
Validation of the commercial coronary computed tomographic angiography artificial intelligence for coronary artery stenosis: a cross-sectional study
Introduction
Coronary artery disease (CAD), also known as coronary atherosclerosis, results in myocardial ischemia and necrosis (1). CAD is currently one of the leading causes of death worldwide (2). Despite the development of drugs and interventional therapies, the clinical prognoses of patients with CAD are still poor. Accurate assessment and management of the risk factors for CAD are very important for optimal treatment selection and the prognosis of patients (3). Although invasive coronary angiography (ICA) is the gold standard for assessing the severity of CAD, it is an invasive examination associated with high medical costs (4).
Coronary computed tomographic angiography (CCTA) is the first-line investigation for suspected chest pain (5). CCTA can clearly show the structure of the heart and coronary artery. A radiologist can use this to assess the degree of coronary artery stenosis and the characteristics of the plaque and classify the patient’s risk (6). CCTA provides a useful noninvasive imaging method for the initial diagnosis of CAD (7,8). However, the diagnosis of CAD using CCTA requires manual image post-processing and subjective visual observation by the radiologist, which demands significant human resources and time and lacks efficiency and accuracy (9).
Artificial intelligence (AI) has rapidly gained popularity over recent years, and the application of AI in the medical field has become commonplace (10). In the cardiovascular field, AI technology could significantly speed up cardiovascular image reconstruction, provide an objective means of image segmentation, and be used to evaluate the degree of vascular stenosis (11,12). AI technology can also be used to assess calcification and the computed tomography fractional flow reserve (CT-FFR) (13). Recent research has focused on the reconstruction and optimization of AI models based on machine learning, including supervised learning, unsupervised learning, and deep learning (DL), which is the main technology of AI. Supervised learning includes many techniques and algorithms, such as artificial neural networks, support vector machines, decision trees, and random forests (14,15). DL includes convolutional neural networks (CNNs), recurrent neural networks, and deep neural networks (16,17). AI technology differs in its applications and limitations for different data types. Therefore, finding an appropriate intelligent mathematical model could facilitate accurate diagnosis of CAD. Some studies have started to address the diagnostic efficiency of AI for CAD and verified the feasibility of this based on CCTA using DL (18,19). There exist a few commercial coronary computed tomographic angiography artificial intelligence (CCTA-AI) platforms that can be used in the cardiovascular clinic for image post-processing and diagnosis. However, there is not enough research that tests the CCTA-AI platforms’ actual levels of diagnostic performance at the segment and lesion levels based on multicenter and multidevice data.
We hypothesized that AI-based assisted analysis is at a level equivalent to that of expert readers with 5–10 years of experience in assessing coronary artery morphology and stenosis. Using ICA as the gold standard, this study compared CCTA-AI with radiological readers to identify the current stage of commercial AI platforms and the role of radiologists by completely external data from different CT models in multicenter hospitals. In addition, this study further verified the generalization ability of contemporary commercial algorithm models. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1115/rc).
Methods
Patient population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Southwest Hospital, Third Military Medical University [Army Medical University (No. KY2020306)] and the medical science research ethics committees of 3 other hospitals [The Second People’s Hospital of Liao Cheng (No. 2021-8); UIanqab Central Hospital (No. 2021-3); LinFen central hospital (ethics approval No. 2021-23-1)]. The requirement for individual consent for this retrospective analysis was waived. This retrospective study involved 4 general hospitals in western [Southwest Hospital, Third Military Medical University (Army Medical University)], eastern (The Second People’s Hospital of Liao Cheng), northeast (UIanqab Central Hospital), and central (LinFen central hospital) China. Patients with suspected chest pain who underwent CCTA and ICA in these hospitals between January 2017 and December 2021 were retrospectively reviewed.
All patients subsequently underwent ICA within 1 month of their CCTA scan. The exclusion criteria were as follows: (I) patients with a history of cardiac surgery, including coronary artery bypass grafting or percutaneous coronary intervention; (II) patients for whom the AI platform was not able to complete the analyses; (III) patients for whom the diagnostic image quality of CCTA or ICA did not meet the requirement [classified as either poor or sufficient (20)]. The flowchart of this study is displayed in Figure 1.
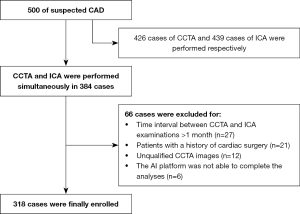
Image acquisition
Patients were scanned on either a second-generation dual-source CT system (SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany), a 64-row CT scanner (Optima CT680, GE Healthcare, Milwaukee, WI, USA), a multidetector CT system (Discovery CT750 HD; GE Healthcare, USA), or a Brilliance CT Elite FHD scanner (Philips Healthcare, Best, The Netherlands). Scan parameters used on each system are shown in Table 1.
