
Functional magnetic resonance imaging reveals dysfunction of the paraspinal muscles in patients with chronic low back pain: a cross-sectional study
Introduction
Chronic low back pain (CLBP) is a condition of the lower back that lasts at least 12 weeks (1), creates significant disability, and is the most common reason for people to seek medical services. The prevalence of CLBP among adults has been reported to be as high as 20.3% (2). It increases linearly from the third decade of life (3), and 80% of the population will have at least 1 episode of CLBP in their lifetime (4).
The lumbar paraspinal muscles are composed of the erector spinae (ES), multifidus (MF), psoas, and quadratus lumbar muscles (5). The paraspinal muscles play an important role in maintaining spinal stability and correct posture (6). Increased fatty infiltration (7) and decreased cross-sectional areas (8) of the paraspinal muscles have been found in patients with CLBP. However, these studies were based on morphological and compositional analyses of the paraspinal muscles, while the physiological status of the paraspinal muscles has been less thoroughly investigated.
With the development of magnetic resonance technology, functional magnetic resonance imaging (fMRI) has emerged with the ability to reflect the physiological functional status of tissues in addition to their anatomical structure (9). T2 mapping is a quantitative assessment technique mainly used to assess articular cartilage in musculoskeletal disorders (10) and can detect changes in cartilage collagen matrix and cartilage water content (11). Based on this ability, it is increasingly used for other conditions such as tumors (12), muscles (13), and intervertebral discs (14).
Blood oxygen level–dependent (BOLD) MRI is based on the concentration of deoxyhemoglobin in the blood of a specific tissue and can determine its transverse relaxation rate R2* (15). It is widely used to monitor blood perfusion in brain disorders (16). Recently, it has been applied for research on muscle diseases (17), blood perfusion of muscles (18), and activation of muscles (19). In addition, BOLD is usually used to assess muscle perfusion after a given stimulation, such as cuff compression (20). BOLD may be a more reliable technique for evaluating lower limb muscle perfusion in patients with peripheral arterial disease at rest and may provide additional information about skeletal muscle perfusion (21).
Above all, BOLD and T2 mapping can find changes in blood metabolic and perfusion levels in tissues. To our knowledge, few studies have combined these 2 techniques to assess the paraspinal muscles in patients with CLBP, and we thus hypothesized that they could be used to evaluate the functional metabolic and perfusion changes in the paraspinal muscles of patients with CLBP. The purpose of this study was to indirectly investigate whether there were functional changes in the metabolism and perfusion of paraspinal muscles in patients with CLPB by measuring fMRI parameters. We present the following article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1106/rc).
Methods
Participants
In this study, we recruited patients with CLPB and asymptomatic participants aged between 20 and 74 years from December 2019 to November 2020. The patients with CLBP were diagnosed in our outpatient clinic and were scheduled to have MRI examinations. The asymptomatic group comprised individuals and hospital staff with no evidence of CLBP or other diseases on physical examination. The 2 groups were matched for age and sex. This prospective cross-sectional study was approved by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University (No. 2018-L-86) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all individual participants. The inclusion criteria were as follows: patients who had a duration of low back pain for at least 12 weeks (or no low back pain in the case of asymptomatic individuals) and who underwent lumbar spine MRI examination, a BMI of 18.5–23.9 kg/m2, a blood pressure of 90–140/60–90 mmHg, a blood oxygen saturation level of ≥94%, and a heart rate under resting conditions of 60–100 bpm. The exclusion criteria were the following: contraindications for MRI examination or inability to cooperate with MRI scanning; visceral low back pain (such as urinary stones); a history of lumbar spine trauma, fractures, tumors, infections, deformities, surgery, or other medical conditions; a family history of neurological or musculoskeletal diseases; pregnancy; people who had professional muscle training before scanning, such as soldiers, athletes, and fitness enthusiasts; habits which could affect energy metabolism (i.e., smoking, a history of alcohol consumption within 3 days of the scan, and drug addiction); and people who had been treated for CLBP within a month before MRI scanning.
