
Percutaneous transhepatic intraluminal forceps biopsy for patients with biliary stricture after endoscopic retrograde approach failure: a retrospective study
Introduction
Biliary stricture has high etiological variability and can result from a variety of benign and malignant diseases. Imaging of the stricture site using modalities such as magnetic resonance imaging (MRI) with cholangiopancreatography (MRCP) and contrast-enhanced can provide a rapid clinical diagnosis (1). However, due to the variability of imaging quality, reaching an exact diagnosis of benign or malignant etiology based on imaging alone continues to be challenging. Between 5% and 15% of patients who undergo surgical resection for suspected malignant biliary stricture are diagnosed with benign pathological findings postoperatively (2,3). A recent guideline for biliary cancer recommended that a pathologic diagnosis should be obtained before any nonsurgical treatment modality is performed (4). For patients with potentially resectable tumors and for those receiving palliative care, a nonsurgical pathologic diagnostic method to determine the pathologic subtype can be valuable in guiding both the choice of therapy and the prognosis of the tumor.
Several nonsurgical pathologic diagnostic techniques are used for biliary strictures, and these vary widely in sensitivity and accuracy. Because biliary tumors in the bile ducts involve intramural growth with poor image occupancy effect, they cannot be accurately punctured using ultrasound (US)- or computed tomography (CT)-guided aspiration biopsy techniques. Consequently, these techniques have a diagnostic positivity rate of only approximately 40.0% (5,6), and put patients at risk of vascular damage and serious complications.
With the development and improvement of endoscopic technology, biopsy guided by endoscopic retrograde cholangiopancreatography (ERCP) has become the mainstream tissue sampling technique for the etiological diagnosis of biliary strictures. Under the guidance of ERCP, an auxiliary guidewire is directed to the biliary stricture site for drainage while brush or forceps biopsy is performed. The specificity of ERCP-guided brush cytology or forceps biopsy for biliary cancer diagnosis can reach 100%; however, the sensitivity varies widely, from 30% to 60% for brush cytology, 43% to 81% for forceps biopsy, and 59.4% for both methods in combination (7). To improve the positivity rate of ERCP-guided biliary biopsy, Tanisaka et al. concluded that intraductal US played an important adjunctive role in the detection of lesions in the biliary biopsies process (8). Alternatively, Tieu et al. used the SpyGlass endoscopy system, which can help to distinguish benign and malignant biliary strictures by observing the structure of the biliary wall to identify distorted tumor vessels, abnormal mucosa, and irregular protruding structures (9). Furthermore, Varadarajulu et al. used a rapid onsite cytologic evaluation (ROSE) technique for tissue sampling from the stricture site for onsite diagnosis (10). However, if ERCP fails due to technical reasons, such as postoperative surgical anatomical changes or failed biliary cannulation, none of the above methods can be performed.
For patients for whom ERCP-guided biliary biopsy fails or percutaneous transhepatic cholangial drainage (PTCD) is planned, our center routinely uses interventional digital subtraction angiography (DSA)-guided percutaneous transhepatic intraluminal forceps biopsy (TIFB). First, a bile duct guidewire is used to establish a skin-duodenal pathway. Then, instead of an endoscope, a 9 F sheath is introduced, and biopsy forceps are reintroduced through the sheath for repeated sampling of the stricture segment, followed by placement of a drainage tube or stent to relieve the obstruction. This method is simple and inexpensive (USD 209.4, versus USD 628.1 for conventional ERCP). Moreover, it can satisfy the goals of both biopsy and drainage.
The present study retrospectively analyzed our center’s 6 years of experience with TIFB following ERCP failure. We present the following article in accordance with the STROBE and STARD reporting checklists (available at https://qims.amegroups.com/article/view/10.21037/qims-22-915/rc).
Methods
Patients
In this retrospective study, clinical data of 240 consecutive patients with biliary strictures who sought further etiological diagnosis at our center between April 2014 and January 2020 were collected. The presence of biliary strictures was confirmed by preoperative diagnostic imaging and blood biochemical tests. After the exclusion of 197 patients who underwent ERCP-guided biliary biopsy, 43 patients [25 males (58.1%), 18 females (41.9%); mean age: 63.2±11.2 years, range: 39–84 years] who received TIFB after ERCP failure were eventually included in the study.
The causes of ERCP failure included altered surgical anatomy (n=21), failed biliary cannulation (n=18), and duodenal obstruction (n=4). The decision to use TIFB for tissue sampling was determined by the oncologist, pathologist, and interventional radiologist at our hospital. The workflow is shown in Figure 1.
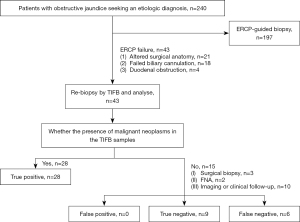
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University, and the requirement to obtain individual consent for this retrospective analysis was waived.