Table 1
| Scan parameters | Siemens dual-source CT flash | GE Optima CT680 | GE Discovery CT750 HD | PHILIPS Brilliance iCT Elite FHD |
|---|---|---|---|---|
| Tube voltage (kV) | 80–100 | 100–120 | 100–120 | 100–120 |
| Tube current (mAs) | Auto | Auto | Auto | Auto |
| Slice thickness (mm) | 0.750 | 0.625 | 0.625 | 0.900 |
| Slice gap (mm) | 0.750 | 0.625 | 0.625 | 0.450 |
| Field of view (cm) | 150–200 | 150–200 | 150–200 | 150–200 |
| Retrospective gating | Both | Both | Both | Both |
| Scan trigger mode | Bolus tracking | Test bolus | Test bolus | Bolus tracking |
| Contrast material volume (mL) | 50–55 | 65–75 | 65–75 | 50–55 |
| Contrast flow rate (g/s) | 5.5–6.0 | 4.5–5.5 | 4.5–5.5 | 4.5–5.5 |
| Contrast concentration (mgI/mL) | 350–370 | 350–370 | 350–370 | 350–370 |
CCTA, coronary computed tomographic angiography.
ICA was obtained using either an Axiom Artis angiography system (Siemens Healthcare, Erlangen, Germany), an Allura Xper FD20 angiography system (Philips Medical Systems, Best, the Netherlands), or an INNOVA 3100 digital plate angiography system (GE Healthcare, Waukesha, WI, USA). The contrast medium was injected manually, and the dosage was 20–30 mL.
Image analysis
In the CCTA-AI part of this study, CCTA images were automatically analyzed using a commercially available CCTA-AI platform package (Coronary Doc, ShuKun Technology, Beijing, China) without any human subjective intervention. This platform can automatically identify vascular segments and detect stenosis using maximum intensity projection, multiplanar reconstruction, curvature plane reconstruction, and volume reconstruction based on a CNN model (18).
In the CCTA reader part of this study, the CCTA images were evaluated by 2 cardiovascular radiologists (with 5–10 years of experience) independently on a post-processing workstation (Syngo Via, Siemens Healthineers, Forchheim, Germany). The radiologists were blinded to each patient’s outcome data. Disagreements were resolved by discussion until a consensus was reached.
ICA was performed with a standard position and image for each vessel according to standard procedures (21). All ICA images were re-evaluated visually by 1 experienced interventional cardiologist (Doctor A) who was blinded to the patient’s CCTA images and clinical information. To ensure reliability, another interventional cardiologist (Doctor B) completed the assessment in a randomly selected group of 40 patients, and interclass correlation coefficients (ICCs) were used to evaluate the reproducibility. An ICC greater than 0.75 indicated good agreement of the data.
Each coronary artery was segmented according to the 18-segment model proposed by the Society of Cardiovascular Computed Tomography (SCCT) (22). After any coronary artery segment with a diameter of less than 2 mm was excluded, the proximal right coronary artery (pRCA), mid-right coronary artery (mRCA), distal right coronary artery (dRCA), right posterior descending artery (R-PDA), proximal left anterior descending artery (pLAD), mid-left anterior descending artery (mLAD), distal left anterior descending artery (dLAD), first diagonal branch (D1), proximal circumflex artery (pCx), first obtuse marginal artery (OM1), and left circumflex artery (LCx) remained. The cutoff values of models 1 and 2, were 50% and 70%, respectively. Model 1 compared stenosis rates between 0–49% and 50–100%. Model 2 compared stenosis rates between 1–69% and 70–100%.
Statistical analysis
Statistical analyses were performed using SPSS 21.0 (IBM Corp., Armonk, NY, USA), where statistical significance was considered at a level of P<0.05. Categorical variables were compared using the χ2 test, whereas continuous variables were compared using the independent-sample t test or Mann–Whitney U test as appropriate following the Kolmogorov–Smirnov test of normality. Models 1 and 2 were used to determine the sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) and their corresponding 95% confidence intervals (CIs). The diagnostic performances of AI for coronary artery stenosis with models 1 and 2 were evaluated using receiver operating characteristic (ROC) curves and quantitatively expressed using the area under the curve (AUC). ROC curve analysis was performed using MedCalc version 19.0 (MedCalc Platform bvba, Ostend, Belgium; https://www.medcalc.org; 2019).
Results
Patient characteristics
After applying strict inclusion and exclusion criteria, 318 patients with suspected chest pain were included in our final analysis (Figure 1). Among the numerous risk factors, hypertension (65%) was the most common, while incidence rates of other risk factors were less than 50%. The average post-processing times of the CCTA-AI platform and CCTA reader were 20.4±4.1 and 1,112.1±122.8 s, respectively. The degree of vascular stenosis and the number of lesion vessels at the patient level of ICA are shown in Table 2. Other detailed demographic and clinical characteristics are shown in Table 2. The ICCs of 2 interventional cardiologists ranged from 0.806 to 0.100, indicating good interobserver assessment reproducibility.
Table 2
| Characteristics | Value |
|---|---|
| Age (years), median ± standard deviation [range] | 62±10 [29–86] |
| Male | 223 [70] |
| Female | 95 [30] |
| Hypertension | 204 [65] |
| Hypercholesterolemia | 74 [23] |
| Diabetes | 123 [39] |
| Current smoking | 122 [38] |
| Current drinking | 78 [25] |
| Family history of CAD | 25 [8] |
| Image quality (4-point Likert scale) | |
| 3 | 127 [40] |
| 4 | 191 [60] |
| The average post-processing time with CCTA-AI(s)# | 20.4±4.1 |
| The average post-processing time with the reader(s)# | 1,112.1±122.8 |
| ICA | |
| Lesion vessels | |
| None | 12 [4] |
| One vessel | 54 [17] |
| Two vessels | 63 [20] |
| Three vessels | 179 [56] |
| Four vessels | 10 [3] |
| The degree of vascular stenosis at the patient level | |
| 0% | 13 [4] |
| 1–24% | 3 [1] |
| 25–49% | 29 [9] |
| 50–69% | 56 [18] |
| 70–99% | 160 [50] |
| 100% | 57 [18] |
Unless otherwise indicated, data are numbers of patients, with percentages. #, data are presented as the mean ± standard deviation. ICA, invasive coronary angiography; CAD, coronary artery disease; CCTA, coronary computed tomographic angiography; AI, artificial intelligence.