MRI examination
MRI of the lumbar spine was performed using a 3.0 T unit (MR750w, GE Healthcare, Chicago, IL, USA). All participants rested for 30 minutes before the scan was performed, and the participant’s abdomen was compressed with an abdominal bandage to reduce respiratory motion artifacts during the MR examination. All participants wore cotton clothing, and we homogenized the magnetic resonance scanner before scanning the functional sequences. According to some studies of patients with CLBP, the ES and MF muscles at the lower lumbar levels (L4–S1 level) have higher fat infiltration throughout the lumbar segments (7). In addition, magnetic resonance studies of the lumbar paraspinal muscles in asymptomatic individuals have also found that the fat distribution of paraspinal muscles has a higher slice correlation and fat infiltration at the lower lumbar levels (22). Based on these findings, we hypothesized that there would be more significant structural changes macroscopically in the paraspinal muscles at the L4–S1 levels. Therefore, the imaging protocols included routine sagittal T2-weighted imaging, BOLD and T2 mapping, where the scanning range of BOLD and T2 mapping included the L4-S1 levels. Sagittal T2-weighted imaging was used to locate the target intervertebral discs, as shown in Figure 1A, while the axial images at the central level of the L4/5 and L5/S1 intervertebral discs were used for data measurement. The scanning parameters are displayed in Table 1.
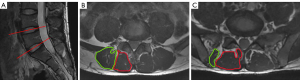
Table 1
| Sequence | TR (ms) | TE (ms) | NEX | FOV (cm2) | Thickness (mm) | Gap size (mm) | Slice number |
|---|---|---|---|---|---|---|---|
| T2WI (sagittal) | 115 | 2,814 | 2 | 32×32 | 4 | 1 | 11 |
| T2 mapping | 628 | 7.1/14.1/21.2/28.3/35.4/42.4/49.5/56.6 | 1 | 24×24 | 4 | 0 | 160 |
| BOLD | 55.5 | 2.0/5.9/9.8/13.7/17.6/21.5 | 2 | 24×24 | 4 | 0 | 120 |
T2WI and T2 mapping–adopted fast spin echo; BOLD-adopted gradient echo. TR, repetition time; TE, echo time; NEX, number of excitations; FOV, field of view; T2WI, T2-weighted imaging; BOLD, blood oxygen level–dependent.
Data measurements
Using the central level of the L4/5 and L5/S1 intervertebral discs as the level of interest, we outlined the contours of the ES and MF muscles using the tracing method, as shown in Figure 1B,1C. The fascia of the muscles and macroscopic fat components at the muscle edges were avoided as much as possible when delineating the region of interest to avoid the influence of fat, but intramuscular fat could not be avoided. In addition, when tracing muscles, we tried to avoid areas where there were artifacts. The R2* and T2 values were measured on the BOLD and T2 mapping sequences, respectively. The R2* and T2 values were calculated automatically using the “R2 star” and “T2 mapping” postprocessing programs, respectively, in the Advantage Workstation 4.6 (GE Healthcare). In addition, we conducted threshold processing for each participant’s image during postprocessing to reduce the impact of noise. All data measurements were performed by 2 experienced radiologists who were blind to the clinical groupings and each other’s measurements.
Statistical analysis
SPSS 26.0 (IBM Corp., Armonk, NY, USA) software was used for the statistical analysis. Normality was verified with the Kolmogorov-Smirnov or Shapiro-Wilk tests. All imaging measurement results are presented as mean ± standard deviation, and a P value <0.05 was considered statistically significant for all measures. Normally distributed data were analyzed with the independent samples t-test, and data that did not conform to normal distribution were analyzed with the Mann-Whitney test. First, we compared the total R2* and T2 values (the sum of 8 muscles at the L4/5 and L5/S1 levels) of the paraspinal muscles between the patients with CLPB and asymptomatic participants. Second, we compared the R2* and T2 values of each level of the paraspinal muscles within each group and between the groups. Third, we performed Pearson correlation analysis between R2* and T2 values and age according to the following scheme: r<0.3, low correlation; 0.3<r<0.5, moderate correlation; 0.5<r<0.7, strong correlation; and r>0.7 very strong correlation (23). In addition, we also compared the differences in R2* and T2 values between the sexes. Finally, intergroup correlation coefficients (ICC) were calculated to test the interrater reliability for the R2* and T2 values. Interobserver variations were estimated using the ICC, with ICC ≥0.9 being excellent, 0.7≤ ICC <0.9 being good, 0.6≤ ICC <0.7 being acceptable, 0.5≤ ICC <0.6 being poor, and ICC <0.5 being unpredictable (5). Good reliability was found for both values (R2*: ICC =0.829; T2 values: ICC =0.853).
According to our preliminary experimental results, the total R2* values of patients with CLBP and asymptomatic participants were 47.2±4.5 and 43.1±1.7 s−1, respectively. Therefore, we set the difference between the mean and standard deviation of the 2 groups as 4.0 and 3.0, respectively; the ratio column between the 2 groups as 1 to 3; and the degree of assurance (power =1−β) as 90%. The bilateral α of the significance level was 0.05. PASS software (NCSS, East Kaysville, UT, USA) calculations indicated that 56 patients with CLBP and 19 asymptomatic participants were required. Ultimately, we recruited 60 patients with CLBP and 20 asymptomatic participants.
