Value of spectral computed tomography-derived quantitative parameters based on full volume analysis in the diagnosis of benign/malignant and pathological subtypes of solitary pulmonary nodules
Introduction
Lung cancer (LC) is the leading cause of cancer-related deaths, with increasing morbidity and mortality worldwide (1,2). As many early-stage LCs are asymptomatic, the greatest opportunity for cure is often missed, and LCs are typically detected at an advanced stage. Early-stage LC manifests predominantly as a solitary pulmonary nodule (SPN), which can be surgically removed to improve survival (3). SPN is defined as a round or oval shadow with a diameter ≤3 cm that is completely surrounded by the pulmonary parenchyma and without other abnormalities (4-6). Previous studies have reported that the prevalence of malignant tumors in patients with SPN ranges from 10% to 70% (7-9). Low-dose computed tomography (CT) is the best screening method for LC and can reduce the death rate from LC by 20% (10). Nevertheless, the qualitative diagnosis of SPNs remains an ongoing diagnostic challenge. Therefore, timely and reliable differentiation of SPNs is crucial for guiding treatment plans and prognosis of patients.
Conventional CT plays an important role in evaluating the imaging signs and enhancement features of SPN, but some benign and malignant SPN have highly similar lesion morphology and enhancement patterns, so it remains a great challenge to distinguish between benign and malignant SPNs (11,12). Spectral CT recently emerged as a considerable advance in the field of CT. Spectral CT imaging is based on multisubstance decomposition algorithm techniques, which can extract the iodine concentration (IC), slope of the spectral decay curve, and effective atomic number (Zeff), to reflect information on tumor angiogenesis and microenvironment. This approach is being increasingly used clinically and has high diagnostic accuracy for many organ diseases (13). Several studies have demonstrated that spectral CT has potential value for distinguishing benign and malignant lung lesions, tissue subtypes, and lymphatic metastases (14-18). However, most previous studies (14-18) have obtained the various parameters from regions of interest (ROIs) drawn manually on the largest lesion slices. This may lead to measurement sampling bias, which is often the only parameter used and cannot reflect information on the tissue characteristics of the entire lesion and, therefore, cannot adequately assess intralesional heterogeneity. A recent radiology study reported that nodule volumes obtained using a semiautomated CT volume method showed greater interobserver agreement, less variability, and improved reproducibility of quantitative analysis compared to manual or semiautomated nodule diameters (19).
To the best of our knowledge, the role of spectral CT-derived multiparameter quantification combined with full-volume analysis for distinguishing SPNs is yet to be reported. This study aimed to evaluate the potential of spectral CT-derived multiparameters based on full-volume acquisition for the differentiation of benign and malignant SPNs. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-979/rc).
Methods
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Ethics Committee of Jiangsu Cancer Hospital approved this study, and individual consent for this retrospective analysis was waived.
Patients
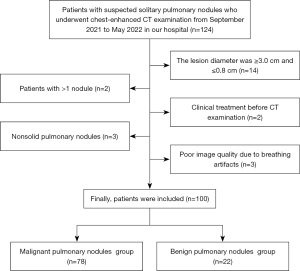
Between September 2021 and May 2022, 124 patients with clinically diagnosed SPNs were enrolled. The inclusion criteria were as follows: (I) suspected SPNs, (II) underwent spectral CT chest-enhanced scan at our hospital, and (III) confirmation by pathology. All cases were confirmed by postoperative pathology, percutaneous biopsy, and bronchial biopsy. The exclusion criteria were as follows: (I) lesion diameter was ≥3.0 cm and ≤0.8 cm (n=14), (II) patients with more than 1 nodule (n=2), (III) clinical treatment before CT examination (n=2), (IV) nonsolid pulmonary nodules (n=3), and (V) poor imaging quality due to respiratory artifacts (n=3). The patient selection flowchart is presented in Figure 1.
We collected and analyzed the data of 100 patients. Among these, 68 cases were confirmed by postoperative pathology, 29 cases by percutaneous biopsy, and 3 cases by bronchoscopic biopsy. Based on the pathological results, the patients were divided into benign, malignant, adenocarcinoma, and squamous cell carcinoma groups for statistical analysis. The cases comprised 22 benign pulmonary nodules (inflammation, n=11; hamartoma, n=5; tuberculosis, n=4; inflammatory pseudotumor, n=2) and 78 malignant pulmonary nodules (adenocarcinoma, n=60; squamous cell carcinoma, n=14; small cell LC, n=2; atypical carcinoid, n=1; and adenosquamous carcinoma, n=1).
CT image acquisition
All patients underwent the same routine protocol using dual-layer spectral CT (IQon; Philips Healthcare, Best, Netherlands). Patients were positioned in the supine position, and the scanning range was from the thoracic entrance to the level of the costophrenic angle. The scanning parameters were as follows: tube voltage, 120 kVp; tube current modulation, three-dimensional (3D) modulation; collimator width, 64×0.625 mm2; matrix, 512×512; scanning field of view, 372 mm; spacing, 0.90; rotation time, 0.50 s; scanning slice thickness, 5 mm; and reconstruction slice thickness, 1 mm. Contrast agent (ioversol, 3.0 mL/kg; iodine, 350 mg/mL; HengRui Medicine, Jiangsu, China) was injected into the antecubital vein at a flow rate of 3 mL/s, followed by a follow-up injection of 20 mL of normal saline at the same flow rate. A chest enhancement scan was performed 50 seconds after completion.