Patient-level analysis
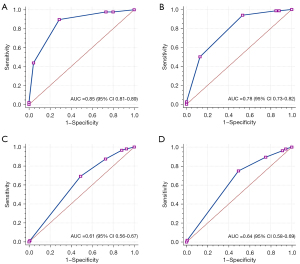
Table 3 describes the detailed data of no visible (0), minimal (1–24%), mild (25–49%), moderate (50–69%), severe stenosis (70–99%), and occluded (100%) of the segment level of vascular stenosis on CCTA-AI and CCTA reader. The specific diagnostic performance parameters of CCTA-AI assessed the detection of vessel stenosis at the patient level, as displayed in Table 4. The ROC results for these comparisons are illustrated in Figure 2. The AUCs (0.85 and 0.78) were obtained by the CCTA-AI platform using the bounds of models 1 and 2. The AUCs obtained by the CCTA reader were 0.61 and 0.64 for models 1 and 2, respectively. At the patient level, the AUC of CCTA-AI was significantly better than that of the CCTA reader.
Table 3
| Vessels stenosis | pRCA | mRCA | dRCA | LM | pLAD | mLAD | dLAD | D1 | pCx | OM1 | LCx |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCTA-AI, n [%] | |||||||||||
| 0 | 111 [35] | 139 [44] | 182 [57] | 201 [63] | 114 [36] | 93 [29] | 288 [90] | 241 [75] | 166 [52] | 272 [85] | 197 [62] |
| 1–24% | 26 [8] | 21 [6] | 18 [6] | 42 [13] | 36 [11] | 14 [4] | 8 [3] | 28 [9] | 29 [9] | 12 [4] | 27 [9] |
| 25–49% | 85 [27] | 80 [25] | 56 [18] | 49 [15] | 73 [23] | 75 [24] | 14 [4] | 21 [7] | 73 [23] | 17 [5] | 44 [14] |
| 50–69% | 64 [20] | 50 [16] | 48 [15] | 20 [6] | 66 [21] | 91 [29] | 7 [2] | 18 [6] | 33 [11] | 14 [4] | 30 [9] |
| 70–99% | 27 [8] | 26 [8] | 14 [4] | 6 [2] | 27 [8] | 44 [14] | 1 [1] | 10 [3] | 17 [5] | 2 [1] | 20 [6] |
| 100% | 5 [2] | 2 [1] | 0 [0] | 0 [0] | 2 [1] | 1 [0] | 0 [0] | 0 [0] | 0 [0] | 1 [1] | 0 [0] |
| CCTA reader, n [%] | |||||||||||
| 0 | 126 [39] | 153 [48] | 209 [66] | 223 [70] | 60 [19] | 120 [38] | 285 [89] | 256 [80] | 153 [48] | 284 [89] | 211 [66] |
| 1–24% | 34 [11] | 28 [9] | 13 [4] | 57 [18] | 26 [8] | 15 [5] | 3 [1] | 16 [5] | 47 [15] | 8 [3] | 15 [5] |
| 25–49% | 72 [23] | 55 [17] | 46 [14] | 29 [9] | 79 [25] | 45 [14] | 15 [5] | 21 [7] | 48 [15] | 12 [4] | 21 [7] |
| 50–69% | 26 [8] | 42 [13] | 21 [7] | 7 [2] | 56 [17] | 59 [18] | 8 [3] | 15 [5] | 26 [8] | 8 [3] | 27 [8] |
| 70–99% | 59 [18] | 40 [13] | 29 [9] | 2 [1] | 97 [31] | 79 [25] | 7 [2] | 10 [3] | 43 [13] | 6 [2] | 39 [12] |
| 100% | 1 [1] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 1 [1] | 0 [0] | 5 [2] |
CCTA, coronary computed tomographic angiography; AI, artificial intelligence; pRCA, proximal right coronary artery; mRCA, mid-right coronary artery; dRCA, distal right coronary artery; pLAD, proximal left anterior descending artery; mLAD, mid-left anterior descending artery; dLAD, distal left anterior descending artery; D1, first diagonal branch; pCx, proximal circumflex artery; OM1, first obtuse marginal artery; LCx, left circumflex artery.
Table 4
| Accuracy parameters | The diagnostic efficacy in model 1 | The diagnostic efficacy in model 2 | |||
|---|---|---|---|---|---|
| CCTA-AI | Reader | CCTA-AI | Reader | ||
| TP | 245 | 192 | 109 | 135 | |
| FP | 13 | 80 | 13 | 78 | |
| FN | 28 | 16 | 108 | 25 | |
| TN | 32 | 30 | 88 | 80 | |
| Se, % [95% CI] | 90 [85–93] | 93 [88–95] | 50 [43–57] | 84 [78–89] | |
| Sp, % [95% CI] | 71 [55–83] | 27 [19–37] | 87 [79–93] | 51 [43–59] | |
| PPV, % [95% CI] | 95 [91–97] | 71 [65–76] | 89 [82–94] | 63 [56–70] | |
| NPV, % [95% CI] | 53 [40–66] | 65 [50–78] | 45 [38–52] | 76 [67–84] | |
| AUC, % [95% CI] | 85 [81–89] | 61 [56–67] | 78 [73–82] | 64 [58–69] | |
Numbers in parentheses are 95% CIs. CCTA, coronary computed tomographic angiography; AI, artificial intelligence; TP, true positive; FP, false positive; FN, false negative; TN, true negative; Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; AUC, the area under the curve; CI, confidence interval.
