
Diagnosing sentinel lymph node metastasis of T1/T2 breast cancer with conventional ultrasound combined with double contrast-enhanced ultrasound: a preliminary study
Introduction
The axillary lymph node is the most common site of local metastasis in patients with breast cancer (BC) and is an important prognostic factor for overall BC survival. Sentinel lymph node biopsy (SLNB) is considered the standard treatment for axillary staging in patients with T1/T2 invasive BC (1,2). If metastatic SLN is present, some patients may require a second axillary surgery (axillary lymph node dissection; ALND) (3,4). Therefore, the identification of appropriate candidates who can avoid an unnecessary SLNB and proceed directly to ALND is quite challenging. Over the years, great efforts have been made to develop new, accurate, noninvasive methods to assess the status of SLNs before SLNB.
Ultrasound (US) examination is the preferred method for evaluating lymph node status in patients with BC. Some studies have analyzed the whole ALNs using US, but the inclusion of all ALNs in T1/T2 BC patients who are clinical axillary negative may lead to an increase in the false-positive rate and a decrease in accuracy in the diagnosis of ALN metastasis (5,6). Zhang et al. (6) used US to analyze the entire ALN and found that the sensitivity, specificity, and accuracy of axillary US were 69.4%, 81.8%, and 77.0%, respectively. Some studies mainly focused on BC features to predict the pathology of ALNs, ignoring the imaging features of ALNs (7,8). As a new technique of US imaging, intravenous contrast-enhanced ultrasound (ICEUS) can obtain more perfusion information, which is helpful for the differential diagnosis of benign and malignant lymph nodes (9,10). After injection of a contrast agent into the intracutaneous layer of the areola (percutaneous contrast-enhanced ultrasound, PCEUS), the sentinel lymph node (SLN) can be traced along the enhanced lymphatic vessels, a method which has been confirmed to have high consistency with the dye method commonly used in the clinic (11,12). Previous studies have shown that the enhancement pattern of PCEUS correlates with SLN metastasis (13,14). The combination of ICEUS and PCEUS (double CEUS) can observe the SLN perfusion from both lymphatic vessels and venous ways, through which the SLN can be evaluated more comprehensively.
The aim of this study was to investigate the value of conventional US combined with double CEUS in diagnosing SLN metastases in patients with T1/T2 BC. In this study, each SLN was labeled to ensure the US features of the lymph node were completely consistent with the pathology. Therefore, we could accurately analyze the relationships between the US features of SLNs and pathology and establish a diagnostic model for SLN metastasis. If this model can be used to diagnose SLN metastasis efficiently and noninvasively before surgery, it can provide more information for clinicians to make treatment plans (surgical plans). We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1175/rc).
Methods
Patients
The prospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (No. 2016108). Informed consent was obtained from all the patients. The participants formed a convenience sample, with the sample size of this study being determined based on feasibility considerations.
A total of 174 female patients with BC treated in the First Affiliated Hospital of Soochow University from July 2017 to December 2021 were initially enrolled in our study. The inclusion criteria were as follows: (I) patients with TI/T2 invasive BC confirmed by pathology, (II) patients with clinically ALN-negative BC (with negative or suspected ALN under US), (III) patients with a single tumor, and (IV) patients scheduled to undergo SLNB. The exclusion criteria were as follows: (I) patients with positive axillary US [absence of fatty hilum and transverse to longitudinal diameter ratio (T/L ratio) ≤1], (II) patients with positive ALN confirmed by pathology, (III) patients with inflammatory BC, (VI) patients allergic to the contrast agent, and (V) patients who had received therapy. The processes of inclusion and exclusion of study participants is shown in Figure 1.
Instruments and methods
Ultrasonography was performed using the Resona7 (Mindray Medical International) and MyLab ClassC (Esaote Group) US systems equipped with high-frequency linear array probes. Conventional US was performed with LA523 or L14-6WU probes. CEUS imaging was performed with LA522 or L11-3U probes. To reduce microbubble destruction, low mechanical index (MI) values were applied (MI 0.02–0.07). The contrast agent used in this study was SonoVue (Bracco SpA). The breast tissue marker needles (Model 864017D, containing a marker approximately 3 mm in diameter) were manufactured by Bard. Nonabsorbent sutures supplied by Ethicon were used. The SLN tracers were carbon nanoparticles suspension injection (Lummy Pharmaceutical Co., Ltd.) and methylthioninium chloride injection (Jumpcan Pharmaceutical Group).