Results
Participant characteristics
In this study, we included 60 patients with CLPB (30 females and 30 males) with a mean age of 46.15±12.19 years and 20 asymptomatic participants (10 females and 10 males) with a mean age of 42.45±15.77 years. All participants were included in the analysis, and no data were lost. There were no significant differences in BMI, heart rate, or oxyhemoglobin saturation between the groups (Table 2).
Table 2
| Characteristics | CLBP (n=60) | Asymptomatic participants (n=20) | P |
|---|---|---|---|
| Age (years) | 46.15±12.19 | 42.45±15.77 | 0.347 |
| Sex | |||
| Female | 30 | 10 | – |
| Male | 30 | 10 | – |
| BMI (kg/m2) | 22.59±1.64 | 20.97±1.60 | 0.825 |
| HR (bmp) | 76.75±7.64 | 77.80±5.02 | 0.575 |
| SPO2 (%) | 95.82±1.57 | 96.00±1.31 | 0.642 |
Data are the mean ± standard deviation except for sex. CLBP, chronic low back pain; BMI, body mass index; HR, heart rate; SPO2, oxyhemoglobin saturation.
Comparisons of R2* and T2 values for paraspinal muscles
We compared the R2* and T2 values for a total of 640 muscles at both levels. The mean R2* values for the 8 muscles of the patients with CLPB (46.7±2.9 s−1) were significantly higher than those of the asymptomatic participants (44.0±2.9 s−1) (P=0.001); the mean T2 values for the 8 muscles of the patients with CLPB (45.4±4.2 ms) were lower than those of the asymptomatic participants (47.1±3.7 ms) but not statistically different (Figure 2A,2B). In both groups, there were no differences in R2* or T2 values between the left and right ES and MF, with all P values >0.05 (Table 3). In the patients with CLPB, the R2* values at the L4/5 (45.9±2.1 s−1) level were lower than those at the L5/S1 (47.4±3.6 s−1) (P=0.007). Although there was a similar trend in the asymptomatic participants, the difference was too small to be statistically significant. The T2 values at the L4/5 level were lower than those at the L5/S1 in both groups but significantly so (Table 4). For the different paraspinal muscles, the R2* values of the ES muscles at both levels were also significantly higher in the patients with CLPB than in asymptomatic participants (L4/5: P=0.001; L5/S1: P=0.035); the R2* values of the MF at both levels for the CLBP group were higher than those for the asymptomatic participants (L4/5: P=0.001; L5/S1: P<0.001) (Figure 3A,3B; Figure 4A-4D). However, the T2 values of the ES and MF in the patients with CLPB were lower at both levels than those in the asymptomatic participants, but not significantly so (P>0.05) (Figure 5A,5B). Finally, concerning different sexes, the R2* and T2 values of the paraspinal muscles were not statistically different in either group (Table 5).
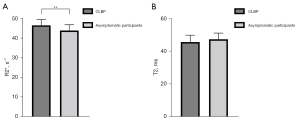
Table 3
| Values | Group | Level | Muscle | Left | Right | P |
|---|---|---|---|---|---|---|
| R2* (s−1) | CLBP | L4–5 | ES | 45.4±2.8 | 45.6±2.7 | 0.824 |
| MF | 46.3±3.3 | 46.6±3.2 | 0.630 | |||
| L5–S1 | ES | 48.3±5.0 | 48.8±5.1 | 0.659 | ||
| MF | 46.2±3.7 | 46.4±3.5 | 0.751 | |||
| Asymptomatic participants | L4–5 | ES | 42.7±3.0 | 43.1±3.2 | 0.674 | |
| MF | 43.0±2.8 | 43.9±3.8 | 0.422 | |||
| L5–S1 | ES | 46.2±4.7 | 45.6±4.1 | 0.674 | ||
| MF | 42.6±2.7 | 42.3±3.1 | 0.787 | |||
| T2 (ms) | CLBP | L4–5 | ES | 45.0±4.3 | 45.4±4.7 | 0.641 |
| MF | 44.7±4.6 | 44.8±4.3 | 0.902 | |||
| L5–S1 | ES | 46.2±5.9 | 46.9±6.0 | 0.558 | ||
| MF | 44.4±4.0 | 44.6±4.0 | 0.870 | |||
| Asymptomatic participants | L4–5 | ES | 46.6±2.8 | 46.7±1.7 | 0.903 | |
| MF | 46.3±4.6 | 46.2±3.9 | 0.959 | |||
| L5–S1 | ES | 47.8±5.4 | 48.5±5.8 | 0.714 | ||
| MF | 45.1±2.5 | 45.0±2.8 | 0.815 |
All values are the mean ± standard deviation. CLBP, chronic low back pain; ES, erector spinae; MF, multifidus.