Segment-level analysis
Coronary artery segments were analyzed based on SCCT. Coronary artery stenosis of CCTA and ICA were categorized by order of the stenosis grade. The segments of pRCA, mRCA, dRCA, LM, pLAD, mLAD, dLAD, D1, pCx, OM1, and LCx were included. The detailed diagnostic parameters of CCTA-AI and CCTA reader assessed using the segment-based analysis were compared as shown in Tables 5-8.
Table 5
| Accuracy parameters | pRCA | mRCA | dRCA | LM | pLAD | mLAD | dLAD | D1 | pCx | OM1 | LCx |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 |
| Prevalence of stenosis, n [%] | 87 [27] | 81 [25] | 38 [12] | 15 [5] | 144 [45] | 121 [38] | 23 [7] | 49 [15] | 70 [22] | 28 [9] | 61 [19] |
| TP | 43 | 37 | 17 | 6 | 90 | 65 | 2 | 9 | 34 | 6 | 45 |
| FP | 44 | 45 | 33 | 3 | 63 | 73 | 13 | 16 | 40 | 8 | 26 |
| FN | 43 | 44 | 21 | 9 | 54 | 56 | 21 | 40 | 40 | 22 | 50 |
| TN | 188 | 192 | 247 | 300 | 111 | 124 | 282 | 253 | 208 | 282 | 197 |
| Se, % [95% CI] | 50 [39–61] | 46 [35–57] | 45 [29–62] | 40 [17–67] | 63 [54–70] | 54 [44–63] | 9 [1–30] | 18 [9–33] | 46 [34–58] | 21 [9–41] | 47 [37–58] |
| Sp, % [95% CI] | 81 [75–86] | 81 [75–86] | 88 [84–92] | 99 [97–100] | 64 [56–71] | 63 [56–70] | 96 [92–98] | 94 [90–96] | 84 [79–88] | 97 [94–99] | 88 [83–92] |
| PPV, % [95% CI] | 49 [39–60] | 45 [34–56] | 34 [22–49] | 67 [31–91] | 59 [51–67] | 47 [39–56] | 13 [2–42] | 36 [19–57] | 46 [34–58] | 43 [19–70] | 63 [51–74] |
| NPV, % [95% CI] | 81 [76–86] | 81 [76–86] | 92 [88–95] | 97 [94–99] | 67 [59–74] | 69 [62–75] | 93 [89–96] | 86 [82–90] | 84 [79–88] | 93 [89–95] | 80 [74–84] |
| AUC, % [95% CI] | 66 [60–71] | 63 [58–69] | 67 [61–72] | 70 [64–75] | 63 [58–69] | 58 [53–64] | 52 [47–58] | 56 [51–62] | 65 [60–71] | 59 [54–65] | 68 [62–73] |
TP, true positive; FP, false positive; FN, false negative; TN, true negative; Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; AUC, the area under the curve; CI, confidence interval; pRCA, proximal right coronary artery; mRCA, mid-right coronary artery; dRCA, distal right coronary artery; pLAD, proximal left anterior descending artery; mLAD, mid-left anterior descending artery; dLAD, distal left anterior descending artery; D1, first diagonal branch; pCx, proximal circumflex artery; OM1, first obtuse marginal artery; LCx, left circumflex artery.
Table 6
| Accuracy parameters | pRCA | mRCA | dRCA | LM | pLAD | mLAD | dLAD | D1 | pCx | OM1 | LCx |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 |
| Prevalence of stenosis, n [%] | 86 [27] | 77 [24] | 34 [11] | 13 [4] | 137 [43] | 114 [36] | 21 [7] | 48 [15] | 66 [21] | 28 [9] | 93 [29] |
| TP | 42 | 38 | 15 | 8 | 65 | 71 | 2 | 8 | 23 | 5 | 30 |
| FP | 44 | 42 | 45 | 14 | 33 | 67 | 5 | 13 | 14 | 9 | 18 |
| FN | 41 | 39 | 19 | 5 | 72 | 43 | 19 | 40 | 43 | 23 | 63 |
| TN | 191 | 199 | 239 | 291 | 148 | 137 | 293 | 257 | 238 | 281 | 207 |
| Se, % [95% CI] | 51 [39–62] | 49 [38–61] | 44 [28–62] | 62 [32–85] | 47 [39–56] | 62 [53–71] | 9 [1–32] | 17 [8–31] | 35 [24–48] | 18 [7–38] | 32 [23–43] |
| Sp, % [95% CI] | 81 [76–86] | 83 [77–87] | 84 [79–88] | 95 [92–97] | 82 [75–87] | 67 [60–73] | 98 [96–99] | 95 [92–97] | 94 [91–97] | 97 [94–98] | 92 [87–95] |
| PPV, % [95% CI] | 49 [38–60] | 48 [36–59] | 25 [15–38] | 36 [18–59] | 66 [56–75] | 51 [43–60] | 29 [5–70] | 38 [19–61] | 62 [45–77] | 36 [14–64] | 63 [47–76] |
| NPV, % [95% CI] | 82 [77–87] | 84 [78–88] | 93 [89–95] | 98 [96–99] | 67 [61–73] | 76 [69–82] | 94 [90–96] | 87 [82–90] | 85 [80–86] | 92 [89–95] | 77 [71–81] |
| AUC, % [95% CI] | 73 [68–78] | 70 [64–75] | 67 [61–72] | 79 [74–84] | 70 [65–75] | 67 [61–72] | 53 [47–59] | 64 [58–69] | 73 [68–78] | 62 [57–68] | 69 [63–74] |
CCTA, coronary computed tomographic angiography; AI, artificial intelligence; TP, true positive; FP, false positive; FN, false negative; TN, true negative; Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; AUC, the area under the curve; CI, confidence interval; pRCA, proximal right coronary artery; mRCA, mid-right coronary artery; dRCA, distal right coronary artery; pLAD, proximal left anterior descending artery; mLAD, mid-left anterior descending artery; dLAD, distal left anterior descending artery; D1, first diagonal branch; pCx, proximal circumflex artery; OM1, first obtuse marginal artery; LCx, left circumflex artery.