Conventional US, double CEUS, and SLN localization
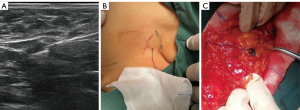
All patients were examined with US in the supine position with their arms in the abduction position to fully expose the affected breast and armpit. The procedure consisted of 4 steps. (I) For PCEUS, the contrast agent was prepared by adding 5.0 mL of normal saline to SonoVue dry powder. The areola skin of each patient was sterilized. A total dose of 4.0 mL of the US contrast agent SonoVue was then injected into the intracutaneous layer of the areola at the 12, 3, 6, and 9 o’clock positions (i.e., 4 injections of 1.0 mL), and the areola area was massaged for 30 seconds. The probe for CEUS (LA522, L11-3U) was then selected, and the contrast mode was applied. First, the lymphatic vesselsnext to the areola were examined. Then, continuous dynamic scanning to the axillary direction along the enhanced lymph channels (LCs) was applied to find the enhanced lymph node (i.e., the SLN), and the filling of contrast medium in SLNs was observed. The videos were saved to a hard disk. (II) For conventional US, the probe for conventional US (LA523, L14-6WU) was selected. The position, shape, echotexture, and internal blood flow of SLNs were then observed and recorded, and the maximum long-axis and the maximum short-axis view of SLNs were selected. (III) For ICEUS, the probe for CEUS (LA522, L11-3U) was selected, and the contrast mode was chosen. After the agents in LCs and SLNs were absorbed, the maximum long-axis section of the lymph nodes was selected, and 2.0 mL of the agents were intravenously injected. The contrast agent perfusion of SLNs was immediately observed for about 90 seconds. The video image was stored on a hard drive. (Ⅳ) For SLN localization, a breast tissue marker needle was modified by tying a suture to the tissue marker and then insertion of the tissue marker with a suture into the positioning needle. After axillary disinfection and subcutaneous local infiltration anesthesia, under the guidance of US, the modified tissue marker needle was punctured into the SLN. The tissue marker with a suture was ejected and left in the lymph node. The end of the suture tied to the marker was exposed to the skin (Figure 2). The above examinations (conventional US and double CEUS) were conducted on the day before the SLNB.
Surgery and pathology
During surgery, 0.1 mL of carbon nanoparticles and 0.5 mL of methylthioninium chloride were treated by intradermal injection around the areola. The SLNB was performed in the axilla to find the stained lymph nodes and the marked lymph nodes along the surgical suture. The labeled node (Figure 2) and other stained nodes were removed separately for pathological examination (hematoxylin and eosin staining). Pathologically, SLNs were divided into the nonmetastatic SLN group and the metastatic SLN group.
Image analysis
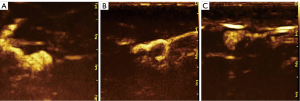
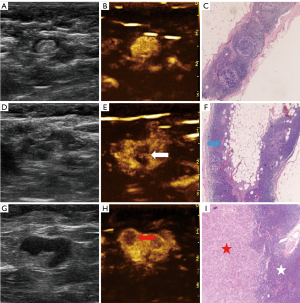
Two senior sonographers analyzed all images independently without knowing the patient’s clinical information or postoperative pathological findings. The maximum section of SLNs was selected, and the longitudinal diameter, transverse diameter, and cortical thickness were measured. All data were measured 3 times, and an average value was obtained. After a review of the PCEUS videos, the enhancement was divided into 3 types (Figure 3) according to the different perfusion features of SLNs: type I, an SLN with homogeneous enhancement; type II, margins of the SLN with annular enhancement and weak or no internal enhancement; and type III, other obvious heterogeneous enhancement types except for annular enhancement. After the videos of ICEUS were reviewed, the enhancement was divided into 3 types (Figure 4): type A, an SLN with obvious and homogeneous enhancement; type B, obvious heterogeneous enhancement with focally weak or no enhancement in the fatty hilum; and type C, obvious heterogeneous enhancement with focally weak or no enhancement in the cortex). Meanwhile, the enhancement direction of SLNs was evaluated: the centrifugal type was enhancement from the fatty hilum to the cortex; the overall type was synchronous enhancement in the cortex and fatty hilum; and the centripetal type was enhancement from the periphery to the center (i.e., the enhancement starting from the cortex part of the lymph node). If the sonographers’ interpretations were inconsistent, an agreement was reached after discussion in order to obtain the final diagnosis.