Table 4
| Group | Values | L4–5 | L5–S1 | P |
|---|---|---|---|---|
| CLBP | R2* (s−1) | 45.9±2.1 | 47.4±3.6 | 0.007** |
| T2 (ms) | 45.7±5.1 | 46.0±5.7 | 0.759 | |
| Asymptomatic participants | R2* (s−1) | 43.8±3.8 | 44.2±3.0 | 0.696 |
| T2 (ms) | 47.1±3.7 | 47.2±4.0 | 0.932 |
All values are the mean ± standard deviation. **, indicates a statistically significant result. CLBP, chronic low back pain.

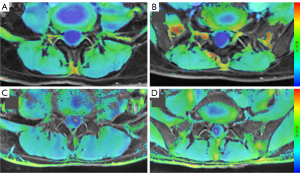

Table 5
| Group | Values | Male | Female | P |
|---|---|---|---|---|
| CLBP | R2* (s−1) | 46.4±2.9 | 46.9±3.0 | 0.767 |
| T2 (ms) | 44.7±4.0 | 46.1±4.4 | 0.317 | |
| Asymptomatic participants | R2* (s−1) | 44.5±3.4 | 43.4±2.5 | 0.230 |
| T2 (ms) | 46.4±3.1 | 47.9±4.3 | 0.407 |
All values are mean ± standard deviation. CLBP, chronic low back pain.
Correlation analysis
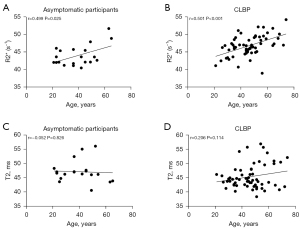
Correlation analysis between R2* and T2 values and age indicated that R2* values increased with age in the asymptomatic participants and showed a moderate correlation (P<0.05; r=0.499) (Figure 6A), while patients with CLBP showed a strong correlation (P<0.05; r=0.501) (Figure 6B). No significant correlations were found for the other group (Figure 6C,6D).
Discussion
This study aimed to indirectly assess the metabolism and perfusion function of paraspinal muscles by measuring R2* and T2 values of the paraspinal muscles in patients with CLBP and asymptomatic participants using fMRI. We found that the R2* values of different muscles in patients with CLBP were significantly higher than those in asymptomatic participants, both overall and at different levels, while the results were the opposite for the T2 values.
The reason for the difference in R2* values between the 2 groups was related to the imaging principle of the BOLD sequence. The BOLD signal in skeletal muscle tissues depends on the ratio between oxyhemoglobin and deoxyhemoglobin in small muscle vessels and can reflect the microcirculation of the tissues (24). Specifically, deoxyhemoglobin is paramagnetic, which leads to heterogeneity of the local magnetic field and can shorten the T2* value of tissues (20), while R2* and T2* have a reciprocal relationship with each other (25). Therefore, when the content of deoxygenated hemoglobin in tissue is higher, the effect of magnetic field inhomogeneity is stronger, which leads to lower T2* values and larger R2* values for the tissue (26). In other words, the amount of deoxyhemoglobin in the tissue is proportional to the R2* values (27). Therefore, based on the results of our study, we could carefully extrapolate and conclude that the deoxyhemoglobin concentration in the paraspinal muscles was higher in patients with CLBP than in asymptomatic participants. However, muscle oxygenation capacity or oxygen metabolism may represent only one aspect of muscle perfusion. This may thus indicate dysfunction of metabolism and perfusion in the paraspinal muscles of patients with CLBP, as studies using muscle biopsies show that blood vessels are reduced in the lumbar MF muscle of patients with chronic degenerative lumbar pathology (28). In addition, we found that the paraspinal muscles at the L5/S1 level in patients with CLBP had more deoxyhemoglobin than at the L4/5, which may be associated with greater fatty infiltration at the L5/S1 level. Of course, many factors affect tissue oxygen metabolism, such as lactate level, blood volume, or hemoglobin concentration (29), so we used strict inclusion and exclusion criteria in this study. In addition, we ensured the participants wore cotton clothes during the scanning and homogenized the magnetic resonance scanner before scanning the functional sequence to ensure the homogeneity of the magnetic field. It has been found that the fiber type of paraspinal muscles changes in patients with CLBP, which may also affect the oxygen metabolism of the tissue (20). We encountered this phenomenon in our study but did not attempt to explain its deeper causes, and thus the specific mechanism needs to be further investigated.