Table 7
| Accuracy parameters | pRCA | mRCA | dRCA | LM | pLAD | mLAD | dLAD | D1 | pCx | OM1 | LCx |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 |
| Prevalence of stenosis, n [%] | 51 [16] | 48 [15] | 24 [8] | 8 [3] | 103 [32] | 78 [25] | 14 [4] | 30 [9] | 47 [15] | 20 [6] | 61 [19] |
| TP | 27 | 16 | 8 | 1 | 54 | 32 | 1 | 5 | 19 | 4 | 24 |
| FP | 24 | 24 | 21 | 1 | 43 | 47 | 13 | 5 | 25 | 2 | 20 |
| FN | 24 | 32 | 16 | 7 | 49 | 46 | 21 | 25 | 28 | 16 | 37 |
| TN | 233 | 246 | 273 | 309 | 172 | 193 | 282 | 283 | 246 | 296 | 237 |
| Se, % [95% CI] | 53 (39–67) | 33 (21–49) | 33 (16–55) | 13 (1–53) | 52 (42–62) | 41 (30–53) | 7 (0–36) | 17 (6–25) | 40 (27–56) | 20 (7–44) | 39 (27–53) |
| Sp, % [95% CI] | 91 (86–94) | 91 (87–94) | 93 (89–95) | 100 (98–100) | 80 (74–85) | 80 (75–85) | 98 (96–99) | 98 (96–99) | 91 (87–94) | 99 (97–100) | 92 (88–95) |
| PPV, % [95% CI] | 53 (39–67) | 40 (25–57) | 28 (13–47) | 50 (3–97) | 56 (45–66) | 41 (30–52) | 14 (1–58) | 50 (20–80) | 43 (29–59) | 67 (24–94) | 55 (39–63) |
| NPV, % [95% CI] | 91 (86–94) | 88 (84–92) | 94 (91–97) | 98 (95–99) | 78 (72–83) | 81 (75–85) | 96 (93–98) | 92 (88–95) | 90 (85–93) | 95 (92–97) | 86 (82–90) |
| AUC, % [95% CI] | 70 (65–75) | 62 (57–68) | 63 (58–68) | 56 (50–62) | 66 (61–71) | 61 (55–66) | 53 (47–58) | 58 (52–63) | 66 (60–71) | 60 (54–65) | 66 (60–71) |
TP, true positive; FP, false positive; FN, false negative; TN, true negative; Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; AUC, the area under the curve; CI, confidence interval; pRCA, proximal right coronary artery; mRCA, mid-right coronary artery; dRCA, distal right coronary artery; pLAD, proximal left anterior descending artery; mLAD, mid-left anterior descending artery; dLAD, distal left anterior descending artery; D1, first diagonal branch; pCx, proximal circumflex artery; OM1, first obtuse marginal artery; LCx, left circumflex artery.
