFD) simulation with interprocedural interaction (if necessary) on a personal computer.
Discussion
In the present study, we evaluated the diagnostic implications of AccuIMR in 163 patients (232 vessels) with different coronary syndromes. The present study had 2 main findings. Firstly, AccuIMR is an angiography-based pressure wire-free method for the assessment of coronary microcirculation with good diagnostic accuracy in identifying abnormal IMR. Secondly, the diagnostic performance of AccuIMR maintained a high level in all patients with different coronary syndromes, including in patients with STEMI, NSTEMI, and CCS.
Substantial studies have shown that FFR could adequately assess epicardial coronary artery lesions. As the “gold standard” for decision-making or clinical management, FFR improves patients’ prognostic outcomes and significantly reduces medical costs in patients with suspected CAD (15-17). However, the epicardial segment of the coronary tree is not the only responsible part for symptoms and adverse outcomes. Microcirculation also plays an important role in patients’ symptoms and adverse events, and it should be noted that CMD cannot be readily treated by PCI (18). With the growing appreciation of microvasculature, several approaches for the assessment of microcirculation have been proposed. However, angiographic modalities have usually been restricted by their qualitative and empirical nature, and Doppler wire-based indexes have been limited by their increased technical complexity and instability. IMR, first described in 2003 (7), is a highly reproducible, readily available, quantitative method for assessing microvascular function independent of the epicardial arteries, which has been considered the “gold standard” of microcirculatory evaluation (19). Hemodynamic changes, such as heart rate, blood pressure, and contractility, have not been shown to affect IMR significantly (20). Measurement at different time points or inclusion or exclusion of Pv have not been shown to have a significant impact on the calculation of IMR (10). A high correlation has also been found in the interobserver analysis of IMR (21). IMR showed superior reproducibility and less hemodynamic dependence compared to CFR, similar to FFR.
IMR has been shown to provide information about the recovery of left ventricular function in patients with STEMI and correlate with CMR imaging (21-24). IMR can be a significant predictor of clinical outcomes, including death, rehospitalization, LVEF change, and heart failure (25,26). In nonobstructive CAD, Lee et al. (27) found that patients with CMD defined by IMR and CFR had worse outcomes than those with normal IMR and CFR. In addition, IMR can also be used in the pathway of elective PCI. Abnormal IMR has been associated with an increased risk of periprocedural myocardial infarction; therefore, measuring IMR before PCI might lead to the use of alternative strategies and reduce periprocedural outcomes (28). Both studies and guidelines have emphasized the importance of CMD in therapeutic and prognostic values (3,29). However, despite compelling evidence of the value and benefit of IMR assessment, its utility in routine clinical practice remains low due to limitations such as the need for a pressure wire, the use of hyperemic agents, the longer procedural time, and the higher cost.
In order to overcome these barriers, a CFD-based calculation of IMR derived from coronary angiography has been proposed (13). De Maria et al. (30) reported a novel approach for the computation of angiography-based IMR (IMRangio) in 45 patients with STEMI, demonstrating a good correlation with invasive IMR measurement. Tebaldi et al. (31) demonstrated an angio-based IMR method (A-IMR) and validated it in 44 patients with CCS, with a sensitivity and specificity of 70.0% and 83.3%, respectively. Another angio-derived IMR technique (caIMR) showed diagnostic accuracy, sensitivity, and specificity of 84.2%, 86.1%, and 81.0%, respectively, in 56 patients with no obstructive coronary arteries (32). Mejia-Renteria et al. (33) compared their angio-IMR with wire-based IMR in 104 patients, which resulted in a sensitivity, specificity, and accuracy of 87.5%, 85.3%, and 85.0%, respectively. The OxAMI cohort study (34) involving non-hyperemic IMRangio (NH IMRangio) showed a good diagnostic performance in identifying IMR >40 U (sensitivity, 77%; specificity, 67%; diagnostic accuracy, 70%), and the NH IMRangio was found to be significantly associated with a higher risk of adverse events in patients with STEMI. However, the validations of recent studies about angio-derived IMR have been limited to a relatively small population of patients or only focused on one specific cohort. In this study, we have extended these findings and demonstrated that angio-based IMR (AccuIMR) can accurately predict CMD in patients with different coronary syndromes, including STEMI, NSTEMI, and CCS.