Image analysis
Images were imported into a Philips workstation (IntelliSpace Portal; Philips Healthcare) for analysis and processing using the 3D semiautomatic segmentation workstation’s own software (Tumor Tracking, Multimodality). Image analysis was performed by a radiologist (LKF, 4 years of radiology experience) and supervised by a senior radiologist (XXD, 8 years of radiology experience), who were blind to the clinicopathological information. All images were acquired in the mediastinal window, and the lesions were identified by reading the images. ROIs of the pulmonary nodules were automatically segmented with the software and underwent normalization processing. Subsequently, a series of parameters were obtained, as follows: CT value of solitary pulmonary nodule at virtual noncontrast was recorded as CTSPN-VNC; enhancement (40 and 70 keV) values were recorded as CTSPN-40 keV and CTSPN-70 keV, respectively; virtual noncontrast of aorta and CT mean of 70 keV were recorded as CTaorta-VNC and CTaorta, respectively. The CT values of virtual plain scans and the aorta were used as a reference. The difference between CT values of the lesion after enhancement and virtual plain scans (Δ40 keV and Δ70 keV), difference between the lesion and aorta (ΔS-A), and contrast enhancement ratio were calculated using the following formulas (CER40 keV and CER70 keV), SPN to arterial enhancement ratio (SAR40 keV and SAR70 keV) (20-22), normalized arterial enhancement fraction (NEF40 keV and NEF70 keV), and spectral curve slope (λ) (23,24).
To account for hemodynamic changes between patients, the IC (mg/mL) and Zeff values were normalized to those of the aorta. The normalized Zeff (NZeff) and normalized IC (NIC) were calculated using the following formulas (25-27):
To assess interobserver repeatability and variability, 50% of the study participants (50 of 100) were randomly selected, and previous measurement procedures were repeated by an additional physician (WMQ, 14 years of radiology experience). Interobserver agreement was assessed using Bland-Altman plots and intraclass correlation coefficients (ICCs).
Statistical analysis
Data were statistically analyzed using the software SPSS 22.0 (IBM Corp., Armonk, NY, USA) and MedCalc15 (MedCalc Software, Mariakerke, Belgium). Continuous variables were expressed as means ± standard deviations. Normality was assessed using the Kolmogorov-Smirnov method, followed by testing for homogeneity of variances using the Levene’s test. Parameters derived from spectral CT of pulmonary nodules were compared between the two groups using an independent samples t-test or Mann-Whitney U test, as appropriate. Receiver operating characteristic (ROC) curve analysis was performed to determine the area under the curve (AUC), accuracy, sensitivity, and specificity. Interobserver agreement for parameters related to CT values was estimated using ICCs (0.000–0.200: poor; 0.201–0.400: fair; 0.401–0.600: moderate; 0.601–0.800: good; 0.801–1.000: excellent). Bland-Altman plot assessment was also performed. Statistical tests were two-tailed, and P<0.05 was considered significant.
Results
Statistical analysis of basic clinical data of benign and malignant SPNs
A total of 100 patients with pathologically diagnosed SPNs were included in this study, including 22 patients with benign SPNs with an average age of 60.591±8.455 years, 13 males and 9 females; and 78 patients with malignant nodules with an average age of 64.551±9.478 years, 38 males and 40 females. We analyzed the differences in age, gender, diameter, and volume between the benign and malignant SPN groups, and there were no significant statistical differences (P=0.308, 0.390, 0.540, and 0.451, respectively). The details are shown in Table 1.
Table 1
| Parameters | Benign SPN (n=22), mean ± SEM | Malignant SPN (n=78), mean ± SEM | P value |
|---|---|---|---|
| Age (years) | 60.591±8.455 | 64.551±9.478 | 0.308 |
| Gender, n | 0.390 | ||
| Male | 13 | 38 | |
| Female | 9 | 40 | |
| Diameter (cm) | 1.450±0.550 | 1.628±0.584 | 0.540 |
| Volume (cm3) | 2.138±2.778 | 2.852±2.793 | 0.451 |
P values were obtained using the chi-square test, independent t-test or Mann-Whitney U-test. P<0.05 indicates statistical significance. SPN, solitary pulmonary nodule; SEM, standard error of the mean.
Differences in spectral CT parameters between benign and malignant SPNs
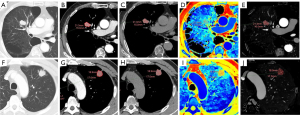
The results of the parameter analysis between the two groups are presented in Table 2. With the exception of ΔS-A(70 keV) (P=0.112), significant differences were observed between malignant and benign groups in the 12 other parameters [Δ40 keV, Δ70 keV, ΔS-A(40 keV), CER40 keV, CER70 keV, SAR40 keV, SAR70 keV, NEF40 keV, NEF70 keV, λ, NZeff, and NIC] (all P<0.05). Representative examples of benign and malignant SPNs are shown in Figure 2.