Equipment
The basic equipment used included a biliary puncture sleeve (including a 21 G Chiba needle, a 0.018-inch platinum microguidewire, a three-piece dilator, a 9-F dilation tube, a 0.035-inch reinforced guidewire, an 8.5-F internal and external drainage tube, and an external fixation device; Cook Medical, Bloomington, IN, USA), a 0.035-inch ultrasmooth guidewire (Terumo, Tokyo, Japan), an Amplatz ultra-stiff guidewire, a 5-F Cobra contrast catheter, and 6.0-mm biopsy forceps (Micro-Tech, Nanjing, China). All procedures were carried out under the guidance of DSA.
TIFB procedure
The extent of the tumor and the anatomy of the bile duct were evaluated by enhanced abdominal CT and/or MRCP. Satisfactory anesthesia was performed by local injection with 2% lidocaine and intravenous 10 mg dezocine injection 10 minutes before the procedure. With the patient lying on the DSA (Siemens, Axiom Artis Zee, Germany) examination table, a 21-G Chiba needle (PTC, Cook Medical) was used to successfully puncture the dilated bile duct under the guidance of US combined with fluoroscopy. Biliary angiography was performed for anatomical observation of the biliary tree. A 0.014-inch platinum guidewire was introduced through the Chiba needle to pass the stricture segment, after which the Chiba needle was withdrawn. A 6-F dilator sheath was introduced along the platinum guidewire to the biliary system. A 0.035-inch guidewire and a 5-F Cobra catheter (Terumo, Tokyo, Japan) were introduced into the biliary duct, and both were passed through the biliary stricture portion to the duodenum. After the reinforcing guidewire (Amplatz, Boston Scientific, Natick, MA, USA) was exchanged, a biopsy channel was established by advancing a 9-F sheath (length 23 cm; Cordis, Miami Lakes, FL, USA) along the stiff guidewire close to the site of the biliary stricture or occlusion. The 6.0-mm biopsy forceps (length 100 cm, Micro-Tech) were then pushed into the 9 F sheath, opened, and pushed forward 5–10 mm into the biliary stricture. The biopsy forceps were tightened to grasp the diseased tissue and then withdrawn, after which the diseased tissue was removed with a needle tip and placed in a prefilled bottle of absolute alcohol or 10% formaldehyde for fixation. This process was repeated three to five times to capture as much diseased tissue as possible (Figures 2-4). Finally, the biopsy forceps were withdrawn and biliary stents or drainage catheters were placed to resolve biliary hypertension.
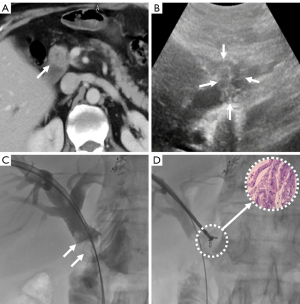
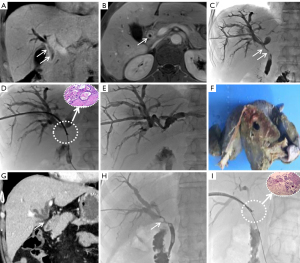
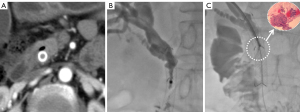
Outcome evaluation and definition
The primary outcomes of this study were the technical success and performance of TIFB. Technical success was defined as successful TIFB completion and the collection of sufficient samples to allow the pathologists to make a diagnosis. Retrospective independent analyses of the tissue samples were performed by two pathologists, with 8 and 9 years of experience, respectively. The performance of TIFB was evaluated based on sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). A biopsy was considered a true positive when the pathologists agreed on the presence of malignant neoplasms in the TIFB samples. In the other cases In the other cases with TIFB with a result other than true positive, surgical biopsy, fine-needle aspiration (FNA) biopsy, or long-term imaging (US, CT, and MRI), and/or clinical follow-up were used. The last follow-up visit was in December 2021. The final diagnostic outcomes were defined as: malignant stricture as confirmed by TIFB biopsy pathology, surgical biopsy pathology, FNA biopsy pathology, and or imaging suggestive of malignancy (increased local abnormalities, abnormal signals in the surrounding lymph nodes, or progression of biliary obstruction) with a clinical course (death or cachexia) of malignant disease during follow-up; and benign stricture as confirmed by surgical pathology, or negative on all cytological tests with no signs of malignancy for at least 12 months of follow-up.
The secondary outcomes included procedure duration, radiation exposure, liver function, and complications. The procedure duration was defined as the time from the start of the operation until a suitable biopsy sample was obtained, and radiation exposure as the radiation dose to which the patient was exposed during this process. Liver function was assessed by determining total bilirubin (TB), direct bilirubin (DB), γ-glutamyl transferase (GGT), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and aspartate aminotransferase (AST) preoperatively (T0) and at 2 weeks postoperatively (T1). In accordance with the Society of Interventional Radiology (SIR) Standards of Practice Committee classification on percutaneous hepatobiliary interventions (11), minor complications were defined as no therapy and no consequence (grade A) or overnight observation only, no therapy, and no consequence (grade B). Major complications were defined as requiring therapy and hospitalization (<48 hours, grade C; >48 hours, grade D), permanent adverse sequelae (grade E), or death (grade F).