The fundamental difference between AccuIMR and the above-mentioned angio-IMR methods may lie in the boundary conditions. The patient-specific mean aortic pressure and blood flow rate derived from hyperemic angiographic data were used for the computation of AccuIMR, which could lead to fewer discrepancies with the measured IMR. For example, the A-IMR used the cQFR in the calculation, which involved conversion from baseline flow to hyperemic flow by an empirical function. The empirical function was derived from patients without CMD; thus, it might not be suitable for patients with microvascular dysfunction. Direct information from hyperemic data could reveal the specific influence of CMD on blood flow. AccuIMR showed good diagnostic performance across the spectrum of coronary syndromes, with a sensitivity, specificity, and accuracy in predicting abnormal IMR of 100.00%, 100.00%, and 100.00%, respectively, in patients with STEMI, 94.44%, 92.54%, and 92.94, respectively, in patients with in NSTEMI, and 88.89%, 94.44%, 93.33%, respectively, in patients with CCS. When considering all vessels, the correlation between IMR and AccuIMR was also good (r=0.76 in all patients; r=0.78 in patients with STEMI; r=0.78 in patients with NSTEMI; r=0.75 in patients with CCS). Notably, AccuIMR showed slightly better agreement with IMR in CCS than that of STEMI and NSTEMI. This could be the result of the correlation between the severity of CMD and acute coronary syndrome (ACS). Microvascular obstruction (MVO) is more likely to occur in patients with ACS, which could lead to very high IMR values. Similarly, it has been reported that CMD could affect the agreement between FFR and angio-based FFR (35). Nevertheless, the agreement and correlation between AccuIMR and IMR remained strong in all patients when using standard cutoff values (IMR >40 U in patients with STEMI; IMR >25 U in patients with NSTEMI and CCS).
It is noteworthy that the optimal IMR threshold has not been determined in patients with STEMI. An IMR >40 U has been reported to be associated with all-cause death or rehospitalization for heart failure at 1 year (25), with all-cause death or heart failure readmissions at 2 years (26), and with post-STEMI major complications at 30 days (36). This has become the most accepted threshold for patients with STEMI. However, more than one-third of patients with STEMI showed discordance between an IMR >40 U and MVO defined by CMR (37). Fearon et al. (38) reported that an IMR ≤32 U could predict recovery of left ventricular function. Lim et al. (39) showed that an IMR ≤33 U optimally correlated with left ventricular wall motion recovery at 6 months. A post-PCI IMR >27 U was most closely associated with MVO (22,40). De Maria et al. (37) demonstrated that patients with an IMR >40 U had no regression in infarct size at follow-up, whereas those with an IMR ≤40 U showed significant regression in infarct size. Although an IMR >40 U could reliably predict major adverse events, it might overlook patients with an IMR ≤40 U who are highly likely to benefit from adjunctive therapy. In this study, AccuIMR showed good diagnostic performance in patients with STEMI with a cutoff of 40 U. The optimal cutoff value to assess the long-term prognosis needs to be further investigated in dedicated studies.
Importantly, a dedicated assessment of coronary microvasculature in the pathway can be an effective tool to reduce symptoms and increase treatment satisfaction and lead to a better quality of life (20). IMR can help medical staff to determine whether microcirculation is the leading cause of symptoms (41), predict periprocedural events for planned PCI (42), and guide adjunctive therapy (29). This study demonstrated that AccuIMR derived from angiography could be a valid and feasible alternative measure to wire-based IMR. Although the potential of AccuIMR has not yet been fully evaluated, the study suggests that this approach can play a role in the therapeutic pathway in the catheterization laboratory.
The main limitation of this study is the relatively small cohort size, which limits the interpretation and conclusions that can be drawn. Secondly, the cutoff value in patients with NSTEMI has not been defined in previous studies, and we simply applied the same cutoff used for CCS. Thirdly, IMR is a well-established index to identify CMD in patients with STEMI, yet its value in patients with NSTEMI and CCS has not been adequately validated. Thus, the ability of AccuIMR in NSTEMI and CCS needs to be confirmed by further studies. A future large-scale, prospective study assessing the prognostic value of AccuIMR is warranted.