Statistical analysis
The interobserver and intraobserver agreements were evaluated by calculating the kappa value or intraclass correlation coefficient (ICC value), respectively. The kappa value was calculated for continuous variables, and the ICC value was calculated for continuous variables. The Shapiro-Wilk test was used to verify the normality of quantitative data. If the data fit the normal distribution, they are expressed as the mean and standard deviation (SD), and the difference was compared with the t-test. If the data did not fit the normal distribution, they are expressed as the median and interquartile range (IQR), and the difference was compared with a nonparametric test. Qualitative data were statistically analyzed with the χ2 test or Fisher exact test. Logistic regression analysis was performed based on the significant variables obtained from univariate analysis. Receiver operating characteristic (ROC) curves were used to assess the accuracy, specificity, sensitivity, and 95% CI of the prediction model. All statistical analyses were performed using SPSS 20.0 (IBM Corp.). A P value <0.05 was regarded as statistically significant. Among the variables included in the multivariate regression, cortical thickness was the continuous variable, and other variables were categorical variables. The references of fatty hilum, blood flow classification, PCEUS enhancement type, ICEUS enhancement type, and ICEUS enhancement direction were central, central, type Ⅰ, type A, and centrifugal, respectively.
A nomogram was formulated based on the multivariate logistic regression analysis results and by using the “rms” package of R version 4.0 (http://www.r-project.org/). The predictive performance of the nomogram was measured with the concordance index (C-index) calibrated with 1,000 bootstrap samples to decrease the overfit bias.
Results
Clinical characteristics of the patients
Four markers were found in the adipose tissue but not the lymph nodes, and these cases were excluded. A total of 163 patients were analyzed in this study, including 152 invasive ductal carcinomas, 4 invasive lobular carcinomas, 4 mucinous adenocarcinomas, and 3 solid papillary carcinomas. The median age was 54.00 years (IQR, 46.00–61.50 years; range, 28–80 years). A total of 165 SLNs were labeled with the modified tissue marker needles. All labeled lymph nodes were stained with dye during surgery. Based on the pathologic findings, 54 SLNs were metastatic lymph nodes, and 111 SLNs were nonmetastatic lymph nodes. No associated adverse events occurred in this study.
The interobserver reproducibility of US image characteristics between the 2 senior sonographers for 165 SLNs was high. The ICC values of breast tumor size, T/L ratio, and cortical thickness were 0.993, 0.995, and 0.996, respectively. The kappa values of fatty hilum, blood flow classification, PCEUS enhancement type, and ICEUS enhancement type and direction were 0.867, 0.938, 0.927, 0.911, and 0.923, respectively.
Ultrasonographic features of SLNs
Table 1 summarizes the differences in age, characteristics of conventional US, PCEUS, and ICEUS between the metastatic SLN group and the nonmetastatic SLN group. The cortical thickness of SLNs in the metastasis group was larger than that of those in the nonmetastatic group (P<0.05). Eccentric or absent fatty hilum and hybrid blood flow were more common in the metastatic group than in the nonmetastatic group. After PCEUS, SLNs tended to show heterogeneous enhancement (type II and III) in the metastatic group. After ICEUS, 55.56% of SLNs in the metastatic group showed overall enhancement, while 75.68% of SLNs in the nonmetastatic group showed centrifugal enhancement. Perfusion defects were observed in the lymph nodes of both groups. However, the perfusion defects were mostly found in the cortex (type C; 10/11) in the metastatic group and mainly in the fatty hilum (type B; 12/13) in the nonmetastatic group. There was no statistical significance between SLNs of the metastatic group and the nonmetastatic group in terms of age, breast tumor size, or T/L ratio (all P>0.05).