Statistical analysis
Continuous variables are presented as the mean ± standard deviation or median with range (min–max). Categorical variables are presented as counts and percentages. Sensitivity, specificity, PPV, NPV, and accuracy (the sum of true positives and true negatives) were calculated and are expressed as percentages. No missing data occurred for any of the indicators for the 43 patients in this study. The continuity-adjusted chi-square test or Fisher’s precision probability test was used to compare differences in TIFB diagnostic accuracy for the two dichotomous variables. Univariate and multivariate logistic regression analyses were used to explore the influences related to true positive results in TIFB diagnosis. For the comparison of liver function at T0 and T1, after the Kolmogorov-Smirnov test for normality, paired t-tests were used for the analysis of normally distributed indicators data such as TB, DB, GGT, and ALT, while non-normally distributed indicators data such as ALP and AST were analyzed by Wilcoxon test. P value <0.05 was considered to indicate a significant difference. All calculations were performed using Microsoft Excel and SPSS 24.0 software package (IBM Corp., Armonk, NY, USA).
Results
Technical success and the diagnostic performance of TIFB
The basic clinical characteristics and therapy parameters of the 43 patients are detailed in Table 1. All the patients had sufficient biopsy samples for the pathologist to make a final diagnosis, with a 100% technical success rate. Neoplastic tissue with tumor cells was found histologically in the tissue samples of 28 (65.12%) of the 43 patients. Among these 28 patients, 19 cases (44.19%) were cholangiocarcinoma, 4 (9.30%) cases were gallbladder cancer, 2 (4.65%) cases were pancreatic cancer, and 3 (6.98%) cases showed metastatic adenocarcinoma. No evidence of the presence of tumor cells was found in the tissue samples of the remaining 15 patients (34.88%), which showed nonspecific infection, inflammation, or fibrous tissue fragments.
Table 1
| Characteristics | Values |
|---|---|
| Age (years), mean ± SD [range] | 63.2±11.2 [39–84] |
| Sex, n (%) | |
| Male | 25 (58.1) |
| Female | 18 (41.9) |
| Cause of ERCP failure, n (%) | |
| Altered surgical anatomy | 21 (48.8) |
| Failed biliary cannulation | 18 (41.9) |
| Duodenal obstruction | 4 (9.30) |
| Location of stricture, n (%) | |
| Intrahepatic bile duct | 7 (16.3) |
| Hilar bile duct | 24 (55.8) |
| Common bile duct | 5 (11.6) |
| Distal bile duct | 7 (16.3) |
| Etiology, n (%) | |
| Cholangiocarcinoma | 20 (46.5) |
| Pancreatic cancer | 6 (14.0) |
| Gallbladder cancer | 4 (9.3) |
| Metastatic adenocarcinoma | 4 (9.3) |
| Benign stricture | 9 (20.9) |
| Length of stricture (cm), median (range) | 3.0 (1.0–4.8) |
| Technical success, % | 100 |
| Number of samples taken during 1 TIFB, mean ± SD [range] | 4.14±0.89 [3–5] |
| Procedure duration (min), mean ± SD [range] | 19.3±7.4 [11–36] |
| Radiation exposure (mGy), mean ± SD [range] | 315.6±35.9 [86.2–526.2] |
| Final diagnosis | |
| Surgical specimen | 3 |
| Follow-up | 10 |
| Other biopsies (FNA) | 2 |
| Major complications (cholangitis), n (%) | 1 (2.33) |
| Minor complications (haemobilia), n (%) | 2 (4.65) |
| Sensitivity, % | 82.35 |
| Specificity, % | 100 |
| PPV, % | 100 |
| NPV, % | 60 |
| Follow-up time (months), median (range) | 21.6 (7.5–60.3) |
| Overall survival (months), median (95% CI) | 41.0 (33.9–48.1) |
SD, standard deviation; ERCP, endoscopic retrograde cholangiopancreatography; TIFB, transhepatic intraluminal forceps biopsy; FNA, fine-needle aspiration; PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval.
In seeking a final diagnosis, these 15 patients opted for surgical sampling (n=3), FNA (n=2), or long-term imaging and/or clinical follow-up (n=10). Among them, 9 cases were ultimately demonstrated to be true negatives with no pathological or histological evidence of malignant tumors. The final diagnoses for the benign samples included: biliary inflammation (n=4), with progressive symptomatic relief during follow-up in addition to symptomatic management and relief of the obstruction observed on MRCP; secondary sclerosing cholangitis (n=2), proven by surgical biopsy; and secondary scar tissue at the biliary gastrointestinal anastomosis (n=3). No increase in lesion size or other signs of malignancy were observed at long-term follow-up. Among the 6 cases with false negatives, 1 case was confirmed as cholangiocarcinoma by surgical sampling, 1 case was confirmed as metastatic adenocarcinoma by FNA biopsy, 1 case was confirmed as pancreatic cancer by FNA biopsy, and the remaining 3 cases were suspected to have pancreatic cancer changes on imaging follow-up.
Overall, 28 (65.12%) TIFB samples were true positives, 9 (20.93%) biopsy samples were true negatives, and 6 (13.95%) biopsy samples were false negatives; no false positives occurred in this study. The overall diagnostic accuracy, sensitivity, and specificity of TIFB were 86.05%, 82.35%, and 100%, respectively. The PPV and NPV of TIFB were 100% and 60%, respectively.