Table 2
| Parameters | Benign SPN (n=22), mean ± SEM | Malignant SPN (n=78), mean ± SEM | P value |
|---|---|---|---|
| Δ40 keV (HU) | 101.03±51.85 | 165.36±46.29 | <0.001 |
| Δ70 keV (HU) | 25.51±15.60 | 47.99±15.16 | <0.001 |
| ΔS-A(40 keV) (HU) | −81.22±53.53 | −19.24±56.75 | <0.001 |
| ΔS-A(70 keV) (HU) | −156.74±43.95 | −136.62±53.96 | 0.112 |
| CER40 keV | 5.30±5.93 | 6.26±3.17 | 0.002 |
| CER70 keV | 1.42±1.87 | 1.83±1.04 | <0.001 |
| SAR40 keV | 0.62±0.25 | 0.95±0.29 | <0.001 |
| SAR70 keV | 0.26±0.11 | 0.38±0.12 | <0.001 |
| NEF40 keV | 0.61±0.30 | 1.05±0.41 | <0.001 |
| NEF70 keV | 0.15±0.08 | 0.31±0.13 | <0.001 |
| λ | 2.52±1.23 | 3.91±1.09 | <0.001 |
| NZeff | 0.79±0.04 | 0.82±0.07 | 0.003 |
| NIC | 0.17±0.08 | 0.31±0.12 | <0.001 |
P values were obtained by independent t-test or Mann-Whitney U-test. P<0.05 indicates statistical significance. CT, computed tomography; SPN, solitary pulmonary nodule; SEM, standard error of the mean; Δ40 keV/70 keV, attenuation difference of the solitary pulmonary nodule between 40 keV/70 keV and virtual noncontrast; HU, Hounsfield unit; ΔS-A(40 keV/70 keV), attenuation difference between the solitary pulmonary nodule in the 40 keV/70 keV and arterial enhancement; CER40 keV/70 keV, contrast enhancement ratio of the 40 keV/70 keV; SAR40 keV/70 keV, solitary pulmonary nodule in the 40 keV/70 keV to arterial enhancement ratio; NEF40 keV/70 keV, normalized arterial enhancement fraction in the 40 keV/70 keV; λ, slope of the spectral attenuation curve; NZeff, normalized effective atomic number; NIC, normalized iodine concentration.
Differences in spectral CT parameters between benign, adenocarcinoma, and squamous cell carcinoma groups
The results of parameter analysis among the three groups of benign, adenocarcinoma, and squamous cell carcinoma are presented in Table 3. With the exception of ΔS-A (70 keV) (P=0.138), the 12 other parameters (Δ40 keV, Δ70 keV, ΔS-A(40 keV), CER40 keV, CER70 keV, SAR40 keV, SAR70 keV, NEF40 keV, NEF70 keV, λ, NZeff, and NIC) were significantly higher in the malignant group than in the benign group (all P<0.05). With the exception of ΔS-A(70 keV), CER40 keV, and CER70 keV (P=0.204, 0.144, and 0.088, respectively), the 10 remaining parameters [Δ40 keV, Δ70 keV, ΔS-A(40 keV), SAR40 keV, SAR70 keV, NEF40 keV, NEF70 keV, λ, NZeff, and NIC] were significantly higher in the squamous cell carcinoma group than in the benign group (all P<0.05). CER70 keV was significantly higher in the adenocarcinoma group than in the squamous cell carcinoma group (P=0.020). No significant between-group differences were noted for the 12 other parameters.
Table 3
| Parameters | Benign (n=22) | Adenocarcinoma (n=60) | Squamous cell carcinoma (n=14) | P value | ||
|---|---|---|---|---|---|---|
| Benign vs. adenocarcinoma | Benign vs. squamous cell carcinoma | Adenocarcinoma vs. squamous cell carcinoma | ||||
| Δ40 keV (HU) | 101.03±51.85 | 167.82±42.56 | 156.66±54.25 | <0.001 | 0.004 | 0.405 |
| Δ70 keV (HU) | 25.51±15.60 | 48.89±13.10 | 43.44±18.94 | <0.001 | 0.004 | 0.322 |
| ΔS-A(40 keV) (HU) | −81.22±53.53 | −17.76±61.64 | −23.41±40.42 | <0.001 | 0.001 | 0.676 |
| ΔS-A(70 keV) (HU) | −156.74±43.95 | −136.69±56.82 | −136.64±47.70 | 0.138 | 0.204 | 0.998 |
| CER40 keV | 5.30±5.93 | 6.53±3.29 | 5.11±1.86 | 0.001 | 0.144 | 0.109 |
| CER70 keV | 1.42±1.87 | 1.92±1.06 | 1.40±0.63 | <0.001 | 0.088 | 0.020 |
| SAR40 keV | 0.62±0.25 | 0.97±0.32 | 0.91±0.20 | <0.001 | 0.001 | 0.994 |
| SAR70 keV | 0.26±0.11 | 0.39±0.14 | 0.36±0.09 | <0.001 | 0.002 | 0.841 |
| NEF40 keV | 0.61±0.30 | 1.08±0.45 | 0.96±0.25 | <0.001 | 0.001 | 0.704 |
| NEF70 keV | 0.15±0.08 | 0.32±0.15 | 0.26±0.09 | <0.001 | 0.001 | 0.385 |
| λ | 2.52±1.23 | 3.96±1.04 | 3.77±1.23 | <0.001 | 0.005 | 0.554 |
| NZeff | 0.79±0.04 | 0.82±0.08 | 0.82±0.04 | 0.006 | 0.020 | 0.918 |
| NIC | 0.17±0.08 | 0.32±0.13 | 0.28±0.08 | <0.001 | 0.001 | 0.508 |
P values were obtained by independent t-test or Mann-Whitney U-test. P<0.05 indicates statistical significance. CT, computed tomography; Δ40 keV/70 keV, attenuation difference of the solitary pulmonary nodule between the 40 keV/70 keV and virtual noncontrast; HU, Hounsfield unit; ΔS-A(40 keV/70 keV), attenuation difference between the solitary pulmonary nodule in the 40 keV/70 keV and arterial enhancement; CER40 keV/70 keV, contrast enhancement ratio of the 40 keV/70 keV; SAR40 keV/70 keV, solitary pulmonary nodule in the 40 keV/70 keV to arterial enhancement ratio; NEF40 keV/70 keV, normalized arterial enhancement fraction in the 40 keV/70 keV; λ, slope of the spectral attenuation curve; NZeff, normalized effective atomic number; NIC, normalized iodine concentration.