Table 1
| Characteristic | Metastatic SLN (n=54) | Nonmetastatic SLN (n=111) | U/χ2 | P value |
|---|---|---|---|---|
| General characteristic | ||||
| Age (years) | 53.50 (45.75–61.50) | 54.00 (46.00–62.00) | 2,995.500 | 0.996 |
| Conventional US characteristics of SLN | ||||
| Breast tumor size (mm) | 21.00 (16.75–26.00) | 20.00 (15.00–27.00) | 2,660.500 | 0.242 |
| T/L ratio | 2.01 (1.66–2.37) | 2.00 (1.77–2.38) | 2,647.500 | 0.225 |
| Cortical thickness (mm) | 4.25 (2.78–6.13) | 2.30 (1.80–3.00) | 961.000 | <0.001 |
| Fatty hilum | 15.854 | <0.001 | ||
| Central | 42 (77.78) | 108 (97.30) | ||
| Eccentric | 10 (18.52) | 2 (1.80) | ||
| Absent | 2 (3.70) | 1 (0.90) | ||
| Blood flow classification | 45.706 | <0.001 | ||
| Central | 29 (53.70) | 107 (96.40) | ||
| Hybrid | 25 (46.30) | 4 (3.60) | ||
| PCEUS | ||||
| Enhancement type | 44.249 | <0.001 | ||
| Type I | 13 (24.07) | 82 (73.88) | ||
| Type II | 9 (16.67) | 15 (13.51) | ||
| Type III | 32 (59.26) | 14 (12.61) | ||
| ICEUS | ||||
| Enhancement type | 19.628 | <0.001 | ||
| Type A | 43 (79.63) | 98 (88.29) | ||
| Type B | 1 (1.85) | 12 (10.81) | ||
| Type C | 10 (18.52) | 1 (0.90) | ||
| Enhancement direction | 19.247 | <0.001 | ||
| Centrifugal | 22 (40.74) | 84 (75.68) | ||
| Overall | 30 (55.56) | 26 (23.42) | ||
| Centripetal | 2 (3.70) | 1 (0.90) | ||
Data are represented as median (interquartile range) or n (%). US, ultrasound; SLN, sentinel lymph node; T/L ratio, transverse to longitudinal diameter ratio; PCEUS, percutaneous contrast-enhanced ultrasound; ICEUS, intravenous contrast-enhanced ultrasound.
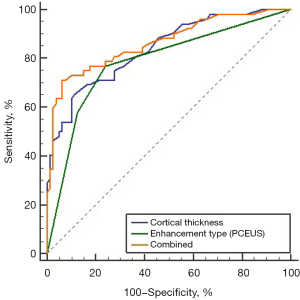
The multivariate logistic regression analysis of the significant variables obtained with univariate analysis in (Table 1) showed that cortical thickness and enhancement type of PCEUS were independent correlated factors of SLN metastasis (Table 2). The ROC curve is shown in Figure 5. The area under the curve (AUC) value of the cortical thickness of SLN for the diagnosis of SLN metastasis was 0.840 (95% CI: 0.775–0.892), the AUC value of the enhanced type of PCEUS for the diagnosis of SLN metastasis was 0.779 (95% CI: 0.707–0.839), and the AUC value of the combined diagnosis was 0.858 (95% CI: 0.795–0.907). The sensitivity of the cortical thickness, the enhanced type of PCEUS, and the combined model was 66.67% (36/54), 75.93% (41/54), and 70.37% (38/54), respectively. The specificity of the cortical thickness, the enhanced type of PCEUS, and the combined model was 88.29% (98/111), 73.87% (82/111), and 93.69% (104/111), respectively.
Table 2
| Characteristics | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| General characteristic | |||||||
| Age (years) | 1.002 | 0.976–1.030 | 0.865 | ||||
| Conventional US characteristics of SLN | |||||||
| Breast tumor size (mm) | 1.016 | 0.981–1.052 | 0.380 | ||||
| T/L ratio | 0.633 | 0.317–1.265 | 0.195 | ||||
| Cortical thickness (mm) | 2.730 | 1.938–3.845 | <0.001* | 2.259 | 1.400–3.646 | 0.001 | |
| Fatty hilum | 5.863 | 1.900–18.093 | 0.002* | ||||
| Blood flow classification | 23.060 | 7.431–71.560 | <0.001* | ||||
| PCEUS | |||||||
| Enhancement type | 3.797 | 2.472–5.831 | <0.001* | 2.341 | 1.394–3.929 | 0.001 | |
| ICEUS | |||||||
| Enhancement type | 2.264 | 1.255–4.085 | 0.007* | ||||
| Enhancement direction | 4.033 | 2.091–7.780 | <0.001* | ||||
*, significant at univariate analysis and onward to multivariate analysis. OR, odds ratio; CI, confidence interval; US ultrasound; SLN, sentinel lymph node; T/L ratio, transverse to longitudinal diameter ratio; PCEUS, percutaneous contrast-enhanced ultrasound; ICEUS, intravenous contrast-enhanced ultrasound.