Analysis of factors related to the diagnostic performance of TIFB
Stratified analysis of the diagnostic accuracy of TIFB is presented in Table 2 and Tables S1-S4. Although TIFB had a higher accuracy for cases with proximal and a length ≥3 cm biliary strictures than those with distal and a length <3 cm biliary strictures, these differences were not significant (P=0.42 and P=0.45, respectively). Furthermore, TIFB showed higher accuracy for cases with suspicious biliary tract invasion (SBTI) on preoperative imaging or intrabiliary malignant origin than it did for cases with no SBTI or extrabiliary malignant origin, and these differences were significant (P=0.007 and P=0.003, respectively).
Table 2
| Variables | S (%) | E (%) | PPV (%) | NPV (%) | A (%) | P valuea |
|---|---|---|---|---|---|---|
| Site of stricture | 0.418 | |||||
| Proximal biliary stricture (n=31)b | 88.00 | 100 | 100 | 66.66 | 90.32 | |
| Distal biliary stricture (n=12)c | 66.67 | 100 | 100 | 50.00 | 75.00 | |
| Imaging performance | 0.007* | |||||
| SBTI on imaging (n=35) | 93.10 | 100 | 100 | 81.82 | 94.29 | |
| No-SBTI on imaging (n=8) | 50.00 | 100 | 100 | 0 | 50.00 | |
| Length of stricture | 0.452 | |||||
| ≥3.0 cm (n=24) | 88.89 | 100 | 100 | 75.00 | 91.67 | |
| <3.0 cm (n=19) | 75.00 | 100 | 100 | 42.86 | 78.95 | |
| Origin of the lesion | 0.003* | |||||
| Intrabiliary malignant (n=24) | – | – | – | – | 95.83 | |
| Extrabiliary malignant (n=10) | – | – | – | – | 50.00 | |
| Benign (n=9) | – | – | – | – | 100 |
a, indicates the difference in the diagnostic accuracy of TIFB in the two dichotomous variables (detailed in the Tables S1-S4); b, proximal biliary stricture includes strictures located in the intrahepatic bile duct and hilar bile duct; c, distal biliary stricture includes strictures located in the common bile duct and distal bile duct; *, the differences were significant by the continuity-adjusted chi-square test or Fisher’s precision probability test. A, accuracy; E, specificity; NPV, negative predictive value; PPV, positive predictive value; S, sensitivity; SBTI, suspicious biliary tract invasion; TIFB, transhepatic intraluminal forceps biopsy.
In addition, excluding the 9 patients with a true negative diagnosis, the predictors associated with a true positive result were further explored among the 34 patients with a positive TIFB diagnosis. As shown in Table 3, univariate analysis revealed that only imaging presentation and origin of malignant lesions were significant among seven variables. The results showed that SBTI on imaging and intrabiliary malignant origin were contributing factors for a true positive result (P=0.02 and P=0.009) and increased the likelihood of a true positive result in TIFB diagnosis [odds ratio (OR): 12.000 (1.623–88.702) and OR: 23.000 (2.183–242.327)]. The subsequent inclusion of these two variables in multivariate analysis (Table 4) revealed that only intrabiliary malignant origin (P=0.02) was an independent contributing factor for a true positive result with TIFB diagnosis.
Table 3
| Variables | True positive (n) | OR (95% CI) | P value |
|---|---|---|---|
| Sex (female/male) | 9/19 | 4.222 (0.648–27.492) | 0.132 |
| Age (<60/≥60 years old) | 12/16 | 0.267 (0.027–2.591) | 0.255 |
| Procedure duration (≥30/<30 min) | 5/23 | 4.600 (0.709–29.841) | 0.110 |
| Site of stricture (distal/proximal biliary stricture) | 22/6 | 3.667 (0.584–23.026) | 0.166 |
| Imaging performance (no-SBTI/SBTI on imaging) | 6/22 | 12.000 (1.623–88.702) | 0.015* |
| Length of stricture (<3.0/≥3.0 cm) | 12/16 | 0.577 (0.090–3.679) | 0.561 |
| Origin of malignant lesion (extrabiliary/intrabiliary) | 23/5 | 23.000 (2.183–242.327) | 0.009* |
*, the differences were significant by univariate logistic regression analysis. SBTI, suspicious biliary tract invasion; OR, odds ratio; CI, confidence interval.
Table 4
| Variables | OR (95% CI) | P value |
|---|---|---|
| Imaging performance | ||
| No-SBTI on imaging | 1 (ref.) | |
| SBTI on imaging | 12.569 (0.973–162.315) | 0.052 |
| Origin of malignant lesion | ||
| Extrabiliary | 1 (ref.) | |
| Intrabiliary | 23.875 (1.626–350.574) | 0.021* |
*, the difference was significant by multivariate logistic regression analysis. SBTI, suspicious biliary tract invasion; OR, odds ratio; CI, confidence interval.