Multiparameter diagnostic performance
The multiparameter diagnostic test characteristics are presented in Table 4 and Figure 3. Among the 12 quantitative parameters that can distinguish between benign and malignant SPN, the AUCs ranged from 0.709 to 0.867, and all of these had high diagnostic efficiency (AUC >0.7). The three quantitative parameters with the highest diagnostic efficacy were NEF70 keV, NIC, and Δ70 keV, and their AUC were 0.867, 0.866, and 0.848, respectively. Among the 12 quantitative parameters, NEF70 keV was the highest. When the NEF70 keV cut-off value was 0.203, the sensitivity was 82.1%, the specificity was 81.8%, and the accuracy was 82.0%. Of the 12 quantitative parameters that could distinguish the benign from the adenocarcinoma groups, the AUCs ranged from 0.699 to 0.874. The three parameters with the highest diagnostic efficacy were Δ70 keV, NEF70 keV, and NIC, and their AUCs were 0.874, 0.873, and 0.872, respectively. Among the 12 quantitative parameters, Δ70 keV was the highest. When the Δ70 keV cut-off value was 33.3, the sensitivity, specificity, and accuracy were 93.3%, 72.7%, and 87.8%, respectively. Among the 10 quantitative parameters that differed between the benign and squamous cell carcinoma groups, their AUC ranged from 0.732 to 0.841, and all of these had high diagnostic efficiency (AUC >0.7). The 3 parameters with the highest diagnostic efficacy were NIC, NEF70 keV, and SAR40 keV, and their AUC were 0.841, 0.834, and 0.821, respectively. Among the 10 quantitative parameters, NIC was the highest. When the NIC cut-off value was 0.2, the sensitivity was 92.9%, the specificity 77.3%, and the accuracy 83.3%.
Table 4
| Groups | Parameters | AUC | Cut-off value | Sensitivity, % | Specificity, % | Accuracy, % | P value |
|---|---|---|---|---|---|---|---|
| Benign vs. malignant | Δ40 keV (HU) | 0.815 | 120.7 | 79.5 | 72.7 | 78.0 | <0.001 |
| Δ70 keV (HU) | 0.848 | 33.3 | 85.9 | 72.7 | 83.0 | <0.001 | |
| ΔS-A(40 keV) (HU) | 0.785 | −63.9 | 76.9 | 72.7 | 76.0 | <0.001 | |
| CER40 keV | 0.721 | 3.754 | 88.5 | 59.1 | 82.0 | 0.004 | |
| CER70 keV | 0.771 | 1.17 | 80.8 | 72.7 | 79.0 | <0.001 | |
| SAR40 keV | 0.823 | 0.626 | 93.6 | 63.6 | 87.0 | <0.001 | |
| SAR70 keV | 0.804 | 0.339 | 56.4 | 95.5 | 65.0 | <0.001 | |
| NEF40 keV | 0.832 | 0.675 | 91.0 | 68.2 | 86.0 | <0.001 | |
| NEF70 keV | 0.867 | 0.203 | 82.1 | 81.8 | 82.0 | <0.001 | |
| λ | 0.793 | 3.293 | 70.5 | 77.3 | 72.0 | <0.001 | |
| NZeff | 0.709 | 0.79 | 75.6 | 59.1 | 72.0 | <0.001 | |
| NIC | 0.866 | 0.2 | 89.7 | 77.3 | 87.0 | <0.001 | |
| Benign vs. adenocarcinoma | Δ40 keV (HU) | 0.833 | 120.7 | 83.3 | 72.7 | 80.5 | <0.001 |
| Δ70 keV (HU) | 0.874 | 33.3 | 93.3 | 72.7 | 87.8 | <0.001 | |
| ΔS-A(40 keV) (HU) | 0.774 | −78 | 83.3 | 63.6 | 78.0 | <0.001 | |
| CER40 keV | 0.746 | 3.754 | 93.3 | 59.1 | 84.1 | 0.001 | |
| CER70 keV | 0.800 | 1.17 | 90.0 | 72.7 | 85.4 | <0.001 | |
| SAR40 keV | 0.823 | 0.595 | 98.3 | 59.1 | 87.8 | <0.001 | |
| SAR70 keV | 0.791 | 0.339 | 50.0 | 95.5 | 62.2 | <0.001 | |
| NEF40 keV | 0.836 | 0.675 | 90.0 | 68.2 | 84.1 | <0.001 | |
| NEF70 keV | 0.873 | 0.203 | 80.0 | 81.8 | 80.5 | <0.001 | |
| λ | 0.807 | 3.293 | 75.0 | 77.3 | 75.6 | <0.001 | |
| NZeff | 0.699 | 0.843 | 40.0 | 95.5 | 54.9 | 0.001 | |
| NIC | 0.872 | 0.2 | 88.3 | 77.3 | 86.6 | <0.001 | |
| Benign vs. squamous cell carcinoma | Δ40 keV (HU) | 0.766 | 120.7 | 71.4 | 72.7 | 72.2 | 0.001 |
| Δ70 keV (HU) | 0.750 | 27.1 | 78.6 | 59.1 | 66.7 | 0.003 | |
| ΔS-A(40 keV) (HU) | 0.819 | −63.9 | 85.7 | 72.7 | 77.8 | <0.001 | |
| SAR40 keV | 0.821 | 0.705 | 92.9 | 68.2 | 77.8 | <0.001 | |
| SAR70 keV | 0.815 | 0.339 | 71.4 | 95.5 | 86.1 | <0.001 | |
| NEF40 keV | 0.818 | 0.71 | 92.9 | 72.7 | 80.6 | <0.001 | |
| NEF70 keV | 0.834 | 0.203 | 85.7 | 81.8 | 83.3 | <0.001 | |
| λ | 0.760 | 3.293 | 64.3 | 77.3 | 72.2 | 0.002 | |
| NZeff | 0.732 | 0.773 | 100.0 | 40.9 | 63.9 | 0.006 | |
| NIC | 0.841 | 0.2 | 92.9 | 77.3 | 83.3 | <0.001 |
P<0.05 indicates statistical significance. ROC, receiver operating characteristic; AUC, area under the curve; Δ40 keV/70 keV, attenuation difference of the solitary pulmonary nodule between the 40 keV/70 keV and virtual noncontrast; HU, Hounsfield unit; ΔS-A(40 keV), attenuation difference between the solitary pulmonary nodule in the 40 keV/70 keV and arterial enhancement; CER40 keV/70 keV, contrast enhancement ratio of the 40 keV/70 keV; SAR40 keV/70 keV, solitary pulmonary nodule in the 40 keV/70 keV to arterial enhancement ratio; NEF40 keV/70 keV, normalized arterial enhancement fraction in 40 keV/70 keV; λ, slope of the spectral attenuation curve; NZeff, normalized effective atomic number; NIC, normalized iodine concentration.

