
Magnetic resonance imaging findings of leiomyomatosis peritonealis disseminata: a case description and literature analysis
Introduction
A 32-year-old female patient experiencing menorrhagia for half a year attended Northwest Women’s and Children’s Hospital. The clinical examination revealed that the patient’s uterus was enlarged to the size of a 4-month pregnant uterus. Multiple nodules could be palpated on the left side of the uterus, and the patient was diagnosed with uterine fibroids according to ultrasound. The laboratory tests showed a slight elevation of cancer antigen 125 and cancer antigen 19-9. The patient had undergone the hysteroscopic resection of submucosal fibroids in 2018 and was pathologically diagnosed as leiomyoma hyaline with degeneration. A pelvic magnetic resonance imagining (MRI) examination was recommended.
Imaging findings
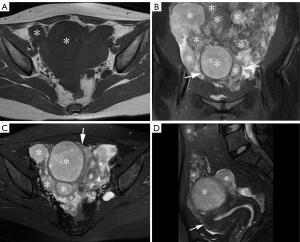
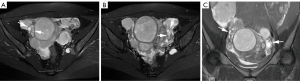
The MRI examination showed that the patient’s uterus was enlarged and irregularly shaped. There were elliptic and round various-sized masses in the myometrium, submucosa, and subserosa of the uterus, as well as in the peritoneum, including the omentum majus. All the masses were isointense or mildly hypointense on T1-weighted imaging (T1WI) (Figure 1A) and had slightly high signal intensities on T2-weighted imaging (T2WI) (Figure 1B-1D). The diameter of the largest mass was 63 mm. Some masses on diffusion-weighted imaging had high signal intensities, and the apparent diffusion coefficient (ADC) value was about (0.88–1.2)×10-3 mm2/s. Both ovaries were visible (Figure 2). A small amount of free fluid was observed in the abdomen and pelvis. Given the appearance of the masses, a diagnosis of leiomyomatosis peritonealis disseminata (LPD) was favored.
Surgery and pathology
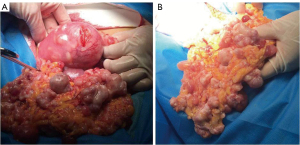
Intraoperatively, the uterus was enlarged to the size of 12 weeks’ gestation with multiple various-sized masses (Figure 3). Multiple various-sized soft masses were observed in the peritoneum, including the omentum majus, ovarian surface, and intestine. Miliary nodules were detected on the surface of the liver, spleen, and stomach. The intraoperative diagnosis was uterine fibroids with LPD. A total hysterectomy accompanied by bilateral adnexectomy, greater omentectomy, and pelvic lesion resection was performed. The pathological diagnosis was clear cell-type leiomyomata.
All the procedures in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent form is available for review by the editorial office of this journal.
Discussion
LPD is a rare and special type of leiomyomatosis, which is characterized by multiple nodules of leiomyoma on the omentum, parietal, and visceral peritoneum (1). The disease was first reported in 1952 (2) and was described and formally named by Taubert in 1965 (3). To date, no more than 200 cases of LPD have been reported (4). This disease mainly occurs in women of reproductive age (5) and is extremely rare in women after menopause and in men (6). LPD is generally considered a benign disease, but it can become malignant and has been reported to have a malignancy rate of about 7% (7).
The exact pathogenesis of LPD is still unclear. Some factors that may lead to the occurrence of LPD include hormonal factors, iatrogenic dissemination, peritoneal metaplasia, and genetic factors (6,8-12). Some scholars have reported that LPD occurs in women with high estrogen levels, which can be caused by pregnancy, estrogen replacement therapy, oral contraception, or functional ovarian tumors (8,9). After the removal of any factors that lead to high hormone levels, LPD lesions tend to shrink or disappear. Thus, estrogen plays an important role in the development of LPD.
In recent years, with the extensive development of laparoscopic technology, the incidence of LPD in patients with laparoscopic myomectomy or subtotal hysterectomy has increased (6,10,11). It is assumed that fragments of leiomyoma are implanted into the abdominal cavity and continue to grow during morcellation. At present, iatrogenic factors are considered an important cause of LPD. Some scholars believe that proliferative leiomyoma nodules may be generated by the metaplasia of mesenchymal stem cells with multi-differentiation potential distribution in the peritoneum (6,12), while others believe that LPD is related to X chromosome inactivation and abnormal chromosome karyotype (8).
In this case, although the patient had no history of laparoscopic surgery, hysteroscopic myomectomy was performed. We believed it could be possible that myoma fragments had entered the abdominal cavity in a retrograde fashion through the fallopian tube during the resection.
The clinical manifestations of LPD are not specific. LPD can manifest as irregular menstruation caused by hysteromyoma, and secondary signs caused by the compression of abdominal and pelvic masses can include abdominal pain, frequent urination, and intestinal obstruction (13,14). The misdiagnosis rate of this disease has been reported to be high in previous reviews (4,10,12), and before advanced imaging, it was difficult to make an accurate diagnosis before surgery. There have been few imaging reports on LPD (15). In this case, the MRI scans showed multiple nodules and masses of different sizes in the uterus and peritoneum, including the greater omentum, parietal, and visceral peritoneum, with clear boundaries. On T2WI, the masses had as lightly high signal intensity due to the cellular components. On T1WI, the masses were mostly iso- to mildly hypointense.