Liver function and complications
The pre- and post-treatment liver function analysis (Figure 5 and Table 5) showed that DB, TB, ALP, GGT, ALT, and AST at T1 decreased than those at T0, respectively, with all these differences being significant (all P<0.001). In total, 1 (2.33%) major complication (grade D) and 2 (4.65%) minor complications (both grade A) occurred in this study. The patient with the major complication experienced postoperative transient cholangitis, which was subsequently treated with antibiotics and continuous drainage; the patient’s inflammation resolved after 2 days. The 2 patients with minor complications experienced hemobilia; in both cases, the condition resolved without treatment within 24 hours.
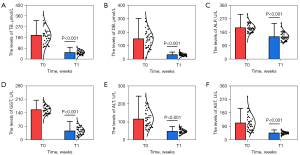
Table 5
| Indicators | T0 | T1 | P value |
|---|---|---|---|
| TB (μmol/L) | 198.81±69.03 | 55.94±17.19 | <0.001 |
| DB (μmol/L) | 150.83±65.59 | 33.77±10.74 | <0.001 |
| ALP (U/L) | 156.53±21.45 | 149.65±29.22 | <0.001 |
| GGT (U/L) | 208.81±29.42 | 46.53±21.45 | <0.001 |
| ALT (U/L) | 112.51±55.39 | 46.21±9.04 | <0.001 |
| AST (U/L) | 117.63±58.74 | 47.39±11.87 | <0.001 |
Data are expressed as mean ± SD. T0, preoperatively; T1, at 2 weeks postoperatively; TB, Total bilirubin; DB, direct bilirubin; ALP, alkaline phosphatase; GGT, γ-glutamyl transferase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SD, standard deviation.
Follow-up
The median follow-up time was 21.6 months (7.5–60.3 months), and the median overall survival was 41.0 months [95% confidence interval (CI): 33.9–48.1]. After successful TIFB, 25 cases underwent biliary stent implantation, 10 cases underwent external drainage, and 8 cases underwent internal and external drainage. Among the 18 cases who received percutaneous drainage, 4 cases were confirmed to have cholangitis on follow-up biopsy and had their drains removed after 4 weeks, 6 cases underwent surgery, and 8 cases could not undergo radical surgery due to advanced tumor. Of the 8 cases for whom radical surgery was not possible, 5 cases chose to have a biliary stent placed and 3 cases chose to have long-term external drainage.
Discussion
Today’s noninvasive imaging techniques, such as US, CT, and MRCP, allow for rapid and sensitive diagnosis of obstructive jaundice (12). However, the etiological diagnosis of biliary strictures remains a clinical challenge, especially in patients with malignant biliary strictures, who are often in the progressive phase at the time of diagnosis and have a poor prognosis (13). Therefore, the nature of the lesion needs to be clarified as much as possible to inform the development of a treatment plan, including surgery, chemotherapy, radiotherapy, and radiofrequency ablation, all of which depend on the pathological diagnosis (14).
For pathological diagnosis, tissue sampling for the cytological and histological characterization of tumor cells and the identification of specific subtypes based on immunohistochemistry is important for accurate diagnostic management of tumors (15). The difficulty in obtaining a biopsy via the ERCP route lies in the insertion of the biopsy forceps through the duodenal papilla into the bile duct obstruction zone. Moreover, if the patient’s anatomy is altered after gastrointestinal surgery or cholangiojejunostomy, or they have concurrent combined duodenal obstructive lesions, the duodenal papilla can be difficult to find, which can result in the failure of endoscopic biopsy. In addition, ERCP-guided biopsies of the proximal biliary system often lack satisfactory accuracy and have limitations for more proximal hilar lesions.
When ERCP-based techniques fail, TIFB is a reasonable alternative for making an etiological diagnosis of a biliary stricture. Through the establishment of a tract via percutaneous hepatobiliary puncture under DSA guidance, the biliary stricture site can form a clear sign of filling defect after contrast injection, allowing for accurate localization of subsequent biopsies (16). The technical success, diagnostic sensitivity, specificity, and accuracy of TIFB in this study were 100%, 82.35%, 100%, and 86.05%, respectively, which is consistent with the results of previous studies using the PTCD approach (technical success: 90–100%; sensitivity: 61–92%; specificity: 100%) (Table 6) (17-23). Similarly, a recent systematic review and meta-analysis from Jeon et al. (24) based on data from 1,762 patients demonstrated a sensitivity of 81% and a specificity of 100% with a diagnostic odds ratio of 85.34 for the detection of malignant biliary strictures by TIFB. Therefore, TIFB holds promise for the future as a preferred means of seeking etiological diagnosis of biliary strictures after ERCP failure.