Some researchers have pointed out that BOLD can noninvasively evaluate muscle perfusion to reflect the microvascular condition of muscles (30). In our study, patients with CLPB showed poor perfusion function in the paraspinal muscles, which might have been related to poor microvascular function. A previous biopsy study found an increase in capillary density after exercise training (31). Therefore, we recommend that some moderate exercise training for the paraspinal muscles of patients with CLBP is beneficial and can improve the perfusion function of the paraspinal muscles.
In this study, the T2 values of the paraspinal muscles in patients with CLPB were shorter than those of the asymptomatic participants, but the difference was not statistically significant. Transverse relaxation times (T2 values) are determined primarily by the water content of the tissue, with higher water content resulting in longer T2 values (32). Muscle is a water-rich tissue and contains approximately 76% water (33). In patients with CLBP, fatty infiltration is one of the major changes in the paraspinal muscles (34). According to related studies, lean muscle area decreases and fat area increases in paraspinal muscles in patients with lumbar degenerative diseases (35). A reduction in the area of pure muscle means a reduction in the water content of the muscle and a corresponding decrease in T2 values. However, fat content also impacts T2 values. One study of the liver found that higher liver fat content was associated with longer T2 time (36), while another study reported the fat fraction of muscle to be positively correlated with T2 values (37). To minimize the effect of fat on measurements, we avoided macroscopically visible fat areas when delineating regions of interest, but fat within the muscle could not be avoided. Because water and fat coexist in muscle, the measured T2 values do not distinguish between the contribution of water and fat. Sinclair et al. assessed the feasibility of new techniques [The IDEAL–Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence] to separate the T2 values of water and fat in order to quantify the T2 values of water alone (38). Muscular fibrosis is also present in the paraspinal muscles of patients with low back pain; indeed, fibrotic gene expression has been shown to be elevated in these patients (39), and the degree of tissue fibrosis has been negatively correlated with T2 values (36). In the present study, however, we could not determine the weight of the pure muscle area reduction, fat infiltration, or fibrosis on T2 values. Because there are many factors affecting T2 values, a difference in T2 values between the 2 groups was not evident in our experiment although we found a trend of faster T2 times in patients with CLBP than in asymptomatic participants. The relatively small sample number might have also influenced our results.
Some studies have indicated that there is asymmetry in the cross-sectional area of the MF muscle in patients with low back pain (40) and that the paraspinal muscles of the dominant arm have a larger cross-sectional area (41). Due to the possible structural asymmetry of the paraspinal muscles, we speculated that the fMRI parameters might change, but we found no difference. This may be related to the fact that we did not determine the dominant arm or leg of the participants, which will be further explored in our later study. Another possible reason for this is that the structural changes in the muscles are out of sync with the functional changes. In addition, it was found that the R2* values of the paraspinal muscles were higher in patients with CLBP, which may indicate dysfunction of metabolism and perfusion. However, long-term metabolism and perfusion disorders can lead to muscle cell atrophy and greater fat content, which may be related to changes in the paraspinal muscle structure after CLBP.
In addition, we found that in the patients with CLPB and asymptomatic participants, R2* values were positively correlated with age, which may mean that microcirculation and perfusion of the paraspinal muscles change with age. One study found that microvascular function decreased with age (30), which partly supports the results of our study. In addition, in living tissues, water and deoxyhemoglobin are not independent, and the two are closely linked to the biological metabolism and can influence each other (19). However, our study did not determine the effect of any interaction that might have occurred.
Our study also has some limitations. First, we used a relatively small sample size. Second, the metabolism and perfusion levels of paraspinal muscles are affected by a variety of factors, and although we controlled for the effects of blood pressure, oxygen saturation, drugs, and smoking, there were still some factors that we could not account for, such as lactate level, blood volume, hemoglobin concentration (29), or extracellular water content (19). Third, because we chose the central level of the disc as the level of interest, the entire paraspinal muscle was not completely assessed.
Conclusions
We found that R2* values were significantly higher in the paraspinal muscles of patients with CLPB than in asymptomatic participants, which may indicate metabolism and perfusion dysfunction in the paraspinal muscles of patients with CLPB.
Acknowledgments
We would like to thank the magnetic resonance technicians of the Department of Medical Imaging of the First Affiliated Hospital of Kunming Medical University for their help with the scans and as well all participants in this study.
