
Schmorl’s node of primarily developmental cause and Schmorl’s node of primarily acquired cause: two related yet different entities
According to the original definition, Schmorl’s node (SN) corresponds histologically to nucleus pulposus herniation into the vertebral spongy bone with thickened trabeculae around the formed node (1,2). Very conflicting literature on the epidemiology of SN have been reported. Several earlier reports noted that, except for the early childhood period, SN prevalence among age groups remains stable (3-6). Dar et al. (4) studied skeleton vertebrae from a normal adult population and noted that SN was more common in men than in women, but SN was age independent. Hilton et al. (5) found no relationship between age and SN with their study of cadaveric spines and thus proposed a developmental pathogenesis for SN. Chen et al. (7) reported that aging was related to greater odds of endplate lesions; however, the prevalence of focal endplate defects remained stable. Sonne-Holm et al. (6) analysed lateral spine radiographs in an adult Caucasian population cohort and did not note any significant correlation between SN and gender, or age. On the other hand, some authors reported the association of SN with age. Wang et al. (8) reported that greater age was associated with the presence of SN among a male cadaver collection. A male predominance of SN had been frequently reported (5,9-11). Üstündağ (11) studied SN in a post-medieval skeletal sample and noted that males were more affected than females, and there was no relationship found between SN and aging. However, a few studies did not observe an association of SN with gender (6,12,13). The correlation between osteoporosis and SN also remains controversial (14-16). In a study on the cadavers of pre-Hispanic inhabitants González-Reimers et al. (14) did not find a relationship between osteoporosis and SN. On the other hand, other evidence suggests osteoporosis is a cause of SN. Mäkitie et al. (15) reported a high prevalence (61%) of SN in their case-control study of 18 patients diagnosed with WNT mutation-induced osteoporosis. Based on a CT study, Güngör et al. (16) suggested that low bone mineral density may be a predisposing factor for the development of SN in patients younger than 40 years. Recently we (17) described a study of thoracic spine MR imaging among community elderly subjects (mean age: 82 years) and noted a number of features of SN paralleled those of osteoporotic vertebral fracture (OVF). SN prevalence in women (55.5%) almost doubled that in men (25.9%). SN was statistically significantly correlated with lower bone mineral density, and subjects with SN were more likely to have OVF. In vertebrae with osteoporosis, the endplate becomes weakened due to the loss of support from trabecular bone and due to thinning of the endplate itself (18), thus this pathway may exist that: osteoporosis → weakened endplate → SN development → osteoporotic endplate fracture (17).
To make sense of these conflicting literature, we suggest that SNs should be classified into two categories: SN of primarily developmental cause (SNd, Figure 1) and SN of primarily acquired cause (SNa, Figure 2). Note that, SN can be considered as a ‘general phenomenon’ (rather than a specific disease entity) where a portion of disc materials herniated through endplate into the vertebral spongy bone, and the surrounding sclerosis in vertebra reflects reactive healing. An analogy can be made to congenital spondylolisthesis, traumatic spondylolisthesis, and degenerative spondylolisthesis (20).
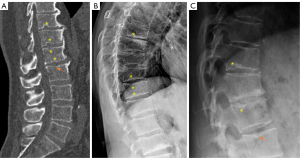
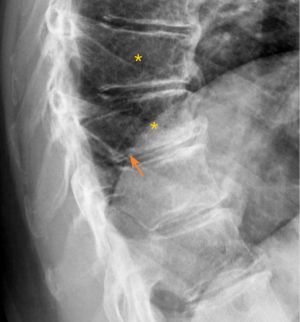
SNd is characterized by that they more likely involve multiple adjacent vertebrae, more likely to be small or modest in size, they likely to have relatively consistent location and more likely involve a posterior portion of the lower endplate (though other locations are also common). Moreover, compared with SNa, SNd tend to have a more solid border on radiograph due to their longstanding and sometimes static nature. It is also possible some SNd and cupid bow may belong to the same spectrum of developmental changes (Figure 3). Note that Pfirrmann and Resnick described cases showing ‘the transition of Schmorl nodes to a cupid’s bow contour’ (21). SNd are known to be a common but not obligate manifestation of Scheuermann disease. SN associated with Scheuermann disease shall belong to SNd. It is also possible that some very ‘tiny’ SNd may not have clinical relevance (Figure 4), and some SNd may not have a true disc material herniation process. Heritability of SN has also been demonstrated (15,22,23). Though the upper endplate is less resistant to compressive pressure and more likely to fracture (24), in a female twin volunteer study (mean: 53 years), for their study subjects Williams et al. (22) noted that SNs (assumed largely SNd) were more prevalent in lower endplate than in upper endplate.