Table 6
| Author/year | D/N | TS (%) | Complications | S (%) | E (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| Oggero (17)/2021 | R/93 | 100 | Cholangitis, 4 (4%); haemobilia, 1 (1%) |
61 | 100 | 100 | 62 |
| Augustin (18)/2020 | R/13 | 100 | Hematoma, 1 (8%); cholangitis, 1 (8%) |
89 | 100 | 100 | 80 |
| Fohlen (19)/2019 | R/50 | 100 | Biliary injury, 1 (2%); haemobilia, 2 (4%); pneumoperitoneum, 1 (2%) |
70 | 100 | 100 | 22 |
| Inchingolo (20)/2019 | P/29 | 90 | None | 92 | 100 | 100 | 71 |
| Warnken (21)/2019 | R/51 | 100 | Hemobilia, 5 (10%) | 66 | 100 | 100 | 58 |
| Mohkam (22)/2017 | R/75 | 100 | – | 75 | 100 | 100 | 55 |
| Park (23)/2017 | R/271 | 100 | Hemobilia, 9 (3%); biloma, 2 (1%) |
77 | 100 | 100 | 26 |
D, design; N, number of patients; R, retrospective study; P, prospective study; TS, technical success; S, sensitivity; E, specificity; PPV, positive predictive value; NPV, negative predictive value.
In this study, analysis showed that the diagnostic accuracy of TIFB was higher for cases with SBTI on imaging and intrabiliary malignant origin than it did for cases with no SBTI or extrabiliary malignant origin (P=0.007 and P=0.003), which is a similar result to those of previous studies (17,19,23,24). Further analysis of the true positive performance of TIFB in the 34 positive diagnoses with TIFB showed that although SBTI on imaging and intrabiliary malignant origin improved the rate of true positive diagnosis in the univariate regression model, only intrabiliary malignant origin was an independent contributor to a true positive diagnosis with TIFB in the multivariate analysis. However, although the 95% CI of intrabiliary malignant origin (1.626–350.574) in the multivariate analysis did not contain 1 (indicating statistical significance), its range was wide, implying that the model was not stable or precise enough. We believe that this was due to the small sample size (n=43) in this study and that enlarging the sample size would improve the accuracy of positive results. In the future, we will conduct a study with a large sample to accurately verify the influence of intrabiliary malignant origin on the diagnostic performance of TIFB. In addition, 83.33% (5/6) of false negatives in the present study were in cases with stricture of extrabiliary malignant origin. In fact, the mechanism of biliary stricture may offer an explanation for the results of the factor analysis of true positive diagnoses by TIFB. Lesions of extrabiliary malignant origin (e.g., pancreatic cancer, malignant lymph node metastases) compress and infiltrate the biliary system externally (25), whereas malignant lesions of intrabiliary origin (e.g., cholangiocarcinoma) grow infiltratively from within the biliary lumen to outside the bile duct or along the biliary wall, and corresponding preoperative imaging changes are exhibited. Sato et al. (26) concluded that another reason for the low true positive appearance for extrabiliary diagnoses by TIFB may be the limited clamping depth of the biopsy forceps. Meanwhile, Park et al. (23) suggested that it may also related to the depth and extent of external tumor infiltration. Therefore, the use of biopsy forceps to obtain more tissue samples in the stenotic segment may be a potential measure to compensate for the poor sensitivity of TIFB in detecting extrabiliary tumors in clinical practice.
In addition, this study found that TIFB showed a slightly better diagnostic performance for proximal biliary strictures than for distal biliary stenosis, and for strictures with a length ≥3.0 cm than for those with a length <3.0 cm. However, further analysis revealed that neither the site nor the length of stricture was a predictor of a true positive TIFB result. This finding was slightly different from the results of previous studies (19,22,23). The reasons for this difference may be related to the biopsy approach and the choice of biopsy forceps adopted at our center and are as follows. First, the 9 F sheath was externally bent by 60% to fit the hilar bend before TIFB was performed. Second, for longer stenoses, our center adopts segmental sampling, which involves separate sampling of the middle-distal and the proximal parts. Third, our center chose a thicker sheath that could accommodate 6-mm biopsy forceps to obtain a sufficient sample volume. Moreover, the number of sampling sessions for TIFB (3–5 times) can be appropriately increased to ensure that an adequate sample volume is obtained, and in this study, the average was 4.14±0.89 times.
For the method of sampling, several studies have demonstrated that forceps biopsy has higher sensitivity than does brush cytology or FNA biopsy (23,27). This may be due to relatively superficial tissue being sampled, fewer cells being obtained, and cytokinesis being present in the obtained cells with brush cytology. Endoscopic ultrasonography (EUS)-FNA is still considered to be the gold standard for tissue sampling of biliary stricture (28), with a sensitivity of 81% for diagnosing the cause of distal stricture (27). A comparison of the diagnostic performances of TIFB and EUS-FNA in proximal biliary stricture showed that they have similar sensitivity (75% vs. 67%) and accuracy (81% vs. 74%) (29). Moreover, TIFB can be easily and quickly implemented based on bile duct decompression access in patients undergoing PTCD after ERCP failure. In addition, EUS-FNA carries the risk of needle tract dissemination, which is detrimental to periportal sampling (24).