ICCs
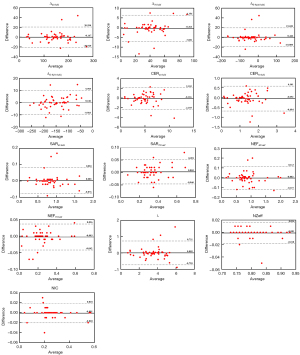
All spectral CT-related parameters had good interobserver agreement for assessing lung nodules, with ICC values of 0.856 to 0.996 (Table 5 and Figure 4).
Table 5
| Parameters | ICC (95% CI) |
|---|---|
| Δ40 keV | 0.981 (0.989–0.967) |
| Δ70 keV | 0.980 (0.966–0.989) |
| ΔS-A(40 keV) | 0.986 (0.975–0.992) |
| ΔS-A(70 keV) | 0.995 (0.992–0.997) |
| CER40 keV | 0.856 (0.759–0.915) |
| CER70 keV | 0.877 (0.792–0.928) |
| SAR40 keV | 0.990 (0.983–0.994) |
| SAR70 keV | 0.978 (0.962–0.988) |
| NEF40 keV | 0.987 (0.978–0.993) |
| NEF70 keV | 0.986 (0.976–0.992) |
| λ | 0.957 (0.925–0.975) |
| NZeff | 0.984 (0.971–0.991) |
| NIC | 0.996 (0.993–0.998) |
ICC, intraclass correlation coefficient; CI, confidence interval; Δ40 keV/70 keV attenuation difference of the solitary pulmonary nodule between the 40 keV/70 keV and virtual noncontrast; ΔS-A(40 keV/70 keV), attenuation difference between the solitary pulmonary nodule in the 40 keV/70 keV and arterial enhancement; CER40 keV/70 keV, contrast enhancement ratio of the 40 keV/70 keV; SAR40 keV/70 keV, solitary pulmonary nodule in the 40 keV/70 keV to arterial enhancement ratio; NEF40 keV/70 keV, normalized arterial enhancement fraction in the 40 keV/70 keV; λ, slope of the spectral attenuation curve; NZeff, normalized effective atomic number; NIC, normalized iodine concentration.
Discussion
This is the first study to demonstrate that CT-derived parameters obtained from spectroscopic CT whole-volume analysis hold potential as reproducible imaging markers for the identification of SPNs. Multiparameter quantitative analyses revealed significant differences in the pulmonary nodules of different pathological types. This approach may help to avoid invasive, time-consuming, and expensive tissue sampling procedures and improve the overall biology of CT scans for characterizing SPNs. These parameters may help to improve discrimination of pulmonary nodule types by radiologists and establish an accurate diagnosis for more effective treatments.
Several studies have demonstrated that the application of enhanced CT-derived CT enhancement values and ratios may reduce the impact of machine and individual differences and may be effective tools for assessing tumor angiogenesis. CT enhancement values and ratios are closely associated with intratumoral microvascular and lymphatic infiltration and can be used as a surrogate marker for preoperative detection of lymphovascular invasion (20,21,28,29).
In the present study, various spectral CT-related parameters were significantly different between benign and malignant pulmonary nodules (P<0.05), with the exception of ΔS-A(70 keV) (P=0.112). Several derived parameters were significantly higher for malignant pulmonary nodules than for benign pulmonary nodules, which is consistent with previous reports (14,16,30). This may be because malignant nodules produce a large amount of angiogenic factors for tumor growth, which stimulates the formation of more microvessels. The increased density of microvessels leads to an increase in capillary perfusion and permeability, causing rapid accumulation of contrast agent in the interstitium after enhancement. Ultimately, this leads to strong contrast enhancement in malignant nodules.
The spectral curve λ reflects the different X-ray absorption rates of tissues with different chemical compositions. Each tissue has a characteristic spectral curve, which demonstrates the dynamic changes in CT values of different substances with different energy levels. In our study, we demonstrated that the slope (value) of malignant nodules was significantly higher than that of benign nodules. This result is consistent with previous studies (14,16), which can be explained by the following mechanism: a steeper energy spectrum curve is associated with a higher slope value, higher percentage of contrast agent iodine in the tumor, and greater attenuation of iodine at lower energies due to the structural and functional immaturity of the malignant tumor vascular network structure. This results in increased vascular permeability and contrast agent leakage and spectral properties, whereby a stronger curve is associated with a steeper decay curve and higher slope.