Table 8
| Accuracy parameters | pRCA | mRCA | dRCA | LM | pLAD | mLAD | dLAD | D1 | pCx | OM1 | LCx |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 | 318 |
| Prevalence of stenosis, n [%] | 50 [16] | 77 [24] | 34 [11] | 6 [2] | 98 [31] | 72 [23] | 12 [4] | 52 [16] | 40 [13] | 20 [6] | 60 [19] |
| TP | 12 | 8 | 3 | 3 | 20 | 19 | 0 | 4 | 5 | 0 | 13 |
| FP | 12 | 14 | 11 | 1 | 8 | 24 | 1 | 2 | 2 | 1 | 7 |
| FN | 38 | 37 | 17 | 3 | 78 | 53 | 12 | 25 | 35 | 20 | 47 |
| TN | 256 | 259 | 287 | 311 | 212 | 222 | 305 | 287 | 276 | 297 | 251 |
| Se, % [95% CI] | 24 (14–38) | 18 (9–33) | 15 (4–39) | 50 (14–86) | 20 (13–30) | 26 (17–38) | 0 (0–30) | 14 (5–33) | 13 (5–28) | 0 (0–20) | 22 (12–35) |
| Sp, % [95% CI] | 96 (92–98) | 95 (91–97) | 96 (93–98) | 100 (98–100) | 96 (93–98) | 90 (86–94) | 100 (98–100) | 99 (97–100) | 99 (97–100) | 100 (98–100) | 97 (94–99) |
| PPV, % [95% CI] | 50 (30–70) | 36 (18–59) | 21 (6–51) | 75 (22–99) | 71 (51–86) | 44 (29–60) | 0 (0–95) | 67 (24–94) | 71 (30–95) | 0 (0–95) | 65 (41–84) |
| NPV, % [95% CI] | 87 (83–91) | 88 (83–91) | 94 (91–97) | 99 (97–100) | 73 (68–78) | 81 (75–85) | 96 (93–98) | 92 (88–95) | 89 (85–92) | 94 (90–96) | 84 (79–88) |
| AUC, % [95% CI] | 75 (70–80) | 74 (68–78) | 69 (64–74) | 88 (83–91) | 70 (65–75) | 65 (60–70) | 58 (53–64) | 66 (60–71) | 70 (65–75) | 64 (59–70) | 69 (64–74) |
CCTA, coronary computed tomographic angiography; AI, artificial intelligence; TP, true positive; FP, false positive; FN, false negative; TN, true negative; Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; AUC, the area under the curve; CI, confidence interval; pRCA, proximal right coronary artery; mRCA, mid-right coronary artery; dRCA, distal right coronary artery; pLAD, proximal left anterior descending artery; mLAD, mid-left anterior descending artery; dLAD, distal left anterior descending artery; D1, first diagonal branch; pCx, proximal circumflex artery; OM1, first obtuse marginal artery; LCx, left circumflex artery.
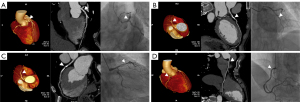
The diagnostic performance of the CCTA-AI platform and CCTA reader at the segment level was moderate for models 1 and 2 (see Figure 3 for specific examples). Furthermore, the AUCs of CCTA-AI were slightly better than those of the CCTA reader in models 1 and 2. With the stenosis cutoff value increasing from model 1 to 2, the diagnostic values of Sp and NPV showed a gradually increasing trend. Conversely, the Se showed a gradually decreasing trend. Meanwhile, the diagnostic performances for segments of large-diameter vessels were better than for narrower ones. For instance, the AUCs of CCTA-AI for identifying stenosis in the pRCA, mRCA, and dRCA segments using model 1 were 0.73, 0.70, and 0.69, respectively.
Discussion
This study aimed to explore the diagnostic performance of one commercialized CCTA-AI platform for the evaluation of coronary artery stenosis compared with a CCTA reader using external multicenter and multidevice data. The main findings of the study can be summarized as follows: (I) at the patient level, the CCTA-AI platform provided a useful tool for the diagnosis of coronary artery stenosis, with significantly improved diagnostic efficiency; and (II) at the segment level, the CCTA-AI platform with models 1 and 2 exhibited moderate diagnostic performance that was slightly better than that of the CCTA reader. In addition, the diagnostic performance in narrow-diameter segments was comparatively weaker.
The results demonstrated that the CCTA-AI platform had a relatively moderate diagnostic performance in the diagnosis of coronary stenosis. The platform was able to accurately identify coronary artery stenosis at the patient level, with results consistent with those of other similar studies (23,24). Thus, the CCTA-AI platform has the potential to be a reliable diagnostic tool for the detection of coronary artery stenosis. In addition, compared with manual processing and reporting times ranging from 18 to 85 minutes, as reported in our and other previous studies (25,26), the mean automatic post-processing time of the CCTA-AI platform was 20.4 seconds. As such, this platform could significantly reduce the work burden of doctors while improving diagnostic efficiency.
The diagnostic performance of CCTA-AI depends on the degree of stenosis at the segment level. At the segment level, compared with model 1, the diagnostic Se of model 2 was generally lower. In contrast, the diagnostic Sp, NPV, and AUC increased. This finding may be related to CCTA itself estimating a relatively high NPV in the judgment of vessel stenosis, particularly in cases of severely stenotic vessels (27). Another factor may be that more severe stenoses tend to exhibit more severe vascular calcification. Consequently, the quantification of stenosis can be overestimated due to artifacts caused by coronary calcification (28). These findings are consistent with those of previous CCTA studies (7,29).
The diagnostic efficacy at the segment level was closely related to the vessel diameter. The Se, PPV, and AUC of large-diameter vessels were comparatively higher than those of narrower ones. Conversely, the Sp and NPV showed the opposite trend. This finding may be due to the impact of CT artifacts in images of narrow vessels caused by cardiac arrhythmia, dense calcifications, or other artifact sources (30). Alternatively, it may be that CCTA has an intrinsically lower accuracy for narrower vessels (especially those <2 mm), as reported previously (29). Thus far, the vast majority of research in this area has focused on diagnostic capacity in the major branches of the coronary artery (31). In summary, the diagnostic performance of narrow-vessel stenosis by CCTA-AI may be relatively limited, which might be down to the nature of CCTA itself, but more external data validation is required to confirm it.