Conclusions
AccuIMR is a pressure wire-free alternative to IMR, offering an easy-to-use, reliable, and time-efficient way to assess coronary microvascular disease in patients with both CCS and ACS. Although further prospective investigation is needed to assess its prognostic value, AccuIMR holds the potential to play a crucial role in risk stratification and patient management during routine clinical practice.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (No. 82070425 to Zhibing Lu), the Natural Science Foundation of Hubei Province of China (No. 2021CFA011 to Zhibing Lu), the Chinese Cardiovascular Association-V.G. Fund (No. 2017-CCA-VG-006 to Chenguang Li), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2020-JKCS-003 to Chenguang Li), and Hangzhou Leading Innovation and Entrepreneurship Team Project (No. TD2022007 to Jianping Xiang).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-961/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-961/coif). ZL reports grants from the National Natural Science Foundation of China (No. 82070425) and the Natural Science Foundation of Hubei Province of China (No. 2021CFA011). CL reports grants from the Chinese Cardiovascular Association-V.G. Fund (No. 2017-CCA-VG-006) and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2020-JKCS-003). YH and JH are employees of ArteryFlow. XL is a co-founder of ArteryFlow. JX is the CEO of ArteryFlow and receives a grant from Hangzhou Leading Innovation and Entrepreneurship Team Project (No. TD2022007). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice Guidelines. The study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University, and the requirement for individual consent for this retrospective study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaski JC, Crea F, Gersh BJ, Camici PG. Reappraisal of Ischemic Heart Disease. Circulation 2018;138:1463-80. [Crossref] [PubMed]
- Ford TJ, Ong P, Sechtem U, Beltrame J, Camici PG, Crea F, Kaski JC, Bairey Merz CN, Pepine CJ, Shimokawa H, Berry CCOVADIS Study Group. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease: Why, How, and When. JACC Cardiovasc Interv 2020;13:1847-64. [Crossref] [PubMed]
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407-77. [Crossref] [PubMed]
- Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas AHEM, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J 2020;41:3504-20. [Crossref] [PubMed]
- Lanza GA. Diagnostic Approach to Patients with Stable Angina and No Obstructive Coronary Arteries. Eur Cardiol 2019;14:97-102. [Crossref] [PubMed]
- Tebaldi M, Biscaglia S, Pecoraro A, Fineschi M, Campo G. Fractional flow reserve implementation in daily clinical practice: A European survey. Int J Cardiol 2016;207:206-7. [Crossref] [PubMed]
- Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation 2003;107:3129-32. [Crossref] [PubMed]
- Chamuleau SA, Siebes M, Meuwissen M, Koch KT, Spaan JA, Piek JJ. Association between coronary lesion severity and distal microvascular resistance in patients with coronary artery disease. Am J Physiol Heart Circ Physiol 2003;285:H2194-200. [Crossref] [PubMed]
- Fearon WF, Aarnoudse W, Pijls NH, De Bruyne B, Balsam LB, Cooke DT, Robbins RC, Fitzgerald PJ, Yeung AC, Yock PG. Microvascular resistance is not influenced by epicardial coronary artery stenosis severity: experimental validation. Circulation 2004;109:2269-72. [Crossref] [PubMed]
- Pagonas N, Gross CM, Li M, Bondke A, Klauss V, Buschmann EE. Influence of epicardial stenosis severity and central venous pressure on the index of microcirculatory resistance in a follow-up study. EuroIntervention 2014;9:1063-8. [Crossref] [PubMed]
- Li C, Leng X, He J, Xia Y, Jiang W, Pan Y, Dong L, Sun Y, Hu X, Wang J, Xiang J, Jiang J. Diagnostic Performance of Angiography-Based Fractional Flow Reserve for Functional Evaluation of Coronary Artery Stenosis. Front Cardiovasc Med 2021;8:714077. [Crossref] [PubMed]