Zeff is a quantitative index derived from the atomic number, which represents the compound atoms of compounds or mixtures of various materials and characterizes tissue composition. In our study, Zeff values were higher for malignant pulmonary nodules than for benign pulmonary nodules, possibly because malignant nodules have abundant densely packed tumor cells with a higher nuclear-to-cytoplasmic ratio (31). In this regard, there is a paucity of studies on the Zeff of pulmonary nodules.
As the main component of contrast agents, iodine directly reflects blood flow and distribution in intravascular and extracellular spaces. IC maps are generally considered to enable assessment of the number and blood flow of blood vessels supplying pulmonary nodules. Standardized NIC parameters with aortic IC minimize the effects of hemodynamic factors on absolute enhancement of lesions between different individuals, thereby increasing comparability between different cases and specificity of iodine-related NICs compared to other indicators. Our study demonstrated that the NIC of malignant nodules was significantly higher than that of benign nodules. Compared to benign nodules, malignant nodules required a greater blood supply to provide tumor cells with more essential nutrients and oxygen, thereby resulting in rapid growth that manifested as a significant increase in microvessels and abundant blood supply.
ROC curve analysis revealed relatively high AUCs (0.709–0.867) of the relevant parameters for discriminating benign and malignant nodules. The three parameters with the highest AUC distributions were NEF70 keV, NIC, and Δ70 keV (AUCs: 0.867, 0.866, and 0.848, respectively). For previous conventional CT, cut-off values of contrast-enhanced CT for discriminating benign and malignant nodules were set at 15–30 Hounsfield units (HUs) (12,32,33). Our cut-off value was almost the same as that of Yi et al. (32) (33.3 and 30 HU, respectively), with a net enhancement of ≥30 HU as the cut-off for distinguishing benign from malignant nodules. The sensitivity, specificity, and accuracy for malignant nodules were 99%, 54%, and 78%, respectively. In a study by Swensen et al. (12) with 15 HU as the threshold, the sensitivity, specificity, and accuracy were 98%, 58%, and 77% respectively. This discrepancy may be related to the injection rate, total volume, and acquisition time of the contrast medium. The mean difference in the CT number of SPNs on non-enhanced weighted mean images and virtual non-enhanced images was 4.3 HU. Moreover, the attenuation value of pulmonary nodules on real plain images was similar to that on virtual plain images. In routine practice, virtual non-enhanced image reconstruction can replace additional non-enhanced CT scans to reduce their radiation dose (34). As 70 keV is equivalent to conventional CT values, we conjecture that Δ70 keV is equivalent to enhanced CT values. In our study, Δ70 keV had a sensitivity, specificity, and accuracy of 85.9%, 72.7%, and 83.0%, respectively, for identifying benign and malignant nodules. The NIC cut-off for distinguishing benign from malignant tumors was 0.2, which is consistent with previous findings (14,16). Wen et al. (14) obtained NIC cut-offs of 0.13 and 0.31 using 25-s and 60-s spectral CT enhanced scans. Our cut-offs using 50-s enhanced scans fell well within this range. Zhang et al. (16) reported NIC cut-offs of 0.21 and 0.30 for 35-s and 90-s enhanced scans, respectively, and our values also fell within this range. In our study, no significant difference was observed between the AUC curves of NEF70 keV, NIC, and Δ70 keV. Although the AUC of the NEF70 keV parameter was the highest, the sensitivity and accuracy were lower than those of NIC. Hence, we considered NIC the most effective potential parameter.
The present study also demonstrated that contrast-enhanced CT-derived multiparameters performed well for differentiating benign tumors from adenocarcinoma and squamous cell carcinoma. However, no significant differences were observed in quantitative multiparameters between adenocarcinoma and squamous cell carcinoma, with the exception of CER70 keV. This is in accordance with some previous reports but not with others (35,36). Theoretically, the neovascularization of adenocarcinoma should be higher than that of squamous cell carcinoma, and the distribution should be relatively uniform, with less necrosis and hemorrhage. Compared to adenocarcinomas, squamous cell carcinomas are larger and more likely to invade blood vessels. The tumor blood vessels of squamous cell carcinoma are more likely to break, shrink, and block due to accumulation and growth, eventually leading to necrosis. Moreover, the degree of enhancement is lower than that of adenocarcinoma. We speculate that this may be related to the following factors. First, we examined SPNs rather than pulmonary masses, as reported in previous studies. Given the small size of the lesions investigated in our study, even squamous cell carcinoma may not have exhibited obvious necrosis. In addition, our software for delineating lesions automatically avoided areas with obvious necrosis and enhancement; therefore, the ROI was predominantly located in lesions with uniform density. This may have resulted in a degree of measurement bias in the results, consistent with the theory of Li et al. (35). In addition, a relationship with a smaller number of squamous cell carcinomas or scanning time has been proposed (36).
Consistent with previous studies, our study demonstrated good interobserver agreement for spectral CT multiparameters measured using a semiautomated full-volume method, indicating good reproducibility and stability for lung nodule assessment (19). Spectral CT parameters in this study were derived from the entire volume of the lesion. However, in most previous studies, only parameter values from 1 or several manually drawn ROIs were used due to technical or software limitations. This approach was unable to reflect the heterogeneity and full information of lesions and precluded a complete characterization of whole tumor biology, which may have impacted the accuracy of delineating lesions (37). A study by Gierada et al. (19) reported that nodule volumes obtained using semiautomated CT volume methods had consistently high interobserver agreement compared with manual or semi-automated nodule diameters. These results were consistent with the expectations that volume measurements were more consistent and less variable compared with size measurements of lung nodules and tumors (19).