Funding: This work was supported by the National Natural Science Foundation of China (No. 82260338), the Applied Basic Research Project of Yunnan Province- Kunming Medical University Joint Fund (No. 202001AY070001-038), the Yunnan Provincial Radiology and Therapy Clinical Medicine Center Project (No. 202102AA100067), and the Doctoral Foundation of the First Affiliated Hospital of Kunming Medical University (No. 2021BS012); these funds were used to recruit participants, delineate regions of interest, search relevant literature, and purchase instruments, respectively.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-1106/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1106/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University (No. 2018-L-86). Informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ho EK, Chen L, Simic M, Ashton-James CE, Comachio J, Wang DXM, Hayden JA, Ferreira ML, Ferreira PH. Psychological interventions for chronic, non-specific low back pain: systematic review with network meta-analysis. BMJ 2022;376:e067718. [Crossref] [PubMed]
- Meucci RD, Fassa AG, Faria NM. Prevalence of chronic low back pain: systematic review. Rev Saude Publica 2015;49:1. [Crossref] [PubMed]
- Faur C, Patrascu JM, Haragus H, Anglitoiu B. Correlation between multifidus fatty atrophy and lumbar disc degeneration in low back pain. BMC Musculoskelet Disord 2019;20:414. [Crossref] [PubMed]
- Wang H, Liu C, Meng Z, Zhou W, Chen T, Zhang K, Wu A. Real-world study for identifying the predictive factors of surgical intervention and the value of magnetic resonance imaging in patients with low back pain. Quant Imaging Med Surg 2022;12:1830-43. [Crossref] [PubMed]
- Kiram A, Hu Z, Man GC, Ma H, Li J, Xu Y, Qian Z, Zhu Z, Liu Z, Qiu Y. The role of paraspinal muscle degeneration in coronal imbalance in patients with degenerative scoliosis. Quant Imaging Med Surg 2022;12:5101-13. [Crossref] [PubMed]
- Fortin M, Videman T, Gibbons LE, Battié MC. Paraspinal muscle morphology and composition: a 15-yr longitudinal magnetic resonance imaging study. Med Sci Sports Exerc 2014;46:893-901. [Crossref] [PubMed]
- Sollmann N, Bonnheim NB, Joseph GB, Chachad R, Zhou J, Akkaya Z, Pirmoazen AM, Bailey JF, Guo X, Lazar AA, Link TM, Fields AJ, Krug R. Paraspinal Muscle in Chronic Low Back Pain: Comparison Between Standard Parameters and Chemical Shift Encoding-Based Water-Fat MRI. J Magn Reson Imaging 2022;56:1600-8. [Crossref] [PubMed]
- Goubert D, De Pauw R, Meeus M, Willems T, Cagnie B, Schouppe S, Van Oosterwijck J, Dhondt E, Danneels L. Lumbar muscle structure and function in chronic versus recurrent low back pain: a cross-sectional study. Spine J 2017;17:1285-96. [Crossref] [PubMed]
- Gao Y, Lu Z, Lyu X, Liu Q, Pan S. A Longitudinal Study of T2 Mapping Combined With Diffusion Tensor Imaging to Quantitatively Evaluate Tissue Repair of Rat Skeletal Muscle After Frostbite. Front Physiol 2021;11:597638. [Crossref] [PubMed]
- Matsuki K, Watanabe A, Ochiai S, Kenmoku T, Ochiai N, Obata T, Toyone T, Wada Y, Okubo T. Quantitative evaluation of fatty degeneration of the supraspinatus and infraspinatus muscles using T2 mapping. J Shoulder Elbow Surg 2014;23:636-41. [Crossref] [PubMed]
- Wuennemann F, Kintzelé L, Braun A, Zeifang F, Maier MW, Burkholder I, Weber MA, Kauczor HU, Rehnitz C. 3-T T2 mapping magnetic resonance imaging for biochemical assessment of normal and damaged glenoid cartilage: a prospective arthroscopy-controlled study. Sci Rep 2020;10:14396. [Crossref] [PubMed]
- Wu Q, Zhu LN, Jiang JS, Bu SS, Xu XQ, Wu FY. Characterization of parotid gland tumors using T2 mapping imaging: initial findings. Acta Radiol 2020;61:629-35. [Crossref] [PubMed]