In this study, the CCTA-AI platform was based on a CNN model. CNN models have been widely used for object detection, classification, and segmentation, including in medical images (32-34). Similarly, in cardiovascular research, CNN models have been used to overcome some important issues. Some limitations of these models have been reported, specifically that CCTA-AI has been shown to have limited performance in the diagnosis of narrow vessels (35). Therefore, it might be necessary to introduce CCTA-AI correction measures to correct or compensate for this systematic deviation. It is important to note that the correction must follow a certain rule, for example, coronary artery disease-reporting and data system (CAD-RADS) or CCTA-AI results. However, this is only a preliminary suggestion, and further large-scale multicenter studies using AI computational modeling are needed to confirm the appropriateness of this course of action.
There were some limitations to this study. Although this was a multicenter and multidevice study involving a relatively large sample, the extent and the distribution of disease severity were relatively small and non-homogeneous. More varied data are required to provide a robust AI model. Second, the study did not assess the nature of the plaque or the CT-FFR when evaluating the prognoses of patients and coronary artery hemodynamic effects, even though these are both crucial pieces of information in the treatment of CAD.
Conclusions
This study shows that the commercial CCTA-AI platform may provide a feasible and useful tool for diagnosing coronary artery stenosis at the patient level, as shown using an external multicenter and multidevice dataset. This technology could significantly improve the diagnostic efficiency of stenosis, and radiologists could use the technology as a one-stop tool for rapid post-processing of images. However, the ability of this technology to be used to make a diagnosis, particularly for smaller branches or distal vessels, still needs to be improved.
Acknowledgments
Funding: This work was supported in part by the Chongqing City Science, Technology, and Health Joint Project (No. 2021msxm341) and in part by the Chongqing City Technology Innovation and Application Development (No. cstc2019jscx-msxmX0126).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-1115/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1115/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Southwest Hospital, Third Military Medical University [Army Medical University (No. KY2020306)] and the medical science research ethics committees of 3 other hospitals [The Second People’s Hospital of Liao Cheng (No. 2021-8); UIanqab Central Hospital (No. 2021-3); LinFen central hospital (No. 2021-23-1)]. The requirement for individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145-58. [Crossref] [PubMed]
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56-e528. [Crossref] [PubMed]
- Head SJ, Milojevic M, Daemen J, Ahn JM, Boersma E, Christiansen EH, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet 2018;391:939-48. [Crossref] [PubMed]
- Bartorelli AL, Andreini D, Mushtaq S, Serruys PW. The revolution project: replacing coronary artery angiography with coronary computed tomography with functional evaluation. Eur Heart J Suppl 2020;22:L15-8. [Crossref] [PubMed]
- Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;144:e368-454. [Crossref] [PubMed]
- Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, et al. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299-308. [Crossref] [PubMed]
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407-77. [Crossref] [PubMed]
- Nicol ED, Norgaard BL, Blanke P, Ahmadi A, Weir-McCall J, Horvat PM, Han K, Bax JJ, Leipsic J. The Future of Cardiovascular Computed Tomography: Advanced Analytics and Clinical Insights. JACC Cardiovasc Imaging 2019;12:1058-72. [Crossref] [PubMed]
- Kang D, Dey D, Slomka PJ, Arsanjani R, Nakazato R, Ko H, Berman DS, Li D, Kuo CC. Structured learning algorithm for detection of nonobstructive and obstructive coronary plaque lesions from computed tomography angiography. J Med Imaging (Bellingham) 2015;2:014003. [Crossref] [PubMed]
- He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med 2019;25:30-6. [Crossref] [PubMed]
- Retson TA, Besser AH, Sall S, Golden D, Hsiao A. Machine Learning and Deep Neural Networks in Thoracic and Cardiovascular Imaging. J Thorac Imaging 2019;34:192-201. [Crossref] [PubMed]
- Krittanawong C, Johnson KW, Rosenson RS, Wang Z, Aydar M, Baber U, Min JK, Tang WHW, Halperin JL, Narayan SM. Deep learning for cardiovascular medicine: a practical primer. Eur Heart J 2019;40:2058-73. [Crossref] [PubMed]
- Rønnow Sand NP, Nissen L, Winther S, Petersen SE, Westra J, Christiansen EH, Larsen P, Holm NR, Isaksen C, Urbonaviciene G, Deibjerg L, Husain M, Thomsen KK, Rohold A, Bøtker HE, Bøttcher M. Prediction of Coronary Revascularization in Stable Angina: Comparison of FFR(CT) With CMR Stress Perfusion Imaging. JACC Cardiovasc Imaging 2020;13:994-1004. [Crossref] [PubMed]