Our study had several limitations. First, it was a single-center retrospective study with a relatively small sample size. Although the sample size of previous studies on SPNs has rarely exceeded 100, larger sample sizes and multicenter validation are warranted. Second, in our study, only 13 parameters obtained from venous phase scan images were used in a single phase to reduce radiation dose. Several studies (14,23) have used dual-phases (arterial and venous phase scans), and we intend to perform dual-phase scanning in the future. However, some studies (14,23) have reported that several parameters such as IC/slope in the venous phase may be superior to those in the arterial phase. Third, our radiologists had experience in evaluating benign pulmonary nodules, and the number of benign pulmonary nodules that were difficult to identify and ended being treated surgically was low. Fourth, we did not include morphological features in this study, because we focused predominantly on the evaluation of pulmonary nodules using quantitative parameters. In this regard, we plan to further investigate the combined features of morphological and quantitative parameters in the future. Finally, our study was performed using a dual-source CT device, and the application of this scanning principle to different types of dual-energy scanners warrants validation in future multicentre large-sample studies.
Conclusions
In conclusion, our findings demonstrate the potential value of spectral CT-related parameters extracted using a full-volume approach for evaluating pulmonary nodules with satisfactory diagnostic performance and reproducibility.
Acknowledgments
Funding: This work was supported by the Nanjing Medical University Imaging Elite Talents Project (No. 320.1140.2022.0510.005) and the Scientific Research Project of Jiangsu Provincial Health Commission (No. Z2022075).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-979/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-979/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Ethics Committee of Jiangsu Cancer Hospital approved this study, and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Weir-McCall JR, Joyce S, Clegg A, MacKay JW, Baxter G, Dendl LM, Rintoul RC, Qureshi NR, Miles K, Gilbert FJ. Dynamic contrast-enhanced computed tomography for the diagnosis of solitary pulmonary nodules: a systematic review and meta-analysis. Eur Radiol 2020;30:3310-23. [Crossref] [PubMed]
- Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. [Crossref] [PubMed]
- Winer-Muram HT. The solitary pulmonary nodule. Radiology 2006;239:34-49. [Crossref] [PubMed]
- Truong MT, Ko JP, Rossi SE, Rossi I, Viswanathan C, Bruzzi JF, Marom EM, Erasmus JJ. Update in the evaluation of the solitary pulmonary nodule. Radiographics 2014;34:1658-79. [Crossref] [PubMed]
- Siegelman SS, Khouri NF, Leo FP, Fishman EK, Braverman RM, Zerhouni EA. Solitary pulmonary nodules: CT assessment. Radiology 1986;160:307-12. [Crossref] [PubMed]
- Khouri NF, Meziane MA, Zerhouni EA, Fishman EK, Siegelman SS. The solitary pulmonary nodule. Assessment, diagnosis, and management. Chest 1987;91:128-33. [Crossref] [PubMed]
- Gould MK, Ananth L, Barnett PGVeterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007;131:383-8. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Erasmus JJ, Connolly JE, McAdams HP, Roggli VL. Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics 2000;20:43-58. [Crossref] [PubMed]
- Swensen SJ, Viggiano RW, Midthun DE, Müller NL, Sherrick A, Yamashita K, Naidich DP, Patz EF, Hartman TE, Muhm JR, Weaver AL. Lung nodule enhancement at CT: multicenter study. Radiology 2000;214:73-80. [Crossref] [PubMed]
- Deniffel D, Sauter A, Fingerle A, Rummeny EJ, Makowski MR, Pfeiffer D. Improved differentiation between primary lung cancer and pulmonary metastasis by combining dual-energy CT-derived biomarkers with conventional CT attenuation. Eur Radiol 2021;31:1002-10. [Crossref] [PubMed]
- Wen Q, Yue Y, Shang J, Lu X, Gao L, Hou Y. The application of dual-layer spectral detector computed tomography in solitary pulmonary nodule identification. Quant Imaging Med Surg 2021;11:521-32. [Crossref] [PubMed]
- Gao L, Lu X, Wen Q, Hou Y. Added value of spectral parameters for the assessment of lymph node metastasis of lung cancer with dual-layer spectral detector computed tomography. Quant Imaging Med Surg 2021;11:2622-33. [Crossref] [PubMed]
- Zhang Y, Cheng J, Hua X, Yu M, Xu C, Zhang F, Xu J, Wu H. Can Spectral CT Imaging Improve the Differentiation between Malignant and Benign Solitary Pulmonary Nodules? PLoS One 2016;11:e0147537. [Crossref] [PubMed]
- Wu L, Cao G, Zhao L, Tang K, Lin J, Miao S, Lin T, Sun J, Zheng X, Spectral CT. Analysis of Solitary Pulmonary Nodules for Differentiating Malignancy from Benignancy: The Value of Iodine Concentration Spatial Distribution Difference. Biomed Res Int 2018;2018:4830659. [Crossref] [PubMed]