Establishment of the ultrasonic nomogram
A nomogram that assessed the risk of SLN metastasis was constructed using the abovementioned independent risk factors (Figure 6). The bootstrap validation method was used for internal validation of the generated model. The nomogram demonstrated good accuracy in estimating the risk of SLN metastasis, with an unadjusted C-index of 0.860 (95% CI: 0.730–0.990) and a bootstrap-corrected C-index of 0.853. In addition, calibration plots graphically showed good agreement for the presence of metastasis between the risk estimation of the nomogram and histopathologic confirmation on surgical specimens (Figure 7). Based on the Youden index, the diagnostic probability cutoff point was set at 0.429, meaning that if the model-diagnostic probability of SLN metastasis was >0.429, the SLN was considered metastatic. Through the analysis of the ROC in the combined model, we obtained a cutoff score of 3.6 mm. The cutoff values for diagnosing metastatic SLNs in the combined model were 3.6 mm and type II in cortical thickness and PCEUS enhancement type, respectively. The sensitivity, specificity, positive predictive value, and negative predictive value were 70.37% (38/54), 93.69% (104/111), 84.44% (38/45), and 86.67% (104/120), respectively. The results of the comparison of surgery and the combined model diagnosis are shown in Table 3.
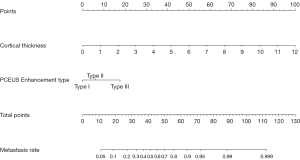
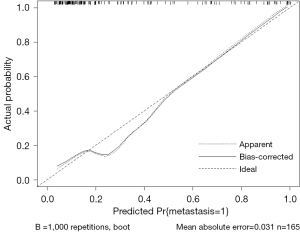
Table 3
| Category | The combined model | Total | Statistical value | |
|---|---|---|---|---|
| Metastatic SLN | Nonmetastatic SLN | |||
| The surgical result | χ2=75.169; P<0.001 | |||
| Metastatic SLN | 38 (70.37%) | 16 (29.63%) | 54 | |
| Nonmetastatic SLN | 7 (6.31%) | 104 (93.69%) | 111 | |
| Total | 45 | 120 | 165 | |
SLN, sentinel lymph node.
Discussion
In this study, we analyzed and summarized the conventional US and CEUS features of metastatic SLNs in patients with BC. Following this, we developed and validated a simple-to-use nomogram-illustrated model for diagnosing the probability of SLN metastasis.
In this study, all labeled lymph nodes were stained, which demonstrated that PCEUS localization of SLNs had a high consistency with the dye method, a finding that was consistent with previous literature reports (11,12). However, during the operation, we found that 4 markers were not in the lymph nodes. A review of US images of these patients found that the SLNs enhanced by PCEUS were small. The reasons these markers were not in the lymph nodes may be that the marker was not successfully inserted into the lymph node due to technical reasons during the puncture or the marker was pulled out of the lymph node during surgery.
The univariate analysis indicated that the cortical thickness and fatty hilum of SLNs were significantly different in the metastatic and nonmetastatic lymph nodes. In addition, the cortex of the metastatic SLN was thicker, and the fatty hilum was prone to eccentricity or disappearance. These findings are consistent with the results of previous studies (15-19). In a previous study, the blood flow in metastatic lymph nodes was mostly peripheral or hybrid (13). In our study, the metastatic lymph nodes mostly showed hybrid blood flow (no peripheral blood flow was found), which may be related to the inclusion criteria. In this study, we mainly focused on patients with negative or suspected positive axillary US preoperatively and excluded patients highly suspected for metastatic lymph nodes according to US (T/L ratio ≤1 and absent fatty hilum), while peripheral blood flow was often present in lymph nodes with absent fatty hilum and a T/L ratio ≤1. In this study, the mean size of breast tumors in the metastatic group (21 mm) was slightly larger than that in the nonmetastatic group (20 mm), but there was no significant statistical difference. There may be 2 reasons for this : first, the amount of data in this study was small and only consisted of 54 cases in the metastasis group; second, cases with a positive US diagnosis (possibly larger breast lesions) were excluded at the time of enrollment in this study.
The enhancement of SLNs traced by PCEUS was divided into 3 types according to the distribution of contrast agents in the lymph nodes, which is inconsistent with the data reported in previous studies (20-22). In our study, patients with enhanced lymphatic vessels but no obvious enhanced lymph nodes were excluded from the study. The results indicated that the enhancement of nonmetastatic SLNs tended to be type I with homogeneous enhancement, while that of metastatic SLNs tended to be type II or III with heterogeneous enhancement. The reason for this finding may be that lymph node metastasis is mainly caused by tumor cells entering lymph nodes through LCs, which leads to the destruction of the normal structure of lymph nodes (23). In this case, the contrast agent cannot flow into the tissue after entering LCs, thus forming filling defects.