- Keller S, Yamamura J, Sedlacik J, Wang ZJ, Gebert P, Starekova J, Tahir E. Diffusion tensor imaging combined with T2 mapping to quantify changes in the skeletal muscle associated with training and endurance exercise in competitive triathletes. Eur Radiol 2020;30:2830-42. [Crossref] [PubMed]
- Zeng F, Zha Y, Li L, Xing D, Gong W, Hu L, Fan Y. A comparative study of diffusion kurtosis imaging and T2* mapping in quantitative detection of lumbar intervertebral disk degeneration. Eur Spine J 2019;28:2169-78. [Crossref] [PubMed]
- Wei X, Hu R, Zhou X, Ni L, Zha D, Feng H, Xu H, Wu X. Alterations of Renal Function in Patients with Diabetic Kidney Disease: A BOLD and DTI Study. Comput Intell Neurosci 2022;2022:6844102. [Crossref] [PubMed]
- Vu C, Chai Y, Coloigner J, Nederveen AJ, Borzage M, Bush A, Wood JC. Quantitative perfusion mapping with induced transient hypoxia using BOLD MRI. Magn Reson Med 2021;85:168-81. [Crossref] [PubMed]
- Lopez C, Taivassalo T, Berru MG, Saavedra A, Rasmussen HC, Batra A, Arora H, Roetzheim AM, Walter GA, Vandenborne K, Forbes SC. Postcontractile blood oxygenation level-dependent (BOLD) response in Duchenne muscular dystrophy. J Appl Physiol (1985) 2021;131:83-94. [PubMed]
- Suo S, Tang H, Lu Q, Zhang L, Ni Q, Cao M, Chen Z, Zhao H, Sun B, Xu J. Blood oxygenation level-dependent cardiovascular magnetic resonance of the skeletal muscle in healthy adults: Different paradigms for provoking signal alterations. Magn Reson Med 2021;85:1590-601. [Crossref] [PubMed]
- Huang YL, Zhou JL, Jiang YM, Zhang ZG, Zhao W, Han D, He B. Assessment of lumbar paraspinal muscle activation using fMRI BOLD imaging and T2 mapping. Quant Imaging Med Surg 2020;10:106-15. [Crossref] [PubMed]
- Törngren K, Eriksson S, Arvidsson J, Falkenberg M, Johnsson A A, Sjoberg C, Lagerstrand K, Nordanstig J. A Reperfusion BOLD-MRI Tissue Perfusion Protocol Reliably Differentiate Patients with Peripheral Arterial Occlusive Disease from Healthy Controls. J Clin Med 2021;10:3643. [Crossref] [PubMed]
- Suo S, Zhang L, Tang H, Ni Q, Li S, Mao H, Liu X, He S, Qu J, Lu Q, Xu J. Evaluation of skeletal muscle microvascular perfusion of lower extremities by cardiovascular magnetic resonance arterial spin labeling, blood oxygenation level-dependent, and intravoxel incoherent motion techniques. J Cardiovasc Magn Reson 2018;20:18. [Crossref] [PubMed]
- Crawford RJ, Filli L, Elliott JM, Nanz D, Fischer MA, Marcon M, Ulbrich EJ. Age- and Level-Dependence of Fatty Infiltration in Lumbar Paravertebral Muscles of Healthy Volunteers. AJNR Am J Neuroradiol 2016;37:742-8. [Crossref] [PubMed]
- Shi L, Yan B, Jiao Y, Chen Z, Zheng Y, Lin Y, Cao P. Correlation between the fatty infiltration of paraspinal muscles and disc degeneration and the underlying mechanism. BMC Musculoskelet Disord 2022;23:509. [Crossref] [PubMed]
- Partovi S, Schulte AC, Staub D, Jacobi B, Aschwanden M, Walker UA, Imfeld S, Broz P, Benz D, Zipp L, Takes M, Jäger KA, Huegli RW, Bilecen D. Correlation of skeletal muscle blood oxygenation level-dependent MRI and skin laser Doppler flowmetry in patients with systemic sclerosis. J Magn Reson Imaging 2014;40:1408-13. [Crossref] [PubMed]
- Partovi S, Schulte AC, Jacobi B, Klarhöfer M, Lumsden AB, Loebe M, Davies MG, Noon GP, Karmonik C, Zipp L, Bongartz G, Bilecen D. Blood oxygenation level-dependent (BOLD) MRI of human skeletal muscle at 1.5 and 3 T. J Magn Reson Imaging 2012;35:1227-32. [Crossref] [PubMed]
- Partovi S, Karimi S, Jacobi B, Schulte AC, Aschwanden M, Zipp L, Lyo JK, Karmonik C, Müller-Eschner M, Huegli RW, Bongartz G, Bilecen D. Clinical implications of skeletal muscle blood-oxygenation-level-dependent (BOLD) MRI. MAGMA 2012;25:251-61. [Crossref] [PubMed]