- Azour L, Ko JP, O'Donnell T, Patel N, Bhattacharji P, Moore WH. Combined whole-lesion radiomic and iodine analysis for differentiation of pulmonary tumors. Sci Rep 2022;12:11813. [Crossref] [PubMed]
- Gierada DS, Rydzak CE, Zei M, Rhea L. Improved Interobserver Agreement on Lung-RADS Classification of Solid Nodules Using Semiautomated CT Volumetry. Radiology 2020;297:675-84. [Crossref] [PubMed]
- Ma Z, Liang C, Huang Y, He L, Liang C, Chen X, Huang X, Xiong Y, Liu Z. Can lymphovascular invasion be predicted by preoperative multiphasic dynamic CT in patients with advanced gastric cancer? Eur Radiol 2017;27:3383-91. [Crossref] [PubMed]
- Yin XD, Huang WB, Lü CY, Zhang L, Wang LW, Xie GH. A preliminary study on correlations of triple-phase multi-slice CT scan with histological differentiation and intratumoral microvascular/lymphatic invasion in gastric cancer. Chin Med J (Engl) 2011;124:347-51. [PubMed]
- Komori M, Asayama Y, Fujita N, Hiraka K, Tsurumaru D, Kakeji Y, Honda H. Extent of arterial tumor enhancement measured with preoperative MDCT gastrography is a prognostic factor in advanced gastric cancer after curative resection. AJR Am J Roentgenol 2013;201:W253-61. [Crossref] [PubMed]
- Deng L, Zhang G, Lin X, Han T, Zhang B, Jing M, Zhou J. Comparison of Spectral and Perfusion Computed Tomography Imaging in the Differential Diagnosis of Peripheral Lung Cancer and Focal Organizing Pneumonia. Front Oncol 2021;11:690254. [Crossref] [PubMed]
- Zhang X, Zheng C, Yang Z, Cheng Z, Deng H, Chen M, Duan X, Mao J, Shen J. Axillary Sentinel Lymph Nodes in Breast Cancer: Quantitative Evaluation at Dual-Energy CT. Radiology 2018;289:337-46. [Crossref] [PubMed]
- Wang X, Liu D, Zeng X, Jiang S, Li L, Yu T, Zhang J. Dual-energy CT quantitative parameters for the differentiation of benign from malignant lesions and the prediction of histopathological and molecular subtypes in breast cancer. Quant Imaging Med Surg 2021;11:1946-57. [Crossref] [PubMed]
- Li H, Wang H, Chen F, Gao L, Zhou Y, Zhou Z, Huang J, Xu L. Detection of axillary lymph node metastasis in breast cancer using dual-layer spectral computed tomography. Front Oncol 2022;12:967655. [Crossref] [PubMed]
- Ha T, Kim W, Cha J, Lee YH, Seo HS, Park SY, Kim NH, Hwang SH, Yong HS, Oh YW, Kang EY, Kim C. Differentiating pulmonary metastasis from benign lung nodules in thyroid cancer patients using dual-energy CT parameters. Eur Radiol 2022;32:1902-11. [Crossref] [PubMed]
- Li LM, Feng LY, Chen XH, Liang P, Li J, Gao JB. Gastric heterotopic pancreas and stromal tumors smaller than 3 cm in diameter: clinical and computed tomography findings. Cancer Imaging 2018;18:26. [Crossref] [PubMed]
- Li Y, Su H, Yang L, Yue M, Wang M, Gu X, Dai L, Wang X, Su X, Zhang A, Ren J, Shi G. Can lymphovascular invasion be predicted by contrast-enhanced CT imaging features in patients with esophageal squamous cell carcinoma? A preliminary retrospective study. BMC Med Imaging 2022;22:93. [Crossref] [PubMed]
- Zegadło A, Żabicka M, Kania-Pudło M, Maliborski A, Różyk A, Sośnicki W. Assessment of Solitary Pulmonary Nodules Based on Virtual Monochrome Images and Iodine-Dependent Images Using a Single-Source Dual-Energy CT with Fast kVp Switching. J Clin Med 2020;9:2514. [Crossref] [PubMed]
- Shen H, Yuan X, Liu D, Huang Y, Wang Y, Jiang S, Zhang J. Multiparametric dual-energy CT for distinguishing nasopharyngeal carcinoma from nasopharyngeal lymphoma. Eur J Radiol 2021;136:109532. [Crossref] [PubMed]
- Yi CA, Lee KS, Kim EA, Han J, Kim H, Kwon OJ, Jeong YJ, Kim S. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004;233:191-9. [Crossref] [PubMed]
- Cha MJ, Lee KS, Kim HS, Lee SW, Jeong CJ, Kim EY, Lee HY. Improvement in imaging diagnosis technique and modalities for solitary pulmonary nodules: from ground-glass opacity nodules to part-solid and solid nodules. Expert Rev Respir Med 2016;10:261-78. [Crossref] [PubMed]
- Chae EJ, Song JW, Seo JB, Krauss B, Jang YM, Song KS. Clinical utility of dual-energy CT in the evaluation of solitary pulmonary nodules: initial experience. Radiology 2008;249:671-81. [Crossref] [PubMed]
- Li X, Meng X, Ye Z. Iodine quantification to characterize primary lesions, metastatic and non-metastatic lymph nodes in lung cancers by dual energy computed tomography: An initial experience. Eur J Radiol 2016;85:1219-23. [Crossref] [PubMed]
- Zhang Z, Zou H, Yuan A, Jiang F, Zhao B, Liu Y, Chen J, Zuo M, Gong L. A Single Enhanced Dual-Energy CT Scan May Distinguish Lung Squamous Cell Carcinoma From Adenocarcinoma During the Venous phase. Acad Radiol 2020;27:624-9. [Crossref] [PubMed]
- Li M, Zhang L, Tang W, Jin YJ, Qi LL, Wu N. Identification of epidermal growth factor receptor mutations in pulmonary adenocarcinoma using dual-energy spectral computed tomography. Eur Radiol 2019;29:2989-97. [Crossref] [PubMed]