Previous studies indicated that the enhancement type of metastatic SLNs tended to be centripetal (24,25). However, our study showed that metastatic SLNs tended to have a synchronous enhancement in the cortex and fatty hilum, which can be described as an overall enhancement. The reason for this finding may be that lymph nodes in this group were mainly nodes with fatty hilum, while some nodes in which fatty hilum completely disappeared were excluded. In comparison, the nodes that were absent of fatty hilum were more likely to have centripetal enhancement. Metastatic lymph nodes were prone to necrosis, which could be seen as filling defects with ICEUS (26). It was also found that the filling defects of metastatic lymph nodes were mostly in the cortical region, while the filling defects of nonmetastatic lymph nodes were mostly in the fatty hilum. The reason for these findings may be that the tumor cells in the metastatic lymph nodes were first deposited in the cortex, and after the formation of the lesions, the US images showed the same hypoecho as the cortical echo, which was difficult to distinguish from the cortex. Therefore, the filling defects caused by tumor ischemia necrosis were mainly in the cortex. Nonmetastatic lymph nodes generally had thinner cortices, and the defects were often shown in the fatty hilum due to inflammation, adipose metaplasia, and other factors.
Logistic regression analysis of the significant variables obtained with univariate analysis showed that cortical thickness and enhancement type of PCEUS were independent predictors of SLN metastasis. The combined diagnosis of cortical thickness and enhancement type in PCEUS showed a better diagnostic efficacy; the ROC curve showed an AUC of 0.858 and a 95% CI of 0.795–0.907. Compared with those of the 2 single models, the AUC value and specificity of the single cortical thickness model were higher (0.840 vs. 0.779 and 88.29% vs. 73.87%), while the sensitivity of the single PCEUS model was higher (66.67% vs. 75.93%). Although the AUC value of the combined model was not significantly increased compared with that of the single cortical thickness model, the sensitivity and specificity were improved (sensitivity 70.37%, specificity 93.69%). Unfortunately, the observation indexes of ICEUS in this study were not included in the final model. This does not mean that ICEUS has no value in identifying SLN metastasis. The number of cases in this study was small; therefore, the quantitative parameters of the ICEUS data were not analyzed. We will try to overcome these problems and improve the model in future research.
Nomograms can provide personalized, evidence-based, and highly accurate risk assessment for all visualized models. The nomogram’s main attraction is its convenience, which can help physicians make management-related decisions. Previous nomograms to predict the risk of SLN metastasis were mostly based on the size, location, immunotyping, and other factors of the breast tumor (7,8). In this study, 2 variables were included in the nomogram to estimate the possibility of metastasis according to the US features of SLNs, which showed good diagnostic efficacy. Patients could be informed of the possibility of SLN metastasis and decide how to manage the ALNs. According to this nomogram, if the diagnostic probability of SLN metastasis is >0.429, the SLN is considered metastatic (27), and targeted biopsy can be performed under US guidance. Then, if a patient has more than 2 SLNs with metastasis, the ALND may be directly operated on instead of the patient undergoing an SLNB. The SOUND (Sentinel Node vs. Observation after Axillary Ultrasound) trial (28) proposed a hypothesis that accurate preoperative axillary ultrasound evaluation may replace SLNB, so that some patients with small BC can be exempted from SLNB. This prospective trial is ongoing. In the near future, if the SOUND trial reports the expected outcomes and the SLNs of patients are all low risk (metastatic probability), the SLNB may be exempted. However, based on the current risk diagnostic model, comparative studies are needed to evaluate the model’s prognostic effect to guide surgical planning.
Our study had some limitations. First, it was a single-center study with a relatively small sample size. Larger, multicenter studies may provide further information with respect to the study. Second, due to the lack of suitable analysis software, the quantitative parameters of the ICEUS data were not analyzed in this study. Third, this model was not externally validated.
Conclusions
Based on the results of multivariate analysis, we developed a nomogram based on the cortical thickness and PCEUS enhancement type of SLNs, which can be used to diagnose SLN metastasis before SLNB. The predictive performance of the nomogram was good. This nomogram can be used as a tool to assist clinicians in assessing SLN metastasis in patients with early-stage BC.