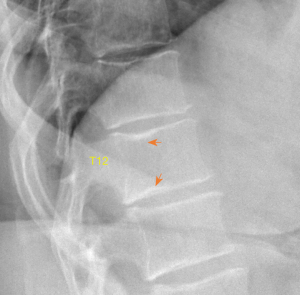
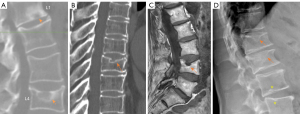
A vertebral endplate consists of perforated cortical bone with a layer of hyaline cartilage bonded to its disc surface. The cortical bone layer contains a network of small cavities which allow bone marrow to lie adjacent to calcified hyaline cartilage for approximately 10% of the central endplate area, which is an important route for metabolite transport into the discs. The nutritional demands of the discs result in that vertebral central endplates are thin and porous, and which can be subject to fracture under stress force even if bone strength is normal. For the pathogenesis of SNa, whatever the cause of the damage to the cartilaginous endplate, to the subchondral bone of the vertebral body, or to both structures, a weakened area is created that is unable to resist the expansive pressure of the adjacent nucleus pulposus. SNa are more likely to be modest or large in size, and their borders are not always clearly defined on radiograph. Trauma and endplate micro-fractures are triggers for SN (25-29). Dar et al. (25) proposed an axial load model which suggests that the human spine must accommodate increased axial forces in addition to balancing the need for spinal mobility and stability, and it may accumulate micro-traumas that can, over time, lead to the formation of SN. In a cohort of children who had suffered from stable compressive vertebral fractures, Möller et al. (26) reported the occurrence of SN at advanced ages (40 years) at adjacent disc levels. In a study of 70 thoracolumbar spines from cadavers of individuals killed in motor vehicle collisions, Fahey et al. (27) reported a link between trauma and the occurrence of SN. Swärd et al. (28) compared vertebral abnormalities in elite gymnasts versus non-athletes, they found SNs in 17 out of 24 (71%) gymnasts with nodes in 57 endplates and in 7 out of 17 (44%) non-athletes with nodes in 23 endplates. Certain pre-existing conditions can facilitate herniation occurs due to axial forces. The theory of weak spot presence within vertebral endplate has been considered. The possible endplate weakness can be due notochord regression, ossification gaps, or vascular channels (2,30,31). The vertebral level distribution of SNa will be similar to traumatic vertebral fracture (high energy trauma) or OVF (low energy trauma) (19,32). SNa less likely involve posterior portion of an endplate which is not a weak point of biomechanics. Among elderly subjects, SNa more likely involve upper endplate which is the same as osteoporotic endplate fracture (24), and SNa are commonly associated with endplate depression. Osteopenic/osteoporotic SN may be a precursor of OVF, a specific type of endplate fracture, or a co-phenomenon for advanced OVF (Figure 5). Tumorous changes of a vertebra can also increase the fragility of an endplate, and lead to disc materials herniation through endplate into the vertebral spongy bone and form SNa (33,34). In these cases, the surrounding sclerosis may not necessarily develop when the SNa is detected.
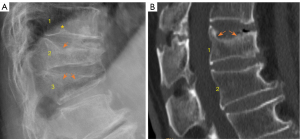
Some earlier authors already discussed SN sub-classifications. Hansson and Roos (35) classified the SNs situated just above or below the nucleus pulposus, symmetrically on both sides of the nucleus with well-rounded smooth bone surroundings as type A nodes. The SNs which are situated asymmetrically in relation to the nucleus and/or surrounded by rough often sclerotic bone and were classified into type B. However, according to our reading of the literature, how our classification can correspond to the classification of Hansson and Roos remains unclear. On the other hand, Hansson and Roos did note that some SNs were associated with lower vertebral bone strength while others were not.
We advocate that, for future studies, SNd and SNa should be separately described as much as possible, as SNd and SNa may have different clinical significance. Small SNd may not have clinical relevance, and some SNd may be well covered with the endplate. SNa are usually associated with endplate fracture, and some SNa may be an indicator of compromised vertebral bone strength (17). On the other hand, the physiopathology of SN formation can be multifactorial; and for some SNs, a definite separation of SNd and SNa may not always be possible. More research is required to elucidate the classification of SNs and their relationship to developmental causes or degenerative causes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-252/coif). YXJW serves as the Editor-in-Chief of Quantitative Imaging in Medicine and Surgery. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schmorl G. Uber die an den wirbelbandscheiben vorkommenden ausdehnungs–und zerreisungsvorgange und die dadurch an ihnen und der wirbelspongiosa hervorgerufenen veranderungen. Verh Dtsch Path Ges 1927;22:250.