- Wan KW, Wong CH, Ip HF, Fan D, Yuen PL, Fong HY, Ying M. Evaluation of the performance of traditional machine learning algorithms, convolutional neural network and AutoML Vision in ultrasound breast lesions classification: a comparative study. Quant Imaging Med Surg 2021;11:1381-93. [Crossref] [PubMed]
- Al'Aref SJ, Anchouche K, Singh G, Slomka PJ, Kolli KK, Kumar A, et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur Heart J 2019;40:1975-86. [Crossref] [PubMed]
- Betancur J, Commandeur F, Motlagh M, Sharir T, Einstein AJ, Bokhari S, Fish MB, Ruddy TD, Kaufmann P, Sinusas AJ, Miller EJ, Bateman TM, Dorbala S, Di Carli M, Germano G, Otaki Y, Tamarappoo BK, Dey D, Berman DS, Slomka PJ. Deep Learning for Prediction of Obstructive Disease From Fast Myocardial Perfusion SPECT: A Multicenter Study. JACC Cardiovasc Imaging 2018;11:1654-63. [Crossref] [PubMed]
- Zreik M, Lessmann N, van Hamersvelt RW, Wolterink JM, Voskuil M, Viergever MA, Leiner T, Išgum I. Deep learning analysis of the myocardium in coronary CT angiography for identification of patients with functionally significant coronary artery stenosis. Med Image Anal 2018;44:72-85. [Crossref] [PubMed]
- Han D, Liu J, Sun Z, Cui Y, He Y, Yang Z. Deep learning analysis in coronary computed tomographic angiography imaging for the assessment of patients with coronary artery stenosis. Comput Methods Programs Biomed 2020;196:105651. [Crossref] [PubMed]
- Li Y, Qiu H, Hou Z, Zheng J, Li J, Yin Y, Gao R. Additional value of deep learning computed tomographic angiography-based fractional flow reserve in detecting coronary stenosis and predicting outcomes. Acta Radiol 2022;63:133-40. [Crossref] [PubMed]
- Dai X, Yu M, Pan J, Lu Z, Shen C, Wang Y, Lu B, Zhang J. Image quality and diagnostic accuracy of coronary CT angiography derived from low-dose dynamic CT myocardial perfusion: a feasibility study with comparison to invasive coronary angiography. Eur Radiol 2019;29:4349-56. [Crossref] [PubMed]
- Dai S, Ding M, Liang N, Li Z, Li D, Guan L, Liu H. Associations of ACE I/D polymorphism with the levels of ACE, kallikrein, angiotensin II and interleukin-6 in STEMI patients. Sci Rep 2019;9:19719. [Crossref] [PubMed]
- Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, Nieman K, Pontone G, Raff GL. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342-58. [Crossref] [PubMed]
- Chen M, Wang X, Hao G, Cheng X, Ma C, Guo N, Hu S, Tao Q, Yao F, Hu C. Diagnostic performance of deep learning-based vascular extraction and stenosis detection technique for coronary artery disease. Br J Radiol 2020;93:20191028. [Crossref] [PubMed]
- Zreik M, van Hamersvelt RW, Wolterink JM, Leiner T, Viergever MA, Isgum I. A Recurrent CNN for Automatic Detection and Classification of Coronary Artery Plaque and Stenosis in Coronary CT Angiography. IEEE Trans Med Imaging 2019;38:1588-98. [Crossref] [PubMed]
- Liu K, Hsieh C, Zhuang N, Gao Y, Li Z, Ren X, Yang L, Zhang J, Budoff MJ, Lu B. Current utilization of cardiac computed tomography in mainland China: A national survey. J Cardiovasc Comput Tomogr 2016;10:76-81. [Crossref] [PubMed]
- Benjamin MM, Rabbat MG. Machine learning-based advances in coronary computed tomography angiography. Quant Imaging Med Surg 2021;11:2208-13. [Crossref] [PubMed]
- Williams MC, Moss A, Nicol E, Newby DE, Cardiac CT. Improves Outcomes in Stable Coronary Heart Disease: Results of Recent Clinical Trials. Curr Cardiovasc Imaging Rep 2017;10:14. [Crossref] [PubMed]
- Murgia A, Balestrieri A, Crivelli P, Suri JS, Conti M, Cademartiri F, Saba L. Cardiac computed tomography radiomics: an emerging tool for the non-invasive assessment of coronary atherosclerosis. Cardiovasc Diagn Ther 2020;10:2005-17. [Crossref] [PubMed]
- Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324-36. [Crossref] [PubMed]
- Abdelrahman KM, Chen MY, Dey AK, Virmani R, Finn AV, Khamis RY, Choi AD, Min JK, Williams MC, Buckler AJ, Taylor CA, Rogers C, Samady H, Antoniades C, Shaw LJ, Budoff MJ, Hoffmann U, Blankstein R, Narula J, Mehta NN. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;76:1226-43. [Crossref] [PubMed]
- Yoneyama H, Nakajima K, Taki J, Wakabayashi H, Matsuo S, Konishi T, Okuda K, Shibutani T, Onoguchi M, Kinuya S. Ability of artificial intelligence to diagnose coronary artery stenosis using hybrid images of coronary computed tomography angiography and myocardial perfusion SPECT. Eur J Hybrid Imaging 2019;3:4. [Crossref] [PubMed]
- McKinney SM, Sieniek M, Godbole V, Godwin J, Antropova N, Ashrafian H, et al. International evaluation of an AI system for breast cancer screening. Nature 2020;577:89-94. [Crossref] [PubMed]
- Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: A cross-sectional study. PLoS Med 2018;15:e1002683. [Crossref] [PubMed]
- Greenspan H, van Ginneken B, Summers RM. Guest Editorial Deep Learning in Medical Imaging: Overview and Future Promise of an Exciting New Technique. IEEE Transactions on Medical Imaging 2016;35:1153-9. [Crossref]
- Muscogiuri G, Van Assen M, Tesche C, De Cecco CN, Chiesa M, Scafuri S, Guglielmo M, Baggiano A, Fusini L, Guaricci AI, Rabbat MG, Pontone G. Artificial Intelligence in Coronary Computed Tomography Angiography: From Anatomy to Prognosis. Biomed Res Int 2020;2020:6649410. [Crossref] [PubMed]