It is important to open the stenosed or occluded segment and allow for complete drainage or stenting to lower bilirubin faster and restore the patient’s liver function. In the present study, all liver function indicators decreased significantly at 2 weeks postoperatively (all P<0.001), which was made possible by the placement of a drainage tube or biliary stent after TIFB. Therefore, TIFB should be considered in cases of failed endoscopic access or those which are suggestive of PTCD. In the reviewed literature, complications associated with TIFB included biopsy site bleeding and biliary fistula (17-23,30). Because the biopsy forceps cannot pass through the guidewire or the hard structure of the forceps, or due to the direction of the bile duct (the biopsy forceps need to pass an angle greater than 90°), the head end of the biopsy forceps tends to push against and slide along the opposite bile duct wall when it is inserted into the bile duct directly. As a result, bile duct injury or even perforation is extremely likely to occur. To avoid bile duct injury, the practice at our center is to pass the soft guidewire through the occluded section first, and then use the biopsy forceps to hold the guidewire and push it into the bile duct obstruction area to complete the biopsy. In the present study, the complications rate with this surgical technique was 6.98% according to the specialized classification of interventional radiology, which is acceptable when compared to the rates reported in other clinical studies (17-23,30).
The limitations of the present study were as follows. First, the study design was retrospective in nature. Second, a larger number of patients and a multicenter study design would have demonstrated a more reliable diagnostic performance and complications rates, and would have provided a reliable statistical analysis for identifying the factors associated with the diagnostic performance of TIFB. Third, we mainly analyzed patients who underwent TIFB after ERCP failure and did not draw a direct comparison regarding the endoscopic pattern. Finally, only patients with benign histological findings were followed up for final diagnosis. We believe that a clinical follow-up period of more than 12 months would be sufficient to exclude other potential diagnoses.
Conclusions
In conclusion, TIFB is a viable method for the etiological diagnosis of biliary stricture with high sensitivity and accuracy and a moderate complications rate. This technique should be considered in cases where ERCP fails or PTCD is indicated.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE and STARD reporting checklists (available at https://qims.amegroups.com/article/view/10.21037/qims-22-915/rc).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-915/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University, and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arrivé L, Hodoul M, Arbache A, Slavikova-Boucher L, Menu Y, El Mouhadi S. Magnetic resonance cholangiography: Current and future perspectives. Clin Res Hepatol Gastroenterol 2015;39:659-64. [Crossref] [PubMed]
- Tummala P, Munigala S, Eloubeidi MA, Agarwal B. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol 2013;47:532-7. [Crossref] [PubMed]
- Roos E, Hubers LM, Coelen RJS, Doorenspleet ME, de Vries N, Verheij J, Beuers U, van Gulik TM. IgG4-Associated Cholangitis in Patients Resected for Presumed Perihilar Cholangiocarcinoma: a 30-Year Tertiary Care Experience. Am J Gastroenterol 2018;113:765-72. [Crossref] [PubMed]
- Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v28-37. [Crossref] [PubMed]
- Pereira SP, Goodchild G, Webster GJM. The endoscopist and malignant and non-malignant biliary obstruction. Biochim Biophys Acta Mol Basis Dis 2018;1864:1478-83. [Crossref] [PubMed]
- Weber A, Schmid RM, Prinz C. Diagnostic approaches for cholangiocarcinoma. World J Gastroenterol 2008;14:4131-6. [Crossref] [PubMed]
- De Moura DTH, Moura EGH, Bernardo WM, De Moura ETH, Baraca FI, Kondo A, Matuguma SE, Almeida Artifon EL. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc Ultrasound 2018;7:10-9. [Crossref] [PubMed]
- Tanisaka Y, Mizuide M, Fujita A, Ogawa T, Suzuki M, Katsuda H, Saito Y, Miyaguchi K, Tashima T, Mashimo Y, Ryozawa S. Diagnostic Process Using Endoscopy for Biliary Strictures: A Narrative Review. J Clin Med 2021; [Crossref] [PubMed]
- Tieu AH, Kumbhari V, Jakhete N, Onyimba F, Patel Y, Shin EJ, Li Z. Diagnostic and therapeutic utility of SpyGlass(®) peroral cholangioscopy in intraductal biliary disease: single-center, retrospective, cohort study. Dig Endosc 2015;27:479-85. [Crossref] [PubMed]
- Varadarajulu S, Bang JY, Hasan MK, Navaneethan U, Hawes R, Hebert-Magee S. Improving the diagnostic yield of single-operator cholangioscopy-guided biopsy of indeterminate biliary strictures: ROSE to the rescue? (with video). Gastrointest Endosc 2016;84:681-7. [Crossref] [PubMed]