LPD is rare, but we believe that it has certain characteristic imaging findings on MRI. First, LPD mostly occurs simultaneously in the uterus and the surrounding peritoneal organs. Due to its homology, the signal intensity of these lesions are similar on MRI. Second, while LPD mostly involves the ovaries, it only occurs on the surface of the ovaries, and the ovarian parenchyma is not invaded due to pathogenesis of LPD. In this case, ovarian parenchyma and follicular signals were clearly observed on MRI. Based on these two characteristic imaging findings, LPD can be distinguished from peritoneal metastases from an ovarian or gastrointestinal primary cancer.
In ovarian cancer, the primary tumor originates from the ovary, and the ovarian lesion may be observed, or the ovary may be distorted by the tumor and thus difficult to identify on MRI. Conversely, ovaries and follicles can often be observed on the MRI of LPD patients, and the mass boundary of LPD is usually clear.
LPD should also be differentiated from peritoneal implantations of gastrointestinal malignancies. Gastrointestinal malignancies have primary tumors of the digestive tract, so it is important to find the primary tumor. In addition, peritoneal implantation lesions of gastrointestinal malignancies generally do not involve the myometrium, while in LPD patients, lesions of the myometrium and peritoneum can often be observed at the same time, and the signal intensities of the lesions on MRI are similar.
According to the pathogenesis of this disease, we believe that LPD has imaging characteristics that could aid in preoperative diagnosis. Further research should be conducted in this area to extend understandings of the etiology, imaging appearance, and preoperative diagnosis of LPD.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1196/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McCarthy CM, Hickey KP. Leiomyomatosis peritonealis disseminata. Eur J Obstet Gynecol Reprod Biol 2017;210:386. [Crossref] [PubMed]
- Willson JR, Peale AR. Multiple peritoneal leiomyomas associated with a granulosa-cell tumor of the ovary. Am J Obstet Gynecol 1952;64:204-8. [Crossref] [PubMed]
- Taubert HD, Wissner SE, Haskins AL. Leiomyomatosis peritonealis disseminata; an unusual complication of genital leiomyomata. Obstet Gynecol 1965;25:561-74. [PubMed]
- Bu H, Jin C, Fang Y, Ma Y, Wang X, Chen J, Chen L. Successful pregnancy after complete resection of leiomyomatosis peritonealis disseminate without recurrence:a case report with next-generation sequencing analysis and literature review. World J Surg Oncol 2020;18:85. [Crossref] [PubMed]
- Gaichies L, Fabre-Monplaisir L, Fauvet R, Alves A, Mulliri A. Leiomyomatosis peritonealisis disseminata: Two unusual cases with literature review. J Gynecol Obstet Hum Reprod 2018;47:89-94. [Crossref] [PubMed]
- Al-Talib A, Tulandi T. Pathophysiology and possible iatrogenic cause of leiomyomatosis peritonealis disseminata. Gynecol Obstet Invest 2010;69:239-44. [Crossref] [PubMed]
- Yang R, Xu T, Fu Y, Cui S, Yang S, Cui M. Leiomyomatosis peritonealis disseminata associated with endometriosis: A case report and review of the literature. Oncol Lett 2015;9:717-20. [Crossref] [PubMed]
- Ordulu Z, Dal Cin P, Chong WW, Choy KW, Lee C, Muto MG, Quade BJ, Morton CC. Disseminated peritoneal leiomyomatosis after laparoscopic supracervical hysterectomy with characteristic molecular cytogenetic findings of uterine leiomyoma. Genes Chromosomes Cancer 2010;49:1152-60. [Crossref] [PubMed]
- Fasih N, Prasad Shanbhogue AK, Macdonald DB, Fraser-Hill MA, Papadatos D, Kielar AZ, Doherty GP, Walsh C, McInnes M, Atri M. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics 2008;28:1931-48. [Crossref] [PubMed]
- Lu B, Xu J, Pan Z. Iatrogenic parasitic leiomyoma and leiomyomatosis peritonealis disseminata following uterine morcellation. J Obstet Gynaecol Res 2016;42:990-9. [Crossref] [PubMed]
- Paul PG, Koshy AK. Multiple peritoneal parasitic myomas after laparoscopic myomectomy and morcellation. Fertil Steril 2006;85:492-3. [Crossref] [PubMed]
- Li J, Dai S. Leiomyomatosis Peritonealis Disseminata: A Clinical Analysis of 13 Cases and Literature Review. Int J Surg Pathol 2020;28:163-8. [Crossref] [PubMed]
- Rieker RJ, Agaimy A, Moskalev EA, Hebele S, Hein A, Mehlhorn G, Beckmann MW, Hartmann A, Haller F. Mutation status of the mediator complex subunit 12 (MED12) in uterine leiomyomas and concurrent/metachronous multifocal peritoneal smooth muscle nodules (leiomyomatosis peritonealis disseminata). Pathology 2013;45:388-92. [Crossref] [PubMed]
- Heinig J, Neff A, Cirkel U, Klockenbusch W. Recurrent leiomyomatosis peritonealis disseminata after hysterectomy and bilateral salpingo-oophorectomy during combined hormone replacement therapy. Eur J Obstet Gynecol Reprod Biol 2003;111:216-8. [Crossref] [PubMed]
- Tanaka YO, Tsunoda H, Sugano M, Satoh T, Yagi H, Minami R, Shiigai M, Inadome Y, Yoshikawa H, Noguchi M, Minami M MR. Br J Radiol 2009;82:e44-7. [Crossref] [PubMed]