- Azzouzi H, Ichchou L. Schmorl's nodes: demystification road of endplate defects-a critical review. Spine Deform 2022;10:489-99. [Crossref] [PubMed]
- Mok FP, Samartzis D, Karppinen J, Luk KD, Fong DY, Cheung KM. ISSLS prize winner: prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: a population-based study of 2449 individuals. Spine (Phila Pa 1976) 2010;35:1944-52. [Crossref] [PubMed]
- Dar G, Peleg S, Masharawi Y, Steinberg N, May H, Hershkovitz I. Demographical aspects of Schmorl nodes: a skeletal study. Spine (Phila Pa 1976) 2009;34:E312-5. [Crossref] [PubMed]
- Hilton RC, Ball J, Benn RT. Vertebral end-plate lesions (Schmorl's nodes) in the dorsolumbar spine. Ann Rheum Dis 1976;35:127-32. [Crossref] [PubMed]
- Sonne-Holm S, Jacobsen S, Rovsing H, Monrad H. The epidemiology of Schmorl's nodes and their correlation to radiographic degeneration in 4,151 subjects. Eur Spine J 2013;22:1907-12. [Crossref] [PubMed]
- Chen L, Battié MC, Yuan Y, Yang G, Chen Z, Wang Y. Lumbar vertebral endplate defects on magnetic resonance images: prevalence, distribution patterns, and associations with back pain. Spine J 2020;20:352-60. [Crossref] [PubMed]
- Wang Y, Videman T, Battié MC. Lumbar vertebral endplate lesions: prevalence, classification, and association with age. Spine (Phila Pa 1976) 2012;37:1432-9. [Crossref] [PubMed]
- Yin R, Lord EL, Cohen JR, Buser Z, Lao L, Zhong G, Wang JC. Distribution of Schmorl nodes in the lumbar spine and their relationship with lumbar disk degeneration and range of motion. Spine (Phila Pa 1976) 2015;40:E49-53. [Crossref] [PubMed]
- Brayda-Bruno M, Albano D, Cannella G, Galbusera F, Zerbi A. Endplate lesions in the lumbar spine: a novel MRI-based classification scheme and epidemiology in low back pain patients. Eur Spine J 2018;27:2854-61. [Crossref] [PubMed]
- Üstündağ H. Schmorl’s Nodes in a Post-Medieval Skeletal Sample from Klostermarienberg, Austria Int J Osteoarchaeol 2009;19:695-710. [Crossref]
- Moustarhfir M, Bresson B, Koch P, Perozziello A, Barreau G, Schouman-Claeys E, Henry-Feugeas MC, Ou P, Dallaudière B. MR imaging of Schmorl's nodes: Imaging characteristics and epidemio-clinical relationships. Diagn Interv Imaging 2016;97:411-7. [Crossref] [PubMed]
- Zehra U, Cheung JPY, Bow C, Lu W, Samartzis D. Multidimensional vertebral endplate defects are associated with disc degeneration, modic changes, facet joint abnormalities, and pain. J Orthop Res 2019;37:1080-9. [Crossref] [PubMed]
- González-Reimers E, Mas-Pascual M, Arnay-De-La-Rosa M, Velasco-Vázquez J, Santolaria-Fernández F. Schmorl nodes: lack of relationship between degenerative changes and osteopenia. Radiology 2002;222:293-4. [Crossref] [PubMed]
- Mäkitie RE, Niinimäki T, Nieminen MT, Schalin-Jäntti C, Niinimäki J, Mäkitie O. Impaired WNT signaling and the spine-Heterozygous WNT1 mutation causes severe age-related spinal pathology. Bone 2017;101:3-9. [Crossref] [PubMed]
- Güngör Ö, Gezer NS, Özdamarlar U, Balcı A. The effect of bone mineral density on development of Schmorl's nodes in young patients. Acta Orthop Traumatol Turc 2020;54:287-92. [Crossref] [PubMed]
- Wáng YXJ, Wang XR, Leung JCS, Yu BWM, Griffith JF, Kwok TCY. Schmorl's nodes are associated with prevalent osteoporotic vertebral fracture and low bone mineral density: a population-based thoracic spine MRI study in older men and women. Quant Imaging Med Surg 2023;13:1914-26. [Crossref] [PubMed]
- Wang YX, Griffith JF. Menopause causes vertebral endplate degeneration and decrease in nutrient diffusion to the intervertebral discs. Med Hypotheses 2011;77:18-20. [Crossref] [PubMed]