Acknowledgments
Funding: This work was supported by the Suzhou Clinical Key Diseases Diagnosis and Treatment Technology Special Project (No. LCZX202104). The fund supported the cost of data analysis, article writing, and publication of the study.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1175/rc).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1175/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approval for this prospective study was granted by the Ethics Committee of the First Affiliated Hospital of Soochow University (No. 2016108). All participants signed a written informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 1994;220:391-8; discussion 398-401. [Crossref] [PubMed]
- Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 2005;23:7703-20. [Crossref] [PubMed]
- Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, Benson AB 3rd, Bosserman LD, Burstein HJ, Cody H 3rd, Hayman J, Perkins CL, Podoloff DA, Giuliano AEAmerican Society of Clinical Oncology Clinical Practice. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2014;32:1365-83. [Crossref] [PubMed]
- Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Morrow M, Hunt KK. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg 2016;264:413-20. [Crossref] [PubMed]
- Chang JM, Leung JWT, Moy L, Ha SM, Moon WK. Axillary Nodal Evaluation in Breast Cancer: State of the Art. Radiology 2020;295:500-15. [Crossref] [PubMed]
- Zhang YN, Wang CJ, Xu Y, Zhu QL, Zhou YD, Zhang J, Mao F, Jiang YX, Sun Q. Sensitivity, Specificity and Accuracy of Ultrasound in Diagnosis of Breast Cancer Metastasis to the Axillary Lymph Nodes in Chinese Patients. Ultrasound Med Biol 2015;41:1835-41. [Crossref] [PubMed]
- Qiu PF, Liu JJ, Wang YS, Yang GR, Liu YB, Sun X, Wang CJ, Zhang ZP. Risk factors for sentinel lymph node metastasis and validation study of the MSKCC nomogram in breast cancer patients. Jpn J Clin Oncol 2012;42:1002-7. [Crossref] [PubMed]
- Luo Y, Zhao C, Gao Y, Xiao M, Li W, Zhang J, Ma L, Qin J, Jiang Y, Zhu Q. Predicting Axillary Lymph Node Status With a Nomogram Based on Breast Lesion Ultrasound Features: Performance in N1 Breast Cancer Patients. Front Oncol 2020;10:581321. [Crossref] [PubMed]
- Ling W, Nie J, Zhang D, Yang Q, Jin H, Ou X, Ma X, Luo Y. Role of Contrast-Enhanced Ultrasound (CEUS) in the Diagnosis of Cervical Lymph Node Metastasis in Nasopharyngeal Carcinoma (NPC) Patients. Front Oncol 2020;10:972. [Crossref] [PubMed]
- Fang F, Gong Y, Liao L, Ye F, Zuo Z, Li X, Zhang Q, Tang K, Xu Y, Zhang R, Chen S, Niu C. Value of Contrast-Enhanced Ultrasound for Evaluation of Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma. Front Endocrinol (Lausanne) 2022;13:812475. [Crossref] [PubMed]
- Miyake T, Shimazu K, Tanei T, Naoi Y, Kagara N, Shimoda M, Kim SJ, Noguchi S. Hookwire-guided Sentinel Lymph Node Biopsy Using Contrast-enhanced Ultrasonography Followed by a One-step Nucleic Acid Amplification (OSNA) Assay for Breast Cancer. Anticancer Res 2019;39:6183-92. [Crossref] [PubMed]
- Hu Z, Cheng X, Li J, Jiang J, Jiang Z, Li H, Li T, Zhang Z, Tan B, Lu M. Preliminary study of real-time three-dimensional contrast-enhanced ultrasound of sentinel lymph nodes in breast cancer. Eur Radiol 2020;30:1426-35. [Crossref] [PubMed]
- Zhao J, Zhang J, Zhu QL, Jiang YX, Sun Q, Zhou YD, Wang MQ, Meng ZL, Mao XX. The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: A prospective study. Eur Radiol 2018;28:1654-61. [Crossref] [PubMed]