- Saad WE, Wallace MJ, Wojak JC, Kundu S, Cardella JF. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol 2010;21:789-95. [Crossref] [PubMed]
- Wu ZY, Jiao DC, Guo FF, Zhang DD, Ren JZ, Han XW. Treatment of biliary stenosis using percutaneous transhepatic cholangiobiopsy with biopsy forceps of varying diameter. Quant Imaging Med Surg 2022;12:207-14. [Crossref] [PubMed]
- Patel P, Rangarajan B, Mangat K. Improved Accuracy of Percutaneous Biopsy Using "Cross and Push" Technique for Patients Suspected with Malignant Biliary Strictures. Cardiovasc Intervent Radiol 2015;38:1005-10. [Crossref] [PubMed]
- Singh A, Gelrud A, Agarwal B. Biliary strictures: diagnostic considerations and approach. Gastroenterol Rep (Oxf) 2015;3:22-31. [Crossref] [PubMed]
- Kapoor BS, Mauri G, Lorenz JM. Management of Biliary Strictures: State-of-the-Art Review. Radiology 2018;289:590-603. [Crossref] [PubMed]
- Ierardi AM, Mangini M, Fontana F, Floridi C, De Marchi G, Petrillo M, Capasso R, Chini C, Cocozza E, Cuffari S, Segato S, Rotondo A, Carrafiello G. Usefulness and safety of biliary percutaneous transluminal forceps biopsy (PTFB): our experience. Minim Invasive Ther Allied Technol 2014;23:96-101. [Crossref] [PubMed]
- Oggero AS, Di Rocco F, Huespe PE, Mullen E, de Santibañes M, Claria RS, María Mazza O, Pekolk J, de Santibañes E, Hyon SH. Impact of Cholestasis on the Sensitivity of Percutaneous Transluminal Forceps Biopsy in 93 Patients with Suspected Malignant Biliary Stricture. Cardiovasc Intervent Radiol 2021;44:1618-24. [Crossref] [PubMed]
- Augustin AM, Steingrüber M, Fluck F, Goetze O, Bley TA, Kickuth R. Percutaneous endobiliary forceps biopsy of biliary strictures for histopathologic examination. Diagn Interv Radiol 2020;26:339-44. [Crossref] [PubMed]
- Fohlen A, Bazille C, Menahem B, Jegonday MA, Dupont B, Le Pennec V, Lubrano J, Guiu B, Pelage JP. Transhepatic forceps biopsy combined with biliary drainage in obstructive jaundice: safety and accuracy. Eur Radiol 2019;29:2426-35. [Crossref] [PubMed]
- Inchingolo R, Spiliopoulos S, Nestola M, Nardella M. Outcomes of percutaneous transluminal biopsy of biliary lesions using a dedicated forceps system. Acta Radiol 2019;60:602-7. [Crossref] [PubMed]
- Warnken EM, Uder M, Stein H, Wucherer M, Lell M, Muschweck H, Adamus R. Transhepatic forceps biopsy after PTCD for histological assessment of bile duct stenoses or occlusions. Z Gastroenterol 2019;57:133-8. [Crossref] [PubMed]
- Mohkam K, Malik Y, Derosas C, Isaac J, Marudanayagam R, Mehrzad H, Mirza DF, Muiesan P, Roberts KJ, Sutcliffe RP. Percutaneous transhepatic cholangiographic endobiliary forceps biopsy versus endoscopic ultrasound fine needle aspiration for proximal biliary strictures: a single-centre experience. HPB (Oxford) 2017;19:530-7. [Crossref] [PubMed]
- Park JG, Jung GS, Yun JH, Yun BC, Lee SU, Han BH, Ko JH. Percutaneous transluminal forceps biopsy in patients suspected of having malignant biliary obstruction: factors influencing the outcomes of 271 patients. Eur Radiol 2017;27:4291-7. [Crossref] [PubMed]
- Jeon TY, Choi MH, Yoon SB, Soh JS, Moon SH. Systematic review and meta-analysis of percutaneous transluminal forceps biopsy for diagnosing malignant biliary strictures. Eur Radiol 2022;32:1747-56. [Crossref] [PubMed]
- Al Mahjoub A, Menahem B, Fohlen A, Dupont B, Alves A, Launoy G, Lubrano J. Preoperative Biliary Drainage in Patients with Resectable Perihilar Cholangiocarcinoma: Is Percutaneous Transhepatic Biliary Drainage Safer and More Effective than Endoscopic Biliary Drainage? A Meta-Analysis. J Vasc Interv Radiol 2017;28:576-82. [Crossref] [PubMed]
- Sato M, Inoue H, Ogawa S, Ohashi S, Maetani I, Igarashi Y, Sakai Y. Limitations of percutaneous transhepatic cholangioscopy for the diagnosis of the intramural extension of bile duct carcinoma. Endoscopy 1998;30:281-8. [Crossref] [PubMed]
- Boos J, Yoo RJ, Steinkeler J, Ayata G, Ahmed M, Sarwar A, Weinstein J, Faintuch S, Brook OR. Fluoroscopic percutaneous brush cytology, forceps biopsy and both in tandem for diagnosis of malignant biliary obstruction. Eur Radiol 2018;28:522-9. [Crossref] [PubMed]
- Moura DTH, de Moura EGH, Matuguma SE, Dos Santos ME, Moura ETH, Baracat FI, Artifon E, Cheng S, Bernardo WM, Chacon D, Tanigawa R, Jukemura J. EUS-FNA versus ERCP for tissue diagnosis of suspect malignant biliary strictures: a prospective comparative study. Endosc Int Open 2018;6:E769-77. [Crossref] [PubMed]
- Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc 2015;81:168-76. [Crossref] [PubMed]
- Jung GS, Huh JD, Lee SU, Han BH, Chang HK, Cho YD. Bile duct: analysis of percutaneous transluminal forceps biopsy in 130 patients suspected of having malignant biliary obstruction. Radiology 2002;224:725-30. [Crossref] [PubMed]