- Jiang J, Tang L, Du C, Leng X, He J, Hu Y, Dong L, Sun Y, Li C, Xiang J, Wang J. Diagnostic performance of AccuFFRangio in the functional assessment of coronary stenosis compared with pressure wire-derived fractional flow reserve. Quant Imaging Med Surg 2022;12:949-58. [Crossref] [PubMed]
- Jiang J, Li C, Hu Y, Li C, He J, Leng X, Xiang J, Ge J, Wang J. A novel CFD-based computed index of microcirculatory resistance (IMR) derived from coronary angiography to assess coronary microcirculation. Comput Methods Programs Biomed 2022;221:106897. [Crossref] [PubMed]
- Gibson CM, Cannon CP, Daley WL, Dodge JT Jr, Alexander B Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation 1996;93:879-88. [Crossref] [PubMed]
- Fearon WF, Bornschein B, Tonino PA, Gothe RM, Bruyne BD, Pijls NH, Siebert UFractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) Study Investigators. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation 2010;122:2545-50. [Crossref] [PubMed]
- Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F. van' t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213-24. [Crossref] [PubMed]
- Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijns W. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 2001;103:2928-34. [Crossref] [PubMed]
- Fearon WF, Kobayashi Y. Invasive Assessment of the Coronary Microvasculature: The Index of Microcirculatory Resistance. Circ Cardiovasc Interv 2017;10:e005361. [Crossref] [PubMed]
- Li W, Takahashi T, Rios SA, Latib A, Lee JM, Fearon WF, Kobayashi Y. Diagnostic performance and prognostic impact of coronary angiography-based Index of Microcirculatory Resistance assessment: A systematic review and meta-analysis. Catheter Cardiovasc Interv 2022;99:286-92. [Crossref] [PubMed]
- Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation 2006;113:2054-61. [Crossref] [PubMed]
- Payne AR, Berry C, Doolin O, McEntegart M, Petrie MC, Lindsay MM, Hood S, Carrick D, Tzemos N, Weale P, McComb C, Foster J, Ford I, Oldroyd KG. Microvascular Resistance Predicts Myocardial Salvage and Infarct Characteristics in ST-Elevation Myocardial Infarction. J Am Heart Assoc 2012;1:e002246. [Crossref] [PubMed]
- Ahn SG, Hung OY, Lee JW, Lee JH, Youn YJ, Ahn MS, Kim JY, Yoo BS, Lee SH, Yoon J, Kwon W, Samady H. Combination of the Thermodilution-Derived Index of Microcirculatory Resistance and Coronary Flow Reserve Is Highly Predictive of Microvascular Obstruction on Cardiac Magnetic Resonance Imaging After ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv 2016;9:793-801. [Crossref] [PubMed]
- Patel N, Petraco R, Dall'Armellina E, Kassimis G, De Maria GL, Dawkins S, Lee R, Prendergast BD, Choudhury RP, Forfar JC, Channon KM, Davies J, Banning AP, Kharbanda RK. Zero-Flow Pressure Measured Immediately After Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction Provides the Best Invasive Index for Predicting the Extent of Myocardial Infarction at 6 Months: An OxAMI Study (Oxford Acute Myocardial Infarction). JACC Cardiovasc Interv 2015;8:1410-21. [Crossref] [PubMed]
- Cuculi F, De Maria GL, Meier P, Dall'Armellina E, de Caterina AR, Channon KM, Prendergast BD, Choudhury RP, Forfar JC, Kharbanda RK, Banning AP. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J Am Coll Cardiol 2014;64:1894-904. [Crossref] [PubMed]
- Fearon WF, Low AF, Yong AS, McGeoch R, Berry C, Shah MG, Ho MY, Kim HS, Loh JP, Oldroyd KG. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation 2013;127:2436-41. [Crossref] [PubMed]
- Carrick D, Haig C, Ahmed N, Carberry J, Yue May VT, McEntegart M, Petrie MC, Eteiba H, Lindsay M, Hood S, Watkins S, Davie A, Mahrous A, Mordi I, Ford I, Radjenovic A, Oldroyd KG, Berry C. Comparative Prognostic Utility of Indexes of Microvascular Function Alone or in Combination in Patients With an Acute ST-Segment-Elevation Myocardial Infarction. Circulation 2016;134:1833-47. [Crossref] [PubMed]
- Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J Am Coll Cardiol 2016;67:1158-69. [Crossref] [PubMed]
- Ng MK, Yong AS, Ho M, Shah MG, Chawantanpipat C, O'Connell R, Keech A, Kritharides L, Fearon WF. The index of microcirculatory resistance predicts myocardial infarction related to percutaneous coronary intervention. Circ Cardiovasc Interv 2012;5:515-22. [Crossref] [PubMed]
- Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol 2018;72:2841-55. [Crossref] [PubMed]
- De Maria GL, Scarsini R, Shanmuganathan M, Kotronias RA, Terentes-Printzios D, Borlotti A, Langrish JP, Lucking AJ, Choudhury RP, Kharbanda R, Ferreira VM, Channon KM, Garcia-Garcia HM, Banning AP. Angiography-derived index of microcirculatory resistance as a novel, pressure-wire-free tool to assess coronary microcirculation in ST elevation myocardial infarction. Int J Cardiovasc Imaging 2020;36:1395-406. [Crossref] [PubMed]
- Tebaldi M, Biscaglia S, Di Girolamo D, Erriquez A, Penzo C, Tumscitz C, Campo G. Angio-Based Index of Microcirculatory Resistance for the Assessment of the Coronary Resistance: A Proof of Concept Study. J Interv Cardiol 2020;2020:8887369. [Crossref] [PubMed]
- Ai H, Feng Y, Gong Y, Zheng B, Jin Q, Zhang HP, Sun F, Li J, Chen Y, Huo Y, Huo Y. Coronary Angiography-Derived Index of Microvascular Resistance. Front Physiol 2020;11:605356. [Crossref] [PubMed]
- Mejia-Renteria H, Lee JM, Choi KH, Lee SH, Wang L, Kakuta T, Koo BK, Escaned J. Coronary microcirculation assessment using functional angiography: Development of a wire-free method applicable to conventional coronary angiograms. Catheter Cardiovasc Interv 2021;98:1027-37. [Crossref] [PubMed]
- Kotronias RA, Terentes-Printzios D, Shanmuganathan M, Marin F, Scarsini R, Bradley-Watson J, Langrish JP, Lucking AJ, Choudhury R, Kharbanda RK, Garcia-Garcia HM, Channon KM, Banning AP, De Maria GL. Long-Term Clinical Outcomes in Patients With an Acute ST-Segment-Elevation Myocardial Infarction Stratified by Angiography-Derived Index of Microcirculatory Resistance. Front Cardiovasc Med 2021;8:717114. [Crossref] [PubMed]
- Mejía-Rentería H, Lee JM, Lauri F, van der Hoeven NW, de Waard GA, Macaya F, et al. Influence of Microcirculatory Dysfunction on Angiography-Based Functional Assessment of Coronary Stenoses. JACC Cardiovasc Interv 2018;11:741-53. [Crossref] [PubMed]
- Fahrni G, Wolfrum M, De Maria GL, Cuculi F, Dawkins S, Alkhalil M, Patel N, Forfar JC, Prendergast BD, Choudhury RP, Channon KM, Banning AP, Kharbanda RK. Index of Microcirculatory Resistance at the Time of Primary Percutaneous Coronary Intervention Predicts Early Cardiac Complications: Insights From the OxAMI (Oxford Study in Acute Myocardial Infarction) Cohort. J Am Heart Assoc 2017.
- De Maria GL, Alkhalil M, Wolfrum M, Fahrni G, Borlotti A, Gaughran L, Dawkins S, Langrish JP, Lucking AJ, Choudhury RP, Porto I, Crea F, Dall'Armellina E, Channon KM, Kharbanda RK, Banning AP. Index of Microcirculatory Resistance as a Tool to Characterize Microvascular Obstruction and to Predict Infarct Size Regression in Patients With STEMI Undergoing Primary PCI. JACC Cardiovasc Imaging 2019;12:837-48. [Crossref] [PubMed]
- Fearon WF, Shah M, Ng M, Brinton T, Wilson A, Tremmel JA, Schnittger I, Lee DP, Vagelos RH, Fitzgerald PJ, Yock PG, Yeung AC. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2008;51:560-5. [Crossref] [PubMed]
- Lim HS, Yoon MH, Tahk SJ, Yang HM, Choi BJ, Choi SY, Sheen SS, Hwang GS, Kang SJ, Shin JH. Usefulness of the index of microcirculatory resistance for invasively assessing myocardial viability immediately after primary angioplasty for anterior myocardial infarction. Eur Heart J 2009;30:2854-60. [Crossref] [PubMed]
- Carrick D, Haig C, Carberry J, May VTY, McCartney P, Welsh P, et al. Microvascular resistance of the culprit coronary artery in acute ST-elevation myocardial infarction. JCI Insight 2016;1:e85768. [Crossref] [PubMed]
- Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, Bolger AF, Beltrame JFAmerican Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology. Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation 2019;139:e891-908. [PubMed]
- Martínez GJ, Yong AS, Fearon WF, Ng MK. The index of microcirculatory resistance in the physiologic assessment of the coronary microcirculation. Coron Artery Dis 2015;26:e15-26. [Crossref] [PubMed]