- Wu G, Zhang R, Mao H, Chen Y, Liu G, Zhang J. The value of blood oxygen level dependent (BOLD) imaging in evaluating post-operative renal function outcomes after laparoscopic partial nephrectomy. Eur Radiol 2018;28:5035-43. [Crossref] [PubMed]
- Shahidi B, Hubbard JC, Gibbons MC, Ruoss S, Zlomislic V, Allen RT, Garfin SR, Ward SR. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J Orthop Res 2017;35:2700-6. [Crossref] [PubMed]
- Jacobi B, Bongartz G, Partovi S, Schulte AC, Aschwanden M, Lumsden AB, Davies MG, Loebe M, Noon GP, Karimi S, Lyo JK, Staub D, Huegli RW, Bilecen D. Skeletal muscle BOLD MRI: from underlying physiological concepts to its usefulness in clinical conditions. J Magn Reson Imaging 2012;35:1253-65. [Crossref] [PubMed]
- Hurley DM, Williams ER, Cross JM, Riedinger BR, Meyer RA, Abela GS, Slade JM. Aerobic Exercise Improves Microvascular Function in Older Adults. Med Sci Sports Exerc 2019;51:773-81. [Crossref] [PubMed]
- Prior SJ, Goldberg AP, Ortmeyer HK, Chin ER, Chen D, Blumenthal JB, Ryan AS. Increased Skeletal Muscle Capillarization Independently Enhances Insulin Sensitivity in Older Adults After Exercise Training and Detraining. Diabetes 2015;64:3386-95. [Crossref] [PubMed]
- Das T, Roos JCP, Patterson AJ, Graves MJ, Murthy R. T2-relaxation mapping and fat fraction assessment to objectively quantify clinical activity in thyroid eye disease: an initial feasibility study. Eye (Lond) 2019;33:235-43. [Crossref] [PubMed]
- Serra-Prat M, Lorenzo I, Palomera E, Yebenes J C, Campins L, Cabre M. Intracellular Water Content in Lean Mass is Associated with Muscle Strength, Functional Capacity, and Frailty in Community-Dwelling Elderly Individuals. A Cross-Sectional Study. Nutrients 2019;11:661. [Crossref] [PubMed]
- Mengiardi B, Schmid MR, Boos N, Pfirrmann CW, Brunner F, Elfering A, Hodler J. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: quantification with MR spectroscopy. Radiology 2006;240:786-92. [Crossref] [PubMed]
- Lee E T, Lee S A, Soh Y, Yoo M C, Lee J H, Chon J. Association of Lumbar Paraspinal Muscle Morphometry with Degenerative Spondylolisthesis. Int J Environ Res Public Health 2021;18:4037. [Crossref] [PubMed]
- Idilman IS, Celik A, Savas B, Idilman R, Karcaaltincaba M. The feasibility of T2 mapping in the assessment of hepatic steatosis, inflammation, and fibrosis in patients with non-alcoholic fatty liver disease: a preliminary study. Clin Radiol 2021;76:709.e13-8. [Crossref] [PubMed]
- Yin L, Xie ZY, Xu HY, Zheng SS, Wang ZX, Xiao JX, Yuan Y. T2 Mapping and Fat Quantification of Thigh Muscles in Children with Duchenne Muscular Dystrophy. Curr Med Sci 2019;39:138-45. [Crossref] [PubMed]
- Sinclair CD, Morrow JM, Janiczek RL, Evans MR, Rawah E, Shah S, Hanna MG, Reilly MM, Yousry TA, Thornton JS. Stability and sensitivity of water T(2) obtained with IDEAL-CPMG in healthy and fat-infiltrated skeletal muscle. NMR Biomed 2016;29:1800-12. [Crossref] [PubMed]
- Shahidi B, Fisch KM, Gibbons MC, Ward SR. Increased Fibrogenic Gene Expression in Multifidus Muscles of Patients With Chronic Versus Acute Lumbar Spine Pathology. Spine (Phila Pa 1976) 2020;45:E189-95. [Crossref] [PubMed]
- Gildea JE, Hides JA, Hodges PW. Size and symmetry of trunk muscles in ballet dancers with and without low back pain. J Orthop Sports Phys Ther 2013;43:525-33. [Crossref] [PubMed]
- Ranson C, Burnett A, O'Sullivan P, Batt M, Kerslake R. The lumbar paraspinal muscle morphometry of fast bowlers in cricket. Clin J Sport Med 2008;18:31-7. [Crossref] [PubMed]