- Wáng YXJ. A summary of our recent evidence-based works on radiographic diagnostics of prevalent osteoporotic vertebral fracture in older men and women. Quant Imaging Med Surg 2023;13:1264-85. [Crossref] [PubMed]
- Wang YXJ, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Translat 2016;11:39-52. [Crossref] [PubMed]
- Pfirrmann CW, Resnick D. Schmorl nodes of the thoracic and lumbar spine: radiographic-pathologic study of prevalence, characterization, and correlation with degenerative changes of 1,650 spinal levels in 100 cadavers. Radiology 2001;219:368-74. [Crossref] [PubMed]
- Williams FM, Manek NJ, Sambrook PN, Spector TD, Macgregor AJ. Schmorl's nodes: common, highly heritable, and related to lumbar disc disease. Arthritis Rheum 2007;57:855-60. [Crossref] [PubMed]
- Rajasekaran S, Kanna RM, Reddy RR, Natesan S, Raveendran M, Cheung KMC, Chan D, Kao PYP, Yee A, Shetty AP. How Reliable Are the Reported Genetic Associations in Disc Degeneration?: The Influence of Phenotypes, Age, Population Size, and Inclusion Sequence in 809 Patients. Spine (Phila Pa 1976) 2016;41:1649-60. [Crossref] [PubMed]
- Che-Nordin N, Deng M, Griffith JF, Leung JCS, Kwok AWL, Zhu YQ, So RHY, Kwok TCY, Leung PC, Wáng YXJ. Prevalent osteoporotic vertebral fractures more likely involve the upper endplate than the lower endplate and even more so in males. Ann Transl Med 2018;6:442. [Crossref] [PubMed]
- Dar G, Masharawi Y, Peleg S, Steinberg N, May H, Medlej B, Peled N, Hershkovitz I. Schmorl's nodes distribution in the human spine and its possible etiology. Eur Spine J 2010;19:670-5. [Crossref] [PubMed]
- Möller A, Maly P, Besjakov J, Hasserius R, Ohlin A, Karlsson MK. A vertebral fracture in childhood is not a risk factor for disc degeneration but for Schmorl's nodes: a mean 40-year observational study. Spine (Phila Pa 1976) 2007;32:2487-92. [Crossref] [PubMed]
- Fahey V, Opeskin K, Silberstein M, Anderson R, Briggs C. The pathogenesis of Schmorl's nodes in relation to acute trauma. An autopsy study. Spine (Phila Pa 1976) 1998;23:2272-5. [Crossref] [PubMed]
- Swärd L, Hellström M, Jacobsson B, Nyman R, Peterson L. Disc degeneration and associated abnormalities of the spine in elite gymnasts. A magnetic resonance imaging study. Spine (Phila Pa 1976) 1991;16:437-43. [Crossref] [PubMed]
- Dimar JR 2nd, Nathan ST, Glassman SD. The spectrum of traumatic Schmorl's nodes: identification and treatment options in 3 patients. Am J Orthop (Belle Mead NJ) 2012;41:427-31. [PubMed]
- Resnick D, Niwayama G. Intravertebral disk herniations: cartilaginous (Schmorl's) nodes. Radiology 1978;126:57-65. [Crossref] [PubMed]
- Chandraraj S, Briggs CA, Opeskin K. Disc herniations in the young and end-plate vascularity. Clin Anat 1998;11:171-6. [Crossref] [PubMed]
- Wáng YXJ, Wang XR, Che-Nordin N, Xu FR, Huang QL. On the possibility of over-diagnosis of osteoporotic vertebral fracture at mid-thoracic level. J Thorac Dis 2019;11:5708-11. [Crossref] [PubMed]
- Grivé E, Rovira A, Capellades J, Rivas A, Pedraza S. Radiologic findings in two cases of acute Schmörl's nodes. AJNR Am J Neuroradiol 1999;20:1717-21. [PubMed]
- Yamaguchi T, Suzuki S, Ishiiwa H, Yamato M, Ueda Y. Schmorl's node developing in the lumbar vertebra affected with metastatic carcinoma: correlation magnetic resonance imaging with histological findings. Spine (Phila Pa 1976) 2003;28:E503-5. [Crossref] [PubMed]
- Hansson T, Roos B. The amount of bone mineral and Schmorl's nodes in lumbar vertebrae. Spine (Phila Pa 1976) 1983;8:266-71. [Crossref] [PubMed]