- Li J, Lu M, Cheng X, Hu Z, Li H, Wang H, Jiang J, Li T, Zhang Z, Zhao C, Ma Y, Tan B, Liu J, Yu Y. How Pre-operative Sentinel Lymph Node Contrast-Enhanced Ultrasound Helps Intra-operative Sentinel Lymph Node Biopsy in Breast Cancer: Initial Experience. Ultrasound Med Biol 2019;45:1865-73. [Crossref] [PubMed]
- Cho N, Moon WK, Han W, Park IA, Cho J, Noh DY. Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: node-to-node correlation with surgical histology and sentinel node biopsy results. AJR Am J Roentgenol 2009;193:1731-7. [Crossref] [PubMed]
- Bedi DG, Krishnamurthy R, Krishnamurthy S, Edeiken BS, Le-Petross H, Fornage BD, Bassett RL Jr, Hunt KK. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. AJR Am J Roentgenol 2008;191:646-52. [Crossref] [PubMed]
- Zhu Y, Zhou W, Zhou JQ, Fei XC, Ye TJ, Huang O, Chen XS, Zhan WW. Axillary Staging of Early-Stage Invasive Breast Cancer by Ultrasound-Guided Fine-Needle Aspiration Cytology: Which Ultrasound Criteria for Classifying Abnormal Lymph Nodes Should Be Adopted in the Post-ACOSOG Z0011 Trial Era? J Ultrasound Med 2016;35:885-93. [Crossref] [PubMed]
- Britton PD, Goud A, Godward S, Barter S, Freeman A, Gaskarth M, Rajan P, Sinnatamby R, Slattery J, Provenzano E, O'Donovan M, Pinder S, Benson JR, Forouhi P, Wishart GC. Use of ultrasound-guided axillary node core biopsy in staging of early breast cancer. Eur Radiol 2009;19:561-9. [Crossref] [PubMed]
- Dudea SM, Lenghel M, Botar-Jid C, Vasilescu D, Duma M. Ultrasonography of superficial lymph nodes: benign vs. malignant. Med Ultrason 2012;14:294-306. [PubMed]
- Liu J, Liu X, He J, Gou B, Luo Y, Deng S, Wen H, Zhou L. Percutaneous contrast-enhanced ultrasound for localization and diagnosis of sentinel lymph node in early breast cancer. Sci Rep 2019;9:13545. [Crossref] [PubMed]
- Niu Z, Xiao M, Ma L, Qin J, Li W, Zhang J, Zhu Q, Jiang Y. The value of contrast-enhanced ultrasound enhancement patterns for the diagnosis of sentinel lymph node status in breast cancer: systematic review and meta-analysis. Quant Imaging Med Surg 2022;12:936-48. [Crossref] [PubMed]
- Guo RQ, Xiang X, Wang LY, Zhu BH, Huang SY, Tang XY, Chen JJ, Qiu L. Percutaneous contrast-enhanced ultrasound for localization and qualitative diagnosis of sentinel lymph nodes in cutaneous malignant melanoma of lower extremities: a preliminary study. Quant Imaging Med Surg 2022;12:366-75. [Crossref] [PubMed]
- Ha SW, Lee HJ, Cho AS, Hwang SI, Lee HJ. Evaluation of lymph node metastasis in a rabbit tumor model: correlations between contrast-enhanced ultrasound and pathologic findings. Ultrasonography 2020;39:60-9. [Crossref] [PubMed]
- Yin SS, Cui QL, Fan ZH, Yang W, Yan K. Diagnostic Value of Arrival Time Parametric Imaging Using Contrast-Enhanced Ultrasonography in Superficial Enlarged Lymph Nodes. J Ultrasound Med 2019;38:1287-98. [Crossref] [PubMed]
- Zhang J, Hao X, Yang Y, Yan CS, Ma C, Xiao M, Gu LS, Wang Y. Evaluation of supplementary diagnostic value of contrast-enhanced ultrasound for lymph node puncture biopsy. J Thorac Dis 2017;9:4791-7. [Crossref] [PubMed]
- Xiang D, Hong Y, Zhang B, Huang P, Li G, Wang P, Li Z. Contrast-enhanced ultrasound (CEUS) facilitated US in detecting lateral neck lymph node metastasis of thyroid cancer patients: diagnosis value and enhancement patterns of malignant lymph nodes. Eur Radiol 2014;24:2513-9. [Crossref] [PubMed]
- Han P, Yang H, Liu M, Cheng L, Wang S, Tong F, Liu P, Zhou B, Cao Y, Liu H, Wang C, Peng Y, Shen D, Wang S. Lymph Node Predictive Model with in Vitro Ultrasound Features for Breast Cancer Lymph Node Metastasis. Ultrasound Med Biol 2020;46:1395-402. [Crossref] [PubMed]
- Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND). Breast 2012;21:678-81. [Crossref] [PubMed]